Defect-rich high-entropy alloy AuCuAgRuNi nanofibers based on d-orbital overlap for biomass value-added conversion†
Ruichen Geng,
Lin Su,
Xiaojun Chu,
Zhihan Zhang,
Ranran Wei,
Yinglong Wang and
Shuli Yin *
*
College of Chemical Engineering, Qingdao University of Science and Technology, Qingdao 266042, P. R. China. E-mail: yinshuli@qust.edu.cn
First published on 14th July 2025
Abstract
Developing electrocatalysts with high activity and selectivity to promote the oxidation of 5-hydroxymethylfurfural (HMF) holds substantial importance yet presents challenges in the realm of biomass upgrading. Herein, the defect-rich high-entropy alloy AuCuAgRuNi nanofibers (AuCuAgRuNi NFs) were synthesized via a facile one-pot wet chemical synthesis. Remarkably, the AuCuAgRuNi NFs demonstrate exceptional electrocatalytic performance for HMF-to-FDCA conversion under ambient conditions, achieving 97.15% yield and 98.40% selectivity with outstanding stability. Theoretical calculations reveal that defect-induced electronic modulation optimizes reactant adsorption, while d-orbital hybridization among multi-metallic components facilitates electron redistribution, synergistically enhancing catalytic activity. Furthermore, HMF oxidation has been successfully integrated with hydrogen evolution in a flow electrolysis cell, and an energy-efficient coupled system has been established, which holds promise for scalable biomass valorization. This research provides valuable insights into the innovative synthesis and inherent catalytic mechanisms of high-entropy alloy systems.
1. Introduction
Global energy shortages and climate change challenges have made it an urgent necessity to innovate advanced technologies for generating sustainable substitutes for fossil fuels.1 5-Hydroxymethylfurfural (HMF) is a versatile compound that can be transformed into valuable chemical intermediates through oxidation reaction, specifically yielding 2,5-furandicarboxylic acid (FDCA) as a important product which is regarded as an environmentally friendly alternative to petroleum-derived and widely-used polyethylene terephthalate.2 Over the past few years, the process of selectively turning HMF into FDCA has rapidly become a hot topic in relevant fields.3,4 Typically, FDCA can be manufactured through the oxidation of HMF, a process usually achieved by traditional thermal catalytic methods.5 Although extensive research has been conducted on HMF oxidation reaction (HMFOR), many methods still rely on severe conditions like high oxygen pressure and high temperatures.6,7 In recent years, the electrocatalytic oxidation of HMF into FDCA has drawn growing interest, thanks to its merits like low energy consumption and gentle operating conditions.8 Significantly, HMF can be coupled with the hydrogen evolution reaction (HER) to simultaneously produce clean hydrogen fuel.9 This not only boosts the economic value but also enhances the energy efficiency. While a great many Ni-based catalysts have been devised for HMFOR, regrettably, those catalysts experience serious and unregulated surface restructuring in alkaline environments.10 This situation can cause a substantial decline in catalytic activity. As a result, crafting sturdy and stable electrocatalysts to realize high-performance HMFOR holds significant importance.High-entropy alloys (HEAs), emerging as significant materials, feature equimolar multi-element compositions (≥5 principal components) with near-isotopic stoichiometry.11,12 The potent cooperative impacts among multiple elements, prominent lattice deformation, and outstanding configurational entropy are anticipated to offer outstanding HMFOR activity and stability upon nanoscale HEAs.13,14 Moreover, a wide compositional range affords increased adaptability in the compositional options for catalyst design and optimization.15–17 The high-entropy stabilization mechanism effectively suppresses metallic leaching in HEAs under harsh electrochemical conditions (pH > 13), thus enhancing corrosion resistance.18 Concurrently, electronic structure tunability through d-band center modulation optimizes HMFOR catalytic efficiency. Even more importantly, atomic-level engineering of nanomaterial architectures enables precise interfacial modulation for boosted electrocatalytic performance.19 Nanofiber architectures enhance catalytic activity through optimized electron/mass transport and improved material utilization, while simultaneously preserving electrocatalytic surface integrity and maximizing accessible active sites.20,21 Consequently, nanofibers hold great promise in the application of HMFOR. Therefore, to strengthen the development of HEAs for catalyzing HMFOR, a crucial first step is to synthesize HEAs nanostructures with precisely controlled morphologies and a wide range of elemental compositions.22–24 Despite its significant importance, it still poses a hard challenge to control the morphology and composition of HEAs structures through mild approaches.
