Amine-functionalized CNTs@mSiO2 with short radical mesochannels for fast and efficient CO2 capture
Liju Bai,
Xiaotong Jiang,
Yimin Deng,
Shuai Wang and
Helei Liu *
*
Joint International Research Laboratory of Carbon Neutrality System and Engineering Management, Beijing Laboratory for System Engineering of Carbon Neutrality, School of Chemistry and Chemical Engineering, Beijing Institute of Technology, Beijing, 102488, China. E-mail: lhl0925@hotmail.com; hl_liu@bit.edu.cn
First published on 23rd July 2025
Abstract
Amine-functionalized adsorbents exhibit significant potential for CO2 capture due to their high efficiency and selectivity. However, their practical application remains constrained by their limited adsorption capacity and slow adsorption kinetics. Herein, we demonstrated an ethylenediamine (ED)-functionalized mesoporous silica-coated carbon nanotube (CNTs@mSiO2-ED) composite for fast and efficient CO2 capture. The novel adsorbent was synthesized through a two-step process involving the synthesis of mesoporous silica-coated carbon nanotubes (CNTs@mSiO2) as amine carriers via an interfacial self-assembly strategy, followed by amine functionalization using a vapor deposition method. Both the carriers and final adsorbents possess abundant short radical mesochannels, offering direct and ultra-short diffusion paths for amines during functionalization and CO2 for adsorption. As a result, the CNTs@mSiO2-ED adsorbent achieved a maximum CO2 adsorption capacity of 68 mL g−1 (∼3 mmol g−1, 298 K, 1 bar) and the facile uptake of CO2, reaching a t50 (the time to achieve 50% of the maximum adsorption capacity) of 1.93 min, 1.70 times higher and 1.32 times shorter than those of ED-functionalized SBA-15, respectively. This work opens up a new avenue for the design of CO2 adsorption materials to overcome the adsorption and reaction kinetics limitations of other solid porous materials envisaged.
Introduction
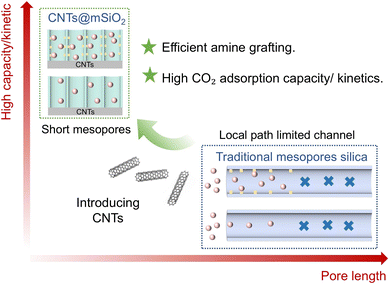
Rising atmospheric CO2 levels are the primary cause of detrimental climate change. CCUS (carbon capture, utilization, and storage) technology represents the most promising and effective short-term solution for reducing CO2 levels. The significance of CCUS in addressing climatic challenges is widely acknowledged.1 Currently, aqueous amine solutions are used in industrial-scale absorption for CO2 capture.2–5 However, this solvent-based method is susceptible to oxidation, corrosion, and degradation, and it involves secondary pollution and significant energy consumption during solvent regeneration, which hinder its broader implementation for CO2 capture.6 To overcome these challenges, many research efforts have been directed toward developing porous solid adsorbents for CO2 capture, including mesoporous silica, zeolites, and metal–organic frameworks (MOFs).7–9 Despite having advantages such as non-corrosive characteristics and lower environmental impact,10,11 solid adsorbents suffer from weak interaction (mainly van der Waals interactions),12–16 making them inadequate to meet the technological requirements for industrial CO2 capture. Therefore, highly polar adsorption sites are incorporated into solid porous materials to enhance their interaction towards CO2, thus reaching the expected adsorption capacity.17 Amidst the array of functionalized solid sorbents, amine-modified solid materials significantly stand out for their exceptional performance in capturing CO2 at low concentrations.18–21 Typically, these adsorbents are synthesized by impregnating amine molecules into a porous material, by grafting amino-silane onto a carrier surface, or by performing in situ polymerization of amine monomers.22–25 The objectives are to enhance adsorption capacity and selectivity. Nevertheless, the reported amine-functionalized adsorbents still exhibit limited CO2 adsorption capacity, which is insufficient to meet the actual industrial demands.Researchers have shown that the pore structure of the carrier material significantly affects the CO2 capture performance of solid amine adsorption materials.26–29 Although microporous materials possess high specific surface areas, their small pore size is comparable to that of amine molecules and can hence hamper amine molecule transport during functionalization, leading to the low modification efficiency of amino sites. The small pore size can also hinder the diffusion of CO2 during adsorption, thereby impeding the utilization of amino sites.30 As a result, the CO2 capture performance is constrained. By contrast, macro-porous materials suffer from insufficient surface areas for amine modification and CO2 adsorption.31 Contrary to these micro- and macro-porous materials, mesoporous materials are considered high potential carriers for solid amine adsorption due to their relatively large pore size as well as their high specific surface area and pore volume.32,33 Until now, a variety of mesoporous materials, including mesoporous silica and mesoporous carbon, have been examined as carriers for solid amine adsorbents, with the main focus on SBA-15 and MCM-41 as typical mesoporous silica materials owing to the high pore structure tunability and abundant surface sites for modification.34 However, these carrier materials often possess an elongated (>300 nm) tubular pore structure, which limits the accessibility to internal pores and hinders mass transport, leading to the insufficient utilization of the amino groups located deep within the pores (Fig. 1). Consequently, the CO2 adsorption performance of the final solid amine adsorption materials is constrained in terms of both adsorption capacity and adsorption kinetics.35,36 There is, therefore, an urgent need to design mesoporous solid amine adsorption materials with enhanced pore surface accessibility for high-performance CO2 capture.
Herein, a novel structured CNTs@mSiO2-ED solid amine adsorbent has been demonstrated for enhanced CO2 capture. As illustrated in Fig. 1, we first synthesized the CNTs@mSiO2 carrier material using a micellar interfacial self-assembly strategy to create abundant mesoporous open channels with a pore size of ∼2.4 nm, a channel length of ∼12 nm, and a specific surface area of 950.1 m2 g−1. Subsequently, the final CNTs@mSiO2-ED solid amine adsorbent was obtained by modifying the carrier material with ED using a simple vapor deposition strategy. Owing to the ultra-short mesoporous channel length and the vertically aligned channel direction, the diffusion kinetics of amine and CO2 molecules in the interior of the mesoporous surface are remarkably enhanced. Experimental results will be compared with those of the previously proposed SBA-15 material. It will be demonstrated that the prepared CNTs@mSiO2-ED solid amine adsorbent possesses a higher amine modification efficiency and CO2 capture performance, as shown by the high CO2 adsorption capacity and its short half-capacity time, significantly exceeding the equivalent capacity and adsorption rate of SBA-15-ED.
Results and discussions
High specific surface area of CNTs@mSiO2
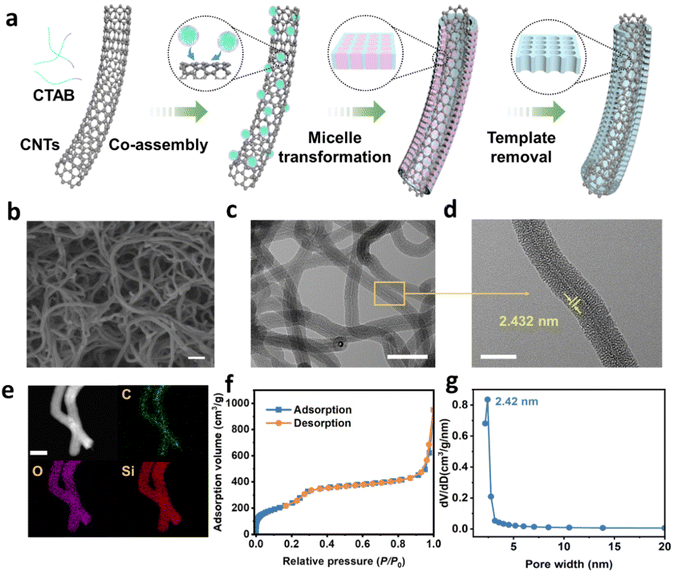
As illustrated in Fig. 2a and S1, the successful synthesis of CNTs@mSiO2 was realized by an interfacial self-assembly strategy, which involved the slow hydrolysis and condensation of silica precursors and the self-assembly process of composite micelles on a CNT surface guided by coulombic interactions. Specifically, cetyltrimethylammonium bromide (CTAB) was first added to deionized water and ethanol to form positively charged micelles under stirring. Subsequently, TEOS, CNTs and ammonia were added, with CNTs as the growth interface and ammonia as the catalyst. Catalyzed by ammonia, tetraethyl orthosilicate (TEOS) slowly hydrolyzed and polymerized, forming negatively charged silica oligomers. The oligomers and CTAB micelles further self-assembled on the CNTs surface via coulombic interactions.37 The kinetically controlled micelle nucleation and slow micelle growth resulted in a shift in the micelle shape from spherical to cylindrical. After further condensation of silica oligomers, vertically aligned mesochannels were formed.33To gain more insights into the vital role of the short radical pore and the chemical environment of the channel in the CNTs@mSiO2, CNTs@mSiO2 with different mesochannel lengths were prepared as the amine carriers (Fig. S2 and Table S2). The CNTs@mSiO2 channels obtained by adding 1.2 mL of TEOS were of a moderate length and size and thus were the main subject of study in this paper. The scanning electron microscopy (SEM) images show that the prepared CNTs@mSiO2 has a uniform nanofilament morphology, with an average diameter of ∼40.0 nm (Fig. 2b). The effective washcoat structure of CNTs by silica is evident in the TEM image shown in Fig. 2c. The radial channel length was measured using ImageJ software, with an average length of approximately 12 nm (Fig. S3). Besides, numerous mesochannels with a diameter of ∼2.4 nm can be identified on the coating. High-resolution magnified TEM images (Fig. 2d) disclose that the mesochannels are vertically aligned in a perpendicular direction to the CNT surface. Energy-dispersive X-ray mappings (Fig. 2e) indicate that C, O, and Si are evenly distributed in CNTs@mSiO2. In addition, the region of C is significantly smaller than that of O and Si, which further confirms that the mesoporous silica layer covers the entire surface of CNTs. The nitrogen adsorption–desorption isotherm of CNTs@mSiO2 exhibits representative type-IV curves, suggesting a typical mesoporous structure (Fig. 2f). The Brunauer–Emmett–Teller (BET) specific surface area and total pore volume of CNTs@mSiO2 are 950.1 m2 g−1 and 0.774 cm3 g−1, respectively. The pore distribution curve calculated by the BJH method demonstrates that the pore size of the mesochannels is centered at 2.4 nm (Fig. 2g).