In our recent research, we have effectively created high-entropy alloy nanowires. These nanowires have a d–d orbital coupling self-complementary effect. When applied to HMF oxidation, nanowires exhibit excellent catalytic activity and structural stability, fully demonstrating the unique advantages and potential of high-entropy alloy materials in the field of biomass conversion. In this research, a defect-rich AuCuAgRuNi NFs catalyst which further enhanced the electro-oxidation performance of HMFOR, thereby improving the yield and selectivity of the product FDCA is introduced. Simultaneously, the overpotential required for AuCuAgRuNi NFs to reach a current density of 10 mA cm−2 is only 1.39 V vs. RHE, and it also has a low Tafel slope of 75.97 mV dec−1. The excellent performance of this catalyst in the HMFOR can be credited to two main aspects. On the one hand, the nanofiber structure of the catalyst increases the reaction area and speeds up the reaction rate. On the other hand, density functional theory (DFT) calculations reveal that the different elements in the high-entropy alloy have d-orbitals that strongly overlap. Also, there are intense interactions among multiple metal components. These factors help optimize the electron transfer between metal sites, which is crucial for enhancing the catalytic performance.
2. Experimental
2.1. Materials and chemicals
Key reagents were sourced from commercial suppliers under strict purity specifications. Chloroauric acid (HAuCl4·4H2O, ≥48%), 4-aminopyridine (4-AP, 98%), copper(II) chloride dihydrate (CuCl2·2H2O, 99.99%), potassium hydroxide (KOH, 90%), and ascorbic acid (AA, ≥99%) were purchased from Aladdin. Nickel(II) chloride hexahydrate (NiCl2·6H2O, 99%), ruthenium(III)-2,4-pentanedionate (C15H21O6Ru, 97%), Nafion solution (5 wt%), ammonium formate (NH4HCO2, AR), 5-hydroxymethylfurfural (C6H6O3, 97%), and silver nitrate (AgNO3, ≥99.8%) were obtained from Macklin.2.2. Sample preparation
To a 7 mL aqueous solution containing 4-AP (0.0475 g), 1 mL aliquots of 20 mM solutions of HAuCl4·4H2O, CuCl2·2H2O, AgNO3, C15H21O6Ru, and NiCl2·6H2O were sequentially added. The resultant mixture was heated in an oil bath maintained at 90 °C. Subsequently, 1 mL of 0.1 M AA was rapidly injected into the reaction mixture, and the solution was allowed to react for 20 minutes. Upon completion, the black precipitate of AuCuAgRuNi NFs was collected by centrifugation at 7700 revolutions per minute (rpm). The collected precipitate was then washed five times with water and ethanol.3. Results and discussion
3.1. Characterizations
Fig. 1a depicts the one-step wet chemical synthesis of AuCuAgRuNi NFs. The metal precursors were thoroughly mixed in a 4-aminophenol (4-AP) aqueous solution and subsequently heated in a 90 °C oil bath. The rapid addition of ascorbic acid (AA) was then carried out and the reaction conditions were maintained for an additional 20 minutes. The morphological features of the AuCuAgRuNi NFs were examined using the transmission electron microscope (TEM) (Fig. 1b). The TEM image with false coloring more clearly displays the nanofibers structure of AuCuAgRuNi NFs (Fig. S1†) and the diameter of AuCuAgRuNi NFs is about 10.82 nm (Fig. 1c). Moreover, energy dispersive X-ray spectroscopy (EDS) analysis demonstrates that the elemental proportions in AuCuAgRuNi NFs are 23.96% Au, 27.60% Cu, 8.59% Ag, 6.52% Ru, and 33.33% Ni, conforming the definition of HEAs (Fig. 1d). Simultaneously, the high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM) image in Fig. 1e coupled with the corresponding elemental mappings illustrate the homogeneous distribution of Au, Cu, Ag, Ru, and Ni elements across the AuCuAgRuNi NFs. This consistent presence of all elements across the nanofibers confirms the successful synthesis of the HEAs.More importantly, high resolution TEM (HRTEM) images show that AuCuAgRuNi NFs exhibit a multitude of structural defects, including amorphous sites (Fig. 2a), twin boundary (Fig. 2b), grain boundary (Fig. 2c), and atomic step (Fig. 2d). Introducing defects offers several benefits in catalysis. Firstly, it can modulate the electronic structure around the catalyst, which optimizes adsorption and enhances electrocatalytic activity. Secondly, other atoms can be inserted into