Amine functionalization of the CNTs@mSiO2
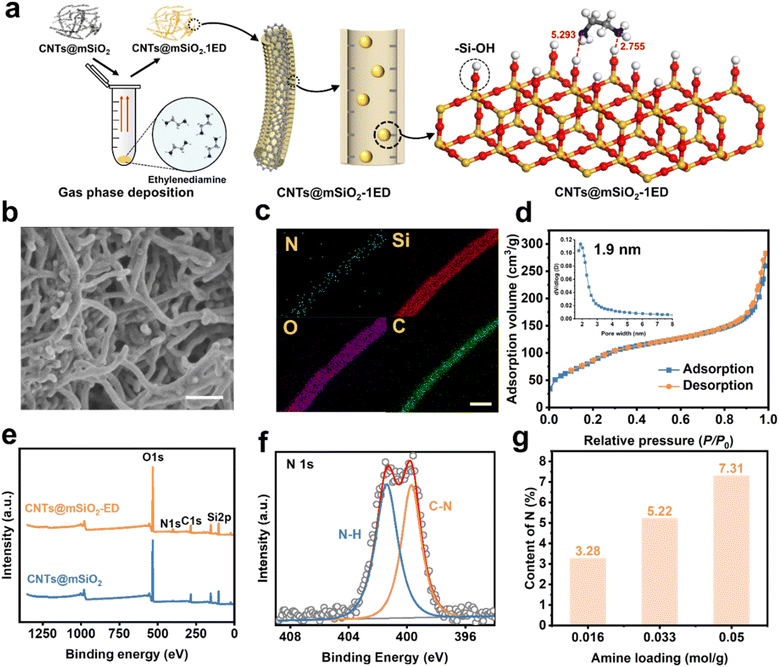
The synthesized CNTs@mSiO2 exhibits a uniform radial mesoporous pore structure, demonstrating it as a promising amine carrier. To elucidate the synergistic effect between amines and the CNTs@mSiO2, a selection of three low-boiling point amines with distinct structural features and different amino groups was made, namely, piperazine (PZ), ethylenediamine (ED) and 1,3-propanediamine (13P). The detailed structural and physical parameters of the three amines are delineated in Table S2. On this basis, employing a vapor deposition method, the amines were converted into vapor molecules using a process of heating (363 K) and vacuuming. CNTs@mSiO2 was treated with amines to obtain the amine-functionalized CNTs@mSiO2-Amine adsorbents, where the vapors subsequently entered the CNTs@mSiO2 pore structure via molecular diffusion and were deposited within the pores (Fig. 3a).38 Notably, ED demonstrated superior amine functionalization efficiency, with a three-dimensional structure of 0.700 nm × 0.640 nm × 0.482 nm. The ED molecules diffuse into the silica mesochannels of CNTs@mSiO2 via vapor deposition, establishing weak interaction forces with the silicon hydroxyl groups on the pore walls via acid–base interactions and hydrogen bonding. This interaction modifies the mesoporous walls, thereby imparting amine functionality (Fig. 3a). As shown in Fig. 3b, the morphology of CNTs@mSiO2 after amino-functionalized modification does not change. Analyzing the distribution of the N element in the EDS mapping diagram of the TEM image reveals that the N element is successfully introduced and evenly distributed on the surface of CNTs@mSiO2 (Fig. 3c). The Brunauer–Emmett–Teller (BET) specific surface area and total pore volume of CNTs@mSiO2-ED are 450.1 m2 g−1 and 0.437 cm3 g−1, respectively. The pore distribution curve analysis, performed using the models based on the non-local density functional theory (NLDFT), shows that the pore size is centered at 1.9 nm (Fig. 3d). In addition, the introduction of ED molecules was further verified by X-ray electron spectroscopy (XPS). Fig. 3e shows the relevant XPS spectrum, in which the N1s peak of CNTs@mSiO2-ED is obvious, which once again proves the successful introduction of amino functional groups.39 The N 1s spectrum of CNTs@mSiO2-ED (Fig. 3f and S4) shows the C–N (399.6 eV) and N–H (401.0 eV) bonds, which are mainly derived from ED. In addition, the content of N in CNTs@mSiO2-ED was varied by adjusting the amount of ED pretreatment. The N contents treated with 0.016, 0.033 and 0.05 mol amine/gCNTs@mSiO2 are 3.28%, 5.22% and 7.31%, respectively (Fig. 3g). However, an excessive loading results in a significant reduction of the specific surface area of CNTs@mSiO2, from an initial 950.1 m2 g−1 to a range of 200–450 m2 g−1, indicating that ED molecules cover the pore structure of CNTs@mSiO2. In this study, 0.033 molAmine/gCNTs@mSiO2 ED was used for amine functionalization.High-performance CO2 adsorption
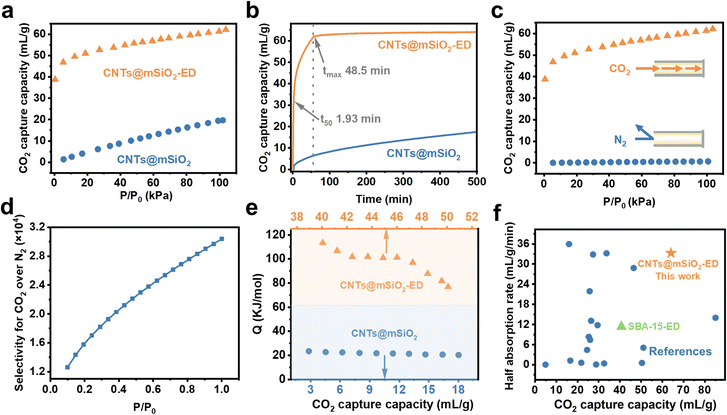
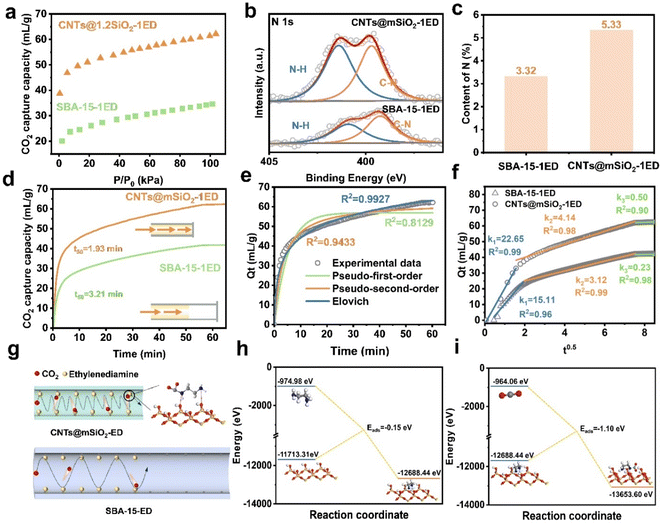