these defect sites to stabilize the structure and create new active centers, further regulating the local electronic environment.25,26 Furthermore, the selected area electron diffraction (SAED) pattern of AuCuAgRuNi NFs displays four clear concentric rings. These rings correspond to the (111), (200), (220), and (311) planes, analogous to typical fcc-phase Au systems, confirming the polycrystalline characteristic of the catalyst (Fig. 2e). HRTEM image shows a lattice spacing of 0.223 nm, matching the (111) plane of the face-centered cubic (fcc) structure (Fig. 2f). Similarly, the X-ray diffraction (XRD) pattern, displays diffraction peaks characteristic of a fcc structure. The four well-defined diffraction peaks observed at 2θ angles of 38.54°, 44.51°, 64.78°, and 77.80° correspond to (111), (200), (220), and (311) crystallographic planes respectively, exhibiting a characteristic face-centered cubic (fcc) arrangement that matches reference patterns for metallic gold. This structural congruence with established fcc systems verifies the successful synthesis of high-entropy alloys (Fig. 2g).19
 | ||
| Fig. 2 (a–d) HRTEM images of AuCuAgRuNi NFs. (e) SAED image of AuCuAgRuNi NFs. (f) HRTEM image of AuCuAgRuNi NFs. (g) XRD spectrum of AuCuAgRuNi NFs. | ||
To elucidate the formation mechanism of AuCuAgRuNi NFs, we conducted a series of controlled experiments, synthesizing samples under diverse reaction conditions. As illustrated in Fig. S2,† TEM images of samples prepared with varying precursor amounts reveal that adjusting precursor dosages significantly impacts AuCuAgRuNi NFs formation. Excessive usage of precursors will affect the formation of nanofibers, highlighting the vital role of optimal precursor ratios in the synthesis of AuCuAgRuNi NFs. Thermal parameters were identified as another key determinant. At low temperatures, sluggish reduction kinetics favored the formation of chemically stable nanoparticles rather than continuous nanofibers. Conversely, when the temperature increases, the morphology of nanofibers will be affected (Fig. S3†). Reaction time also plays a crucial role: insufficient time restricts atomic diffusion, preventing complete structure formation, while overly long reaction times promote grain growth, degrading the desired nanofiber morphology (Fig. S4†). These findings collectively underscore that precisely controlled reaction conditions are essential for the successful fabrication of AuCuAgRuNi NFs.
X-ray photoelectron spectroscopy (XPS) was utilized to examine the chemical oxidation states of AuCuAgRuNi NFs. Fig. 3a shows the presence of elements such as Au, Cu, Ag, Ru, and Ni in AuCuAgRuNi NFs. As depicted in Fig. 3b, the XPS spectrum for Au 4f can be separated into two distinct peak, specifically the Au0 4f5/2 and Au0 4f7/2 peaks which located at 87.6 and 83.8 eV.27 The XPS spectrum for Cu 2p also exhibits two sets of peaks, identified as Cu 2p1/2 (954.2, 951.9 eV) and Cu 2p3/2 (934.6, 931.9 eV) (Fig. 3c). This peak pattern can correspond to the chemical states of Cu+/Cu0 and Cu2+. Combining with the Cu LMM auger spectrum, the kinetic energies located at 573.9 eV, corresponding to Cu+ which verifies the presence of Cu+ (Fig. S5†). The presence of Cu+ and Cu2+ is most likely due to the oxidation of the material upon exposure to air. Moreover, the peaks located at 962.9 and 943.2 eV are attributed to the satellite peaks.28 As shown in Fig. 3d, the Ag 3d spectrum features two distinct peaks at 373.7 and 367.6 eV, which are indicative of the presence of Ag0. Additionally, the binding energies at 375.2 and 369.1 eV are characteristic of Ag+.29 In the Ru 3p spectrum, the bands at 485.20 and 465.05 eV correspond to Ru0 3p1/2 and Ru0 3p3/2. Additionally, two higher binding energy peaks in the Ru 3p spectrum are located at 489.8 and 468.70 eV, which are in good agreement with Ru4+ 3p1/2 and Ru4+ 3p3/2 (Fig. 3e).30 The Ni 2p spectrum reveals the presence of both Ni0 and Ni2+ species. The binding energies of 873.5 and 855.5 eV correspond to Ni0 2p1/2 and 2p3/2, respectively, while 876.0 and 858.1 eV are assigned to Ni2+ 2p1/2 and 2p3/2, respectively. Furthermore, the additional peaks observed at 879.8 and 861.6 eV are identified as satellite peaks associated with the Ni 2p transitions (Fig. 3f).31 The synergistic interaction between the metallic states and oxidized states of Cu, Ag, Ru, and Ni in AuCuAgRuNi NFs facilitates efficient electron transfer, thereby significantly enhancing their electrocatalytic activity.31,32
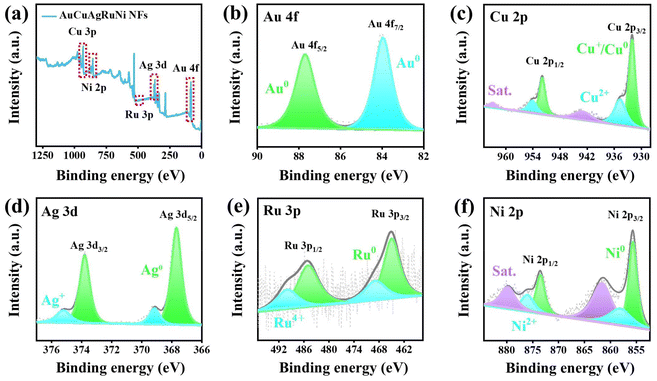 | ||
| Fig. 3 (a) XPS survey spectrum of AuCuAgRuNi NFs. (b) Au 4f, (c) Cu 2p, (d) Ag 3d, (e) Ru 3p, (f) Ni 2p XPS spectra of AuCuAgRuNi NFs. | ||
3.2. Performance in electrochemical reactions and theoretical analysis
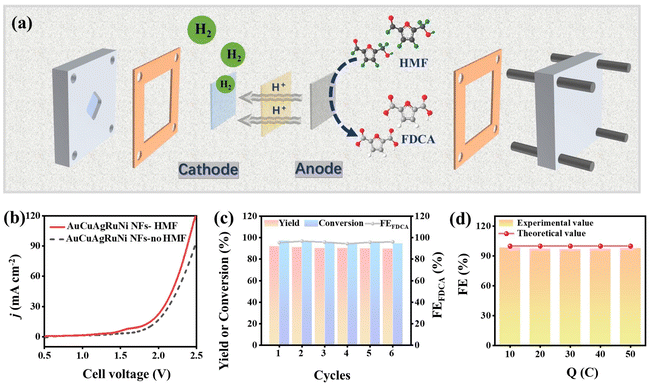
The electrocatalytic activity for HMFOR was assessed using a typical three-electrode setup (Fig. S6†). The HMFOR was initially contrasted with the oxygen evolution reaction (OER) via linear sweep voltammetry (LSV) cures, considering that the OER acts as a competing reaction. As shown in Fig. 4a, the AuCuAgRuNi NFs occurred at approximately 1.64 V vs. RHE in 1 M KOH to achieve 10 mA cm−2. When HMF was introduced, the requisite potential dropped to 1.39 V vs. RHE, which was 250 mV lower than that of the OER for achieving a comparable current density (Fig. S7†). This finding indicates that the addition of HMF modified the thermodynamic properties of the reaction, rendering the HMF oxidation process more favorable compared to the OER. Remarkably, AuCuAgRuNi NFs have an oxidation peak at 1.40 V vs. RHE approximately, which is due to the oxidation of Ni2+ to Ni3+.33 In addition, under the identical voltage, the current density obtained by the control sample AuCuAgRu alloy is much lower than that of AuCuAgRuNi NFs (Fig. S8†). For example, at 1.50 V vs. RHE, the difference in the current density reached by the two is 23.57 mA cm−2 (Fig. 4b). It reveals that Ni performs a crucial function in the HMFOR. Specifically, AuCuAgRuNi NFs exhibit a relatively small Tafel slope of 75.97 mV dec−1, which implies more favorable kinetics for the HMFOR process (Fig. 4c). This characteristic is crucial as it directly correlates with the reaction rate and efficiency, suggesting that AuCuAgRuNi NFs can potentially drive the HMFOR at a faster pace and with greater ease compared to the AuCuAgRu alloy. The charge transfer resistance (Rct) serves as a crucial performance metric and was consequently measured using electrochemical impedance spectroscopy (EIS). Fig. 4d presents the nyquist plots of AuCuAgRuNi NFs and AuCuAgRu alloy in 1 M KOH with 10 mM HMF. Notably, the Rct of AuCuAgRuNi NFs is markedly reduced in 1 M OKH with 10 mM HMF. This indicates that AuCuAgRuNi NFs exhibit a more rapid charge-transfer kinetics for the HMFOR in comparison to the AuCuAgRu alloy. Additionally, LSV tests were conducted on samples with varying precursor amounts, temperatures, and reaction times to investigate the different conditions on HMFOR performance. The results show that AuCuAgRuNi NFs synthesized under typical conditions exhibit higher HMFOR activity, indicating that appropriate synthesis conditions significantly influence HMFOR activity (Fig. S9†).High-performance liquid chromatography (HPLC) was utilized to identify the oxidation products and quantify the corresponding yield and selectivity of FDCA. The standard curve of FDCA was fitted by HPLC in Fig. S10.† Additionally, error bars are included to represent the standard deviation of at least three independent experiments. When the voltage is too low, both the yield and selectivity of FDCA are less than 60% (Fig. 4e). When the voltage is too high, although the conversion rate is relatively high, the faradaic efficiency of FDCA (FEFDCA) gradually declines (Fig. S11†). Therefore, on the premise of ensuring a relatively high yield and selectivity of FDCA, we choose the minimum voltage of 1.50 V vs. RHE having a yield of 97.15% along with a selectivity reaching 98.40%. Meanwhile, at 1.50 V vs. RHE, both the yield and selectivity of FDCA for the AuCuAgRu alloy are much inferior to those of AuCuAgRuNi NFs (Fig. 4f). After 6 h of chronoamperometric (i–t) test, AuCuAgRuNi NFs possess the highest residual current density (Fig. S12†). Surprisingly, after 2000 cyclic voltammetry (CV) cycles, the LSV curves and morphology of AuCuAgRuNi NFs have significant slight changes, which proves its excellent stability (Figs S13 and S14†). These studies indicate that AuCuAgRuNi NFs have excellent HMFOR performance.