The CO2 adsorption capacity of the CNTs@mSiO2-Amine adsorbent at 1 bar and 298 K was investigated under different amine species, treatment temperatures and amine addition amounts. The CO2 adsorption capacity ranges from 40 to 68 mL g−1 (Fig. S5). The performance is much higher than that of the pristine CNTs@mSiO2 absorbent (19.1 mL g−1), validating the effectiveness of vapor deposition for amine impregnation. Notably, among the different CNTs@mSiO2-Amine absorbents, CNTs@mSiO2-ED shows the highest CO2 adsorption capacity of 68 mL g−1, which is 2.56 times that of the CNTs@mSiO2 absorbent (Fig. 4a). This is because the deposited amino groups can react with CO2 to form carbamates, thereby enhancing CO2 capture. The influence of the temperature on the CO2 capture capacity was investigated. With the temperature increasing from 273 K to 315 K, the CO2 capture capacity decreases from 76 to 45 mL g−1, indicating a clear thermodynamic control of the CO2 adsorption hand (Fig. S5).40 The kinetic adsorption performance of adsorbents for gas molecules is a vital factor in the actual separation process. Considering this, the CO2 dynamic adsorption isotherms of CNTs@mSiO2 and CNTs@mSiO2-ED were measured at 298 K and 1 bar. The adsorption kinetics curve shown in Fig. 4b reveals that CNTs@mSiO2-1ED exhibits rapid CO2 adsorption with a t50 (the time to achieve 50% of the maximum adsorption capacity) of 1.93 min and an equilibrium time of about 48.5 min, which are much faster than those of the pristine CNTs@mSiO2 absorbent (3.21 min). The dynamic saturation adsorption of CO2 is more than 68 mL g−1 and that of N2 is almost negligible (Fig. 4c). The CO2/N2 selectivity was calculated using the ideal adsorbed solution theory (IAST) (eqn S1), which thermodynamically predicts the behavior of mixed gas adsorption based on single–component equilibrium sorption isotherms. These calculations were conducted at 298 K and 1 bar to simulate conditions typical of flue gas. The selectivities derived from IAST quantify the adsorbent's preferential uptake of CO2 over N2 in binary gas mixtures. The calculated selectivity of CNTs@mSiO2-ED reaches up to over 3.6 × 104 in the entire pressure range from close to zero to 1 bar (Fig. 4d).41 The ultra-high selectivity and uptake ratio of CO2 further indicate that CNTs@mSiO2-ED is an outstanding candidate for capturing CO2 from CO2/N2 mixtures. To verify the binding affinity of CNTs@mSiO2-ED for CO2, the isosteric adsorption enthalpy (Qst) of CNTs@mSiO2-ED for CO2 was calculated using the Clausius–Clapeyron equation. As shown in the supplementary equation (eqn S2),42 the Qst of CO2 at a near-zero loading in CNTs@mSiO2-ED is 115 kJ mol−1. It is worth noting that the calculated Qst of CNTs@mSiO2-ED is much higher than that of CNTs@mSiO2 (21 kJ mol−1), suggesting stronger host–guest interactions between CNTs@mSiO2-ED and CO2 (Fig. 4e). The CO2 adsorption process of CNTs@mSiO2-ED exhibits multiple adsorption stages, with chemical adsorption involving two primary aminos as the dominating ones. Compared to other reported amine-modified mesoporous silica absorbents, the CNTs@mSiO2-ED demonstrates superior CO2 adsorption performance and a rapid adsorption rate (as shown in Fig. 4f and Table S3). These results solidly demonstrate that CNTs@mSiO2-ED can break the trade-off between the adsorption capacity and adsorption kinetics. The adsorption behaviour is attributed to the short radical mesochannels, implying the ideal screening ability of CNTs@mSiO2-ED.Short adsorption path-enhanced dynamics