In biomass upgrading technology, enhancing the selectivity of target products is crucial. The electro-oxidation process of HMF involves two main pathways, attributed to the coexistence of carbonyl and hydroxyl groups in its molecular structure (Fig. 5a). In pathway 1, the aldehyde group of HMF is preferentially oxidized, forming 5-hydroxymethylfurancarboxylic acid (HMFCA) as an intermediate product. In pathway 2, the hydroxymethyl part of HMF is oxidized with preference, yielding 2,5-diformylfuran (DFF) as an intermediate product. Ultimately, both HMFCA and DFF are further oxidized to produce 5-formyl-2-furancarboxylic acid (FFCA), which is then converted into FDCA. To further study the HMF reaction pathway under the action of AuCuAgRuNi NFs, HPLC chromatography is used to determine the peak time and the content of organic substances (Fig. S15†). As the electrolysis duration increased at 1.50 V vs. RHE, the concentration of HMF progressively diminished, while the concentration of FDCA correspondingly rose, signifying the effective conversion of HMF into FDCA (Fig. 5b). Concurrently, both HMFCA and FFCA were detected during electrolysis, yet DFF has never been detected from start to finish, suggesting that the electrooxidation of HMF on the AuCuAgRuNi NFs electrode predominantly followed pathway (Fig. 5c). Stability test serves as a key determinant for gauging catalyst performance. To assess this, 10 i–t tests were conducted at a constant potential of 1.50 V vs. RHE to evaluate the stability of the AuCuAgRuNi NFs catalyst during the HMFOR. As shown in Fig. 5d and Fig. S16,† the AuCuAgRuNi NFs electrode exhibited excellent durability. There is no obvious change in the FDCA yield, HMF conversion, and FEFDCA. The improvement in the HMFOR performance of AuCuAgRuNi NFs is due to the lattice strain caused by the abundant structural defects in the nanofibers, which accelerates the processes of charge transfer and mass transfer. Therefore, AuCuAgRuNi NFs exhibit higher activity than other materials (Table S1†).
DFT calculations were utilized to elucidate the orbital hybridization patterns, providing a deeper understanding of the active sites, electronic structure, and catalytic performance of the catalysts during the entire HMFOR process. After geometric optimization, the AuCuAgRuNi NFs exhibit a subtle positional distortion for each constituent element (Fig. 5e and Fig. S17†). Despite these minor distortions, the overall structure retains its stability, thereby confirming the robustness of the AuCuAgRuNi NFs configuration. To achieve a more profound understanding of the electronic structures, the partial projected density of states (PDOS) for each constituent element in the AuCuAgRuNi NFs has been depicted (Fig. 5f). An evident overlap could be observed between the d-orbitals of varied elements. The robust interplay among the multi-metal constituents not only expedites electron transfer across distinct metal sites but also furnishes numerous active sites for electrocatalytic processes. Remarkably, the d-orbitals of these elements cross the Fermi level (EF), suggesting the possibility of electron transfer between the surface of the AuCuAgRuNi NFs and adsorbates. This trait is beneficial for enhancing the efficiency of electrocatalytic reactions.34,35 Concurrently, the d-band center also discloses the balance within the electronic structure influenced by the varied elemental composition, as depicted in Fig. 5g. The results show that the d-band center of Cu-3d is highly close to that of the HEAs. The d-band centers of Rh-4d and Ni-3d orbitals are situated near the EF, implying that these sites exhibit strong adsorption capacities. Other elements with either higher or lower d-band centers ensure the oxidative capacity of HEAs for HMFOR.