The excellent CO2 adsorption performance of CNTs@mSiO2-ED can be attributed to the delicately designed CNTs@mSiO2 amine carrier with short radical mesochannels, which ensures a high amine functionalization efficiency and a short CO2 diffusion path. To gain more insights into the vital role of the short radical pore and the chemical environment of the channel in the CNTs@mSiO2-ED for the adsorption of CO2. As a control, the conventional silica material SBA-15 was employed as the amine carrier to prepare the SBA-15-ED absorbent using the same vapor deposition method. Despite the larger pore size (∼5 nm), which is favorable for amine vapor and CO2 penetration, SBA-15-ED exhibits a much lower amine functionalization efficiency, CO2 capture capacity and adsorption kinetics than CNTs@mSiO2-ED. As shown in Fig. 5a and b, the N element content analysis determined from the XPS spectrum demonstrates that SBA-15-ED has an N content of 3.32%, much lower than that of CNTs@mSiO2-ED at 5.33%. Consequently, SBA-15-ED shows an insufficient CO2 capture capacity of 35 mL g−1 due to inadequate amine sites for the chemical adsorption of CO2 (Fig. 5c). To evaluate the adsorption kinetics of CNTs@mSiO2-ED and SBA-15-ED, we quantified the t50 from the CO2 adsorption plot. At a relative pressure close to 1.0 bar, the t50 are 1.93 min and 3.21 min, respectively, at 298 K. The t50 ofCNTs@mSiO2-ED is 40.0% shorter than that of SBA-15 (Fig. 5d). In addition, CNTs@mSiO2 with different mesochannel lengths were prepared as amine carriers. The CO2 capture capacity decreases and the t50 increases with an increase in the mesochannel length, further confirming the superiority of the short diffusion path for high capacity and fast CO2 capture (Fig. S7). The plot of CO2 adsorption amount over time reveals the relationship between the structure of the substance and the adsorption properties and predicts the adsorption process and the results by kinetic modelling, and the equations of the four models are shown in eqn S3–6 of the SI.43 The specific parameters are shown in Table S4. As shown in Fig. 5e, the R2 values of the proposed pseudo-first-order kinetic model and the proposed pseudo-second-order kinetic model are close to 0.99, indicating that the adsorption process of CNTs@mSiO2 is not dominated by pure physical adsorption or chemical adsorption. The R2 of the Elovich kinetic model is 0.9927, proving its adequacy. In addition, an internal diffusion model was used to characterize the different reaction stages of CO2 adsorption (Fig. 5f and Table S4). The results show that CNTs@mSiO2-ED is controlled by both physical adsorption and chemical adsorption. In the early stage of adsorption, CO2 molecules diffuse into the pores, a part of the CO2 molecules undergo physical adsorption, and the rest of the gas molecules undergo chemical adsorption. During chemisorption, CO2 reacts with the amino sites provided by ED to form carbamate, so the adsorption capacity at this stage is significantly enhanced, and with the progress of the adsorption time, more and more affinity sites are occupied, and the diffusion resistance of CO2 molecules gradually increases (Fig. 5g). Cyclic stability is crucial for solid amine adsorption materials. The strength of the bond between the amino group and the material, as well as the bond strength between the CO2 and the amino group, significantly impacts the CO2 adsorption cycle in CNTs@mSiO2-ED. Based on this, the adsorption energy of silica hydroxyl on ED in the CNTs@mSiO2 channel was calculated using first principles (Fig. S8). The calculated results show that CNTs@mSiO2 requires −0.15 eV to adsorb ethylenediamine (Fig. 5h). In conclusion, ED can modify the surface of the CNTs@mSiO2 to increase the number of active sites for amino group, thus promoting the CO2 adsorption process. The adsorption energy between the amine-modified carrier material and CO2 is calculated as −1.10 eV, showing a strong binding force, which further explains the reason for the high selectivity of CO2/N2 (Fig. 5i).
Conclusions
In this study, we demonstrated a carbon nanotube-reconstructed mesoporous silica with amino-functionalized sites for efficient and fast CO2 capture. In CNTs@mSiO2 with ultra-short mesochannels as carriers, both amine and CO2 diffusion paths were effectively shortened, thereby realizing efficient functionalization and fast CO2 capture. Ultimately, the obtained CNTs@mSiO2-ED adsorbent achieved a maximum CO2 adsorption capacity of 68 mL g−1 (298 K, 1 bar) and a facile uptake of CO2 (t50 1.93 min), which are, respectively, 1.70 times higher and 1.32 times faster than those of SBA-15-ED. This work has far-reaching significance and provides novel inspiration for the design of solid amine adsorbents with superior pore structures for high-performance CO2 adsorption.Experiments and methods
Materials and chemicals
Carbon nanotubes (CNTs) (BET = 281.2 m2 g−1) were obtained from the Beijing Advanced Innovation Centre for Smart Matter Science and Engineering of the Beijing University of Chemical Technology. Cetyltrimethylammonium bromide (CTAB, 99%), ethylenediamine (ED), piperazine (PZ), 1,3-propanediamine (13P) and tetraethyl orthosilicate (TEOS, AR) were purchased from Aldrich. All chemicals were used as received without further purification. Deionized water was used for all experiments.Synthesis of the carrier CNTs@mSiO2
First, 0.3 g of CTAB was dispersed in 80 mL of deionized water and then sonicated until the solution became clear. Next, 100 mg of CNTs was added to the solution and sonicated for 60 min. After adding 70 mL of anhydrous ethanol and 1 mL of ammonia to the suspension, the entire suspension was sonicated for 10 min. Finally, 10 mL of a TEOS ethanol solution was added, and the reaction was carried out at room temperature and 2000 rpm for 7 hours. When the reaction was complete, the final sample was washed three times using a hydrochloric acid–ethanol solution with stirring at 333 K and then freeze-dried to obtain the final sample, named CNTs@mSiO2, where “m” represents the volume of TEOS added.Vapor-phase deposition for amine impregnation
The amine-functionalized modification was carried out by vapor deposition. Firstly, CNTs@mSiO2 was vacuumed at 393 K to remove residual moisture and other volatile components. After pretreatment, amines were added under vacuum with a special device and heated to 363 K for amine functionalization. The obtained material was named CNTs@mSiO2−xAmine. Different structural amines were used for amine functionalization, mainly including PZ, ED and 13P, as shown in Table S1.Gas adsorption measurements
The CO2 adsorption capacity and adsorption kinetics analyses were carried out using Hiden Analytical IGA100C and Kubor 1000, respectively. Before gas adsorption measurements, the nonvolatile solvent molecules in the adsorbent should be removed by heating (373 K) for 1 h, thus achieving full activation. Then, the static adsorption and dynamic adsorption tests were carried out at 298 K. Among them, the dynamic adsorption was measured according to the change in the mass.Characterization of CNTs@mSiO2 and CNTs@mSiO2-ED
CNTs@mSiO2 and CNTs@mSiO2-1ED were characterized to assess their structural properties. N2 physisorption was performed at 77 K for relative pressures ranging from 10−5 to 0.99 using a surface area and porosity analyzer (Autosorb-iQ-MP). The pore size distribution was calculated from the adsorption branch of the isotherms using the Barrett–Joyner-Halenda (BJH) formula. The micropore size distribution was calculated using the non-local density functional theory (NLDFT). Transmission electron microscopy (TEM) images were acquired on a JEM-2100F transmission electron microscope with an accelerating voltage of 200 kV equipped with a post-column Gatan imaging filter (GIF-Tridium). The samples for TEM measurements were suspended in ethanol and supported on a carbon film on a Cu grid. Scanning electron microscopy (SEM) images were taken using a ZEISS Gemini-SEM 360. The –NH2 sites were determined by in situ FT-IR experiments of pyridine adsorption and calculated from the quantitative analyses of the characteristic adsorption IR bands at 1455 and 1545 cm−1. X-ray photoelectron spectroscopy (XPS) was performed on an AXIS ULTRA DLD XPS system with a MONO Al source (Shimadzu Corp.) to characterize the NH2-modified mesoporous silica.Author contributions
Liju Bai: writing – original draft, software, investigation, data curation. Xiaotong Jiang: methodology. Yimin Deng: writing – review & editing. Shuai Wang: investigation. Helei Liu: writing – review & editing, validation, funding acquisition.Conflicts of interest
The authors declare no competing financial interest.Data availability
The data supporting the findings of this paper are available within the main text, the supplementary information (see DOI: https://doi.org/10.1039/d5ta04410c), or from the corresponding authors upon reasonable request.Acknowledgements
The authors gratefully acknowledge the support by the National Key Research and Development Program of China (No.2024YFB4106200), Projects of International Cooperation and Exchanges NSFC (W2512003) and Hebei Natural Science Foundation (B2024105009). The instrumental support from the Analysis and Testing Center of Beijing Institute of Technology is highly appreciated.References
- C. Chao, Y. Deng, R. Dewil, J. Baeyens and X. Fan, Renew. Sustain. Energy Rev., 2020, 138, 110490 CrossRef
.