3.3. Electrocatalytic performance for HMFOR & HER
To optimize the electrocatalytic performance, we engineered a continuous-flow electrolysis system integrating HMFOR–HER synergistic catalysis, establishing an energy-efficient platform for coupled electrochemical transformations. The AuCuAgRuNi NFs serve as the anode for the HMFOR in the electrolytic cell, while the Pt mesh acts as the cathode (Fig. 6a). Fig. 6b illustrates the LSV curves for both the HMFOR–HER and OER–HER systems. The findings indicate that the HMFOR–HER system consistently achieves higher current densities compared to the OER–HER system across the entire range of potentials tested (Fig. S18†). The HMFOR–HER integrated configuration demonstrates superior energy economy, with the synergistic anodic process significantly enhancing overall cell efficiency. The HMFOR performance test of the coupling system conducted at 1.80 V showed that the yield of FDCA, the conversion rate of HMF, and the FEFDCA all remained above 90% without significant fluctuations. The system demonstrated excellent stability over 6 cycles (Fig. 6c). The hydrogen generation was efficiently collected via the drainage method, achieving a yield that closely aligns with the theoretical predictions. The faradaic efficiency can reach 98% (Fig. 6d). The HMFOR–HER coupling system offers new insights into the concurrent enhancement of biomass upgrading and hydrogen generation, thereby contributing to the development of cleaner energy solutions.4. Conclusion
In summary, defect-engineered AuCuAgRuNi nanoflowers (NFs), synthesized via a facile one-pot approach, demonstrate remarkable catalytic efficiency for the electrochemical oxidation of HMF to FDCA, delivering a 97.15% yield with 98.40% selectivity under ambient conditions and showcasing practical viability. Defect engineering modulates the electronic configuration, optimizing adsorption dynamics and substantially boosting electrocatalytic activity. DFT calculations reveal that d-orbital hybridization and synergistic multi-metallic interactions drive efficient electron transfer across the catalyst. This study offers a strategic paradigm for sustainable biomass valorization through rational design of high-entropy electrocatalysts.Author contributions
Ruichen Geng: data curation, writing – original draft. Lin Su: methodology. Xiaojun Chu: supervision. Zhihan Zhang: data curation. Ranran Wei: supervision. Yinglong Wang: supervision. Shuli Yin: funding acquisition, writing – review & editing.Conflicts of interest
The authors report no declarations of interest.Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its ESI.†Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 22408193), and the Natural Science Foundation of Shandong Province (No. ZR2023QB076).References
- Y. Lu, C.-L. Dong, Y.-C. Huang, Y. Zou, Z. Liu, Y. Liu, Y. Li, N. He, J. Shi and S. Wang, Identifying the Geometric Site Dependence of Spinel Oxides for the Electrooxidation of 5-Hydroxymethylfurfural, Angew. Chem., Int. Ed., 2020, 59, 19215–19221 CrossRef CAS PubMed
.
- J. Lei, H. Zhang, J. Yang, J. Ran, J. Ning, H. Wang and Y. Hu, Structural designs and mechanism insights into electrocatalytic oxidation of 5-hydroxymethylfurfural, J. Energy Chem., 2025, 100, 792–814 CrossRef CAS
.
- G. Liu, T. Nie, Z. Song, X. Sun, T. Shen, S. Bai, L. Zheng and Y.-F. Song, Pd Loaded NiCo Hydroxides for Biomass Electrooxidation: Understanding the Synergistic Effect of Proton Deintercalation and Adsorption Kinetics, Angew. Chem., Int. Ed., 2023, 62, e202311696 CrossRef CAS PubMed
.
- Y. Wang, H. He, H. Lv, F. Jia and B. Liu, Two-dimensional single-crystalline mesoporous high-entropy oxide nanoplates for efficient electrochemical biomass upgrading, Nat. Commun., 2024, 15, 6761 CrossRef CAS PubMed
.
- M. G. Davidson, S. Elgie, S. Parsons and T. J. Young, Production of HMF, FDCA and their derived products: a review of life cycle assessment (LCA) and techno-economic analysis (TEA) studies, Green Chem., 2021, 23, 3154–3171 RSC
.
- M. Kim, Y. Su, A. Fukuoka, E. J. M. Hensen and K. Nakajima, Aerobic Oxidation of 5-(Hydroxymethyl)furfural Cyclic Acetal Enables Selective Furan-2,5-dicarboxylic Acid Formation with CeO2-Supported Gold Catalyst, Angew. Chem., Int. Ed., 2018, 57, 8235–8239 CrossRef CAS PubMed
.