- X. Sun, X. Shen, H. Wang, F. Yan, J. Hua, G. Li and Z. Zhang, Nat. Commun., 2024, 15, 5068 CrossRef CAS
.
- L. Bai, S. Wang, D. Zhao, W. Wu, D. Chen, M. Li, T. M. Aminabhavi and H. Liu, Chem. Eng. Sci., 2024, 296, 120271 CrossRef CAS
.
- Y. Ji, R. Y. Xie, C. Wu, X. Y. Liu, X. J. Zhang and L. Jiang, J. CO2 Util., 2023, 69, 102422 CrossRef CAS
.
- S. Chen, L. Chen, L. Zhang and X. Li, Sep. Purif. Technol., 2025, 353, 128623 CrossRef CAS
.
- F. Vega, A. Sanna, B. Navarrete, M. M. Maroto-Valer and V. J. Cortés, Greenhouse Gases:Sci. Technol., 2014, 4, 707–733 CrossRef CAS
.
- Z. Zhu, H. Tsai, S. T. Parker, J.-H. Lee, Y. Yabuuchi, H. Z. H. Jiang, Y. Wang, S. Xiong, A. C. Forse, B. Dinakar, A. Huang, C. Dun, P. J. Milner, A. Smith, P. Guimarães Martins, K. R. Meihaus, J. J. Urban, J. A. Reimer, J. B. Neaton and J. R. Long, J. Am. Chem. Soc., 2024, 146, 6072–6083 CrossRef CAS PubMed
.
- L. Zhang, Q. Lei, M. Yi, Z. Zhang, X. Lian, J. Xu, S. Zhang, L. Li, B. Li and X. H. Bu, Angew. Chem., Int. Ed., 2024, 137, e202421753 CrossRef
.
- A. Rajendran, G. K. H. Shimizu and T. K. Woo, Adv. Mater., 2023, 36, 2301730 CrossRef
.
- T. D. Bennett, F.-X. Coudert, S. L. James and A. I. Cooper, Nat. Mater., 2021, 20, 1179–1187 CrossRef CAS
.
- R. L. Siegelman, E. J. Kim and J. R. Long, Nat. Mater., 2021, 20, 1060–1072 CrossRef CAS PubMed
.
- G. Rim, P. Priyadarshini, M. Song, Y. Wang, A. Bai, M. J. Realff, R. P. Lively and C. W. Jones, J. Am. Chem. Soc., 2023, 145, 7190–7204 CrossRef CAS
.
- Y. Zhang, S. Zhao, L. Li, J. Feng, W. Qiu, Z. Wei, X. Li, Z. Huang and H. Lin, Gas Sci. Eng., 2024, 123, 205251 CrossRef CAS
.
- S. Krachuamram, K. C. Chanapattharapol and N. Kamonsutthipaijit, Microporous Mesoporous Mater., 2021, 310, 110632 CrossRef CAS
.
- M. A. Sakwa-Novak and C. W. Jones, ACS Appl. Mater. Interfaces, 2014, 6, 9245–9255 CrossRef CAS
.
- Z. Zhao, Y. Zhang, R. M. Othman, W. Ha, J. Wang, T. Wang, L. Zhong, J. Wang and W.-P. Pan, Process Saf. Environ. Prot., 2024, 186, 151–165 CrossRef CAS
.
- Z. Zheng, Y. S. Wang, M. Wang, G. H. Zhao, G. P. Hao and A. H. Lu, Nat. Commun., 2024, 15, 8919 CrossRef CAS PubMed
.
- J. S. A. Carneiro, G. Innocenti, H. J. Moon, Y. Guta, L. Proaño, C. Sievers, M. A. Sakwa-Novak, E. W. Ping and C. W. Jones, Angew. Chem., Int. Ed., 2023, 62, e202302887 CrossRef CAS PubMed
.
- K. Min, W. Choi, C. Kim and M. Choi, Nat. Commun., 2018, 9, 726 CrossRef
.
- P. Zang, J. Tang, H. Zhang, X. Wang, P. Zhao, L. Cui, J. Chen, P. Zhao and Y. Dong, Sep. Purif. Technol., 2024, 331, 125622 CrossRef CAS
.