- B. Tharat, L. Ngamwongwan, T. Seehamongkol, B. Rungtaweevoranit, J. Nonkumwong, S. Suthirakun, K. Faungnawakij, N. Chanlek, A. Plucksacholatarn, W. Nimsaila, C. Prommin and A. Junkaew, Hydroxy and surface oxygen effects on 5-hydroxymethylfurfural oxidation to 2,5-furandicarboxylic acid on β-MnO2: DFT, microkinetic and experiment studies, Nanoscale, 2024, 16, 678–690 RSC
.
- B. J. Taitt, D.-H. Nam and K.-S. Choi, A Comparative Study of Nickel, Cobalt, and Iron Oxyhydroxide Anodes for the Electrochemical Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid, ACS Catal., 2019, 9, 660–670 CrossRef CAS
.
- S. Barwe, J. Weidner, S. Cychy, D. M. Morales, S. Dieckhöfer, D. Hiltrop, J. Masa, M. Muhler and W. Schuhmann, Electrocatalytic Oxidation of 5-(Hydroxymethyl)furfural Using High-Surface-Area Nickel Boride, Angew. Chem., Int. Ed., 2018, 57, 11460–11464 CrossRef CAS PubMed
.
- H. G. Cha and K.-S. Choi, Combined biomass valorization and hydrogen production in a photoelectrochemical cell, Nat. Chem., 2015, 7, 328–333 CrossRef CAS PubMed
.
- T. A. A. Batchelor, J. K. Pedersen, S. H. Winther, I. E. Castelli, K. W. Jacobsen and J. Rossmeisl, High-Entropy Alloys as a Discovery Platform for Electrocatalysis, Joule, 2019, 3, 834–845 CrossRef CAS
.
- Y. Wang, X.-Y. Zhang, H. He, J.-J. Chen and B. Liu, Ordered Mesoporous High-Entropy Intermetallics for Efficient Oxygen Reduction Electrocatalysis, Adv. Energy Mater., 2024, 14, 2303923 CrossRef CAS
.
- J. Zhang, C. Wang, S. Huang, X. Xiang, Y. Xiong, B. Xu, S. Ma, H. Fu, J. Kai, X. Kang and S. Zhao, Design high-entropy electrocatalyst via interpretable deep graph attention learning, Joule, 2023, 7, 1832–1851 CrossRef CAS
.
- A. Ludwig, Maximize mixing in highly polyelemental solid solution alloy nanoparticles, Matter, 2021, 4, 2100–2101 CrossRef CAS
.
- T. A. A. Batchelor, T. Löffler, B. Xiao, O. A. Krysiak, V. Strotkötter, J. K. Pedersen, C. M. Clausen, A. Savan, Y. Li, W. Schuhmann, J. Rossmeisl and A. Ludwig, Complex-Solid-Solution Electrocatalyst Discovery by Computational Prediction and High-Throughput Experimentation, Angew. Chem., Int. Ed., 2021, 60, 6932–6937 CrossRef CAS PubMed
.
- Y. Yao, Z. Liu, P. Xie, Z. Huang, T. Li, D. Morris, Z. Finfrock, J. Zhou, M. Jiao, J. Gao, Y. Mao, J. Miao, P. Zhang, R. Shahbazian-Yassar, C. Wang, G. Wang and L. Hu, Computationally aided, entropy-driven synthesis of highly efficient and durable multi-elemental alloy catalysts, Sci. Adv., 2020, 6, eaaz0510 CrossRef CAS PubMed
.
- K. Deng, Z. Lian, W. Wang, J. Yu, H. Yu, Z. Wang, Y. Xu, L. Wang and H. Wang, Lattice Strain and Charge Redistribution of Pt Cluster/Ir Metallene Heterostructure for Ethylene Glycol to Glycolic Acid Conversion Coupled with Hydrogen Production, Small, 2024, 20, 2305000 CrossRef CAS PubMed
.
- Y. Yang, X. Sun, G. Han, X. Liu, X. Zhang, Y. Sun, M. Zhang, Z. Cao and Y. Sun, Enhanced Electrocatalytic Hydrogen Oxidation on Ni/NiO/C Derived from a Nickel-Based Metal–Organic Framework, Angew. Chem., Int. Ed., 2019, 58, 10644–10649 CrossRef CAS PubMed
.
- X. Fu, J. Zhang, S. Zhan, F. Xia, C. Wang, D. Ma, Q. Yue, J. Wu and Y. Kang, High-Entropy Alloy Nanosheets for Fine-Tuning Hydrogen Evolution, ACS Catal., 2022, 12, 11955–11959 CrossRef CAS
.
- K. Deng, J. Yu, Q. Mao, R. Yang, H. Yu, Z. Wang, J. Wang, L. Wang and H. Wang, Superlattices in Ru Metallene Nanobelts for Robust Hydrogen Evolution, Adv. Funct. Mater., 2025, 35, 2420728 CrossRef CAS
.