- O. Shekhah, Y. Belmabkhout, Z. Chen, V. Guillerm, A. Cairns, K. Adil and M. Eddaoudi, Nat. Commun., 2014, 5, 4228 CrossRef CAS
.
- X. Jin, J. Ge, L. Zhang, Z. Wu, L. Zhu and M. Xiong, Inorganics, 2022, 10, 87 CrossRef CAS
.
- X. Suo, Y. Yu, S. Qian, L. Zhou, X. Cui and H. Xing, Angew. Chem., Int. Ed., 2021, 60, 6986–6991 CrossRef CAS PubMed
.
- J. Huang, Y. Huang, X. Yu, Y. Zhao, X. Wu, P. Liang, Z. Li, H. Wang and Q. Xia, Small, 2024, 21, 2411010 CrossRef
.
- S. Fan, C. Liang, F. Feng, K. Wong, K. Wang, S. Jia, N. Bhuwania, S. Zhang and S. Zhang, Angew. Chem., Int. Ed., 2024, 137, e202421028 CrossRef
.
- G. Zainab, A. A. Babar, N. Ali, A. A. Aboalhassan, X. Wang, J. Yu and B. Ding, J. Colloid Interface Sci., 2020, 561, 659–667 CrossRef CAS PubMed
.
- H. Dan, H. Lei, J. Luo, Z. Guo, Y. Xiang, Y. Chen and Y. Ding, J. Environ. Chem. Eng., 2023, 11, 111379 CrossRef CAS
.
- Y. Li, J. Li, X. Wang, Y. Ma, W. Guan and T. Qian, Mater. Chem. Phys., 2016, 182, 402–408 CrossRef CAS
.
- F. Lou, A. Zhang, G. Zhang, L. Ren, X. Guo and C. Song, Appl. Energy, 2020, 264, 114637 CrossRef CAS
.
- G. Song, C. Li, T. Wang, K. H. Lim, F. Hu, S. Cheng, E. Hondo, S. Liu and S. Kaw, Small, 2023, 20, 2305517 CrossRef PubMed
.
- J. Zhu, Q. Zhang, L. guo, Y. Zhao, R. Zhang, L. Liu and J. Yu, Chem. Eng. J., 2022, 434, 134662 CrossRef CAS
.
- Z. Zhao, P. Zhang, Y. Zhao, L. Wang, J. Zhang, F. Bu, W. Zhou, R. Zhao, X. Zhang, Z. Lv, Y. Liu, Y. Xia, W. Zhang, T. Zhao, D. Chao, W. Li and D. Zhao, Nat. Protoc., 2024, 1–42 Search PubMed
.
- Y. Yang, S. Zhou, Z. Lv, C. T. Hung, Z. Zhao, T. Zhao, D. Chao, B. Kong and D. Zhao, J. Am. Chem. Soc., 2024, 146, 19580–19589 CrossRef CAS PubMed
.
- H. Shi, R. Cao, H. Zhang, J. Yang, Y. Fu and J. Chen, Adv. Funct. Mater., 2024, 35, 2415211 CrossRef
.
- J. Weng, C. Zhu, B. Zhao, W. Tang, X. Lu, F. Liu, M. Wu, Y. Ding and P.-X. Gao, Nat. Commun., 2024, 15, 5541 CrossRef CAS
.
- H. Liu, X. Jiang, R. Idem, S. Dong and P. Tontiwachwuthikul, AIChE J., 2022, 68, e17816 CrossRef CAS
.
- Y. Liu, W. Li, D. Shen, C. Wang, X. Li, M. Pal, R. Zhang, L. Chen, C. Yao, Y. Wei, Y. Li, Y. Zhao, H. Zhu, W. Wang, A. M. El-Toni, F. Zhang and D. Zhao, Chem. Mater., 2015, 27, 5577–5586 CrossRef CAS
.
- F. Zhao, B. Zhu, L. Wang and J. Yu, J. Colloid Interface Sci., 2024, 659, 486–494 CrossRef CAS
.
- W. Li and D. Fu, Chem. Phys. Lett., 2024, 843, 141244 CrossRef CAS
.
- S. Hu, Y. Miao, Y. Guo, H. Wu and Y. Miao, Chem. Eng. Sci., 2025, 302, 120737 CrossRef CAS
.
- S. Zhao, Z. Zhao, Z. Zha, Z. Jiang, Z. Wang and M. D. Guiver, Adv. Funct. Mater., 2024, 34, 2314469 CrossRef CAS
.
- X. An, K. Zhao, W. Pang, W. Zhang, L. Wang, T. Guo and D. Fu, Chem. Eng. J., 2022, 431, 133877 CrossRef CAS
.
- M. Liao, S. Long, P. Zou, K.-Y. Zhang, H.-C. Liang, K. Jiang, D. Xie and F.-M. Yang, Energy Fuels, 2023, 37, 15867–15878 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2025 |