- H. Lv and B. Liu, Multidimensionally ordered mesoporous intermetallics: Frontier nanoarchitectonics for advanced catalysis, Chem. Soc. Rev., 2024, 53, 11321–11333 RSC
.
- G. Feng, F. Ning, J. Song, H. Shang, K. Zhang, Z. Ding, P. Gao, W. Chu and D. Xia, Sub-2 nm Ultrasmall High-Entropy Alloy Nanoparticles for Extremely Superior Electrocatalytic Hydrogen Evolution, J. Am. Chem. Soc., 2021, 143, 17117–17127 CrossRef CAS PubMed
.
- L. Fan, Y. Ji, G. Wang, J. Chen, K. Chen, X. Liu and Z. Wen, High Entropy Alloy Electrocatalytic Electrode toward Alkaline Glycerol Valorization Coupling with Acidic Hydrogen Production, J. Am. Chem. Soc., 2022, 144, 7224–7235 CrossRef CAS PubMed
.
- X. Min, H. Lv, Y. Yamauchi and B. Liu, Porous Metal Nanocrystal Catalysts: Can Crystalline Porosity Enable Catalytic Selectivity?, CCS Chem., 2022, 4, 1829–1842 CrossRef CAS
.
- Y. Zhang, J. Liu, Y. Xu, C. Xie, S. Wang and X. Yao, Design and regulation of defective electrocatalysts, Chem. Soc. Rev., 2024, 53, 10620–10659 RSC
.
- H. Li, J. Lai, Z. Li and L. Wang, Multi-Sites Electrocatalysis in High-Entropy Alloys, Adv. Funct. Mater., 2021, 31, 2106715 CrossRef CAS
.
- S. Jeong, A. J. Branco, S. W. Bollen, C. S. Sullivan and M. B. Ross, Universal pH electrocatalytic hydrogen evolution with Au-based high entropy alloys, Nanoscale, 2024, 16, 11530–11537 RSC
.
- Y. Xin, H. Fu, L. Chen, Y. Ji, Y. Li and K. Shen, High-Entropy Engineering
in Hollow Layered Hydroxide Arrays to Boost 5-Hydroxymethylfurfural Electrooxidation by Suppressing Oxygen Evolution, ACS Cent. Sci., 2024, 10, 1920–1932 CrossRef CAS PubMed
.
- D. Fan, K. Guo, Y. Zhang, Q. Hao, M. Han and D. Xu, Engineering high-entropy alloy nanowires network for alcohol electrooxidation, J. Colloid Interface Sci., 2022, 625, 1012–1021 CrossRef CAS PubMed
.
- F. Lu, L. Zong, G. Zhang, P. Li, K. Fan, S. Jiang, X. Chen, P. Liu and L. Wang, Ultrafine high entropy alloys with Ru activated sites for highly durable and industrial grade electrocatalytic water splitting, Composites, Part B, 2025, 301, 112501 CrossRef CAS
.
- Y. Xin, L. Chen, Y. Li and K. Shen, Highly selective electrosynthesis of 3,4-dihydroisoquinoline accompanied with hydrogen production over three-dimensional hollow CoNi-based microarray electrocatalysts, Nano Res., 2024, 17, 2509–2519 CrossRef CAS
.
- M. Hu, J. Li, T. Liu, Z. Wu and Y. Du, Insights into the formation and growth of high entropy PdPtSnPbNi nanowires to obtain catalysts with high alcohol electrocatalytic oxidation activity, J. Colloid Interface Sci., 2024, 675, 481–487 CrossRef CAS PubMed
.
- Y. Song, W. Xie, Y. Song, H. Li, S. Li, S. Jiang, J. Y. Lee and M. Shao, Bifunctional integrated electrode for high-efficient hydrogen production coupled with 5-hydroxymethylfurfural oxidation, Appl. Catal., B, 2022, 312, 121400 CrossRef CAS
.
- H. Li, M. Sun, Y. Pan, J. Xiong, H. Du, Y. Yu, S. Feng, Z. Li, J. Lai, B. Huang and L. Wang, The self-complementary effect through strong orbital coupling in ultrathin high-entropy alloy nanowires boosting pH-universal multifunctional electrocatalysis, Appl. Catal., B, 2022, 312, 121431 CrossRef CAS
.
- C. Zhan, Y. Xu, L. Bu, H. Zhu, Y. Feng, T. Yang, Y. Zhang, Z. Yang, B. Huang, Q. Shao and X. Huang, Subnanometer high-entropy alloy nanowires enable remarkable hydrogen oxidation catalysis, Nat. Commun., 2021, 12, 6261 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5qi00895f |
| This journal is © the Partner Organisations 2025 |