Electrochemical properties of a ferrocene-containing poly(vinyl alcohol)-based redox hydrogel and its application as a matrix for electrochromic devices†
Yutaka Ohsedo *a and
Riri Eguchib
*a and
Riri Eguchib
aDivision of Engineering, Faculty of Engineering, Nara Women's University, Kitauoyahigashi-machi, Nara 630-8506, Japan. E-mail: ohsedo@cc.nara-wu.ac.jp
bFaculty of Human Life and Environment, Nara Women's University, Japan
First published on 21st July 2025
Abstract
For the creation of novel electrochemically active redox hydrogel materials, this study developed a novel redox-active hydrogel for wearable electronics by incorporating a ferrocene/hydroxypropyl-β-cyclodextrin (Fc/CD) inclusion complex into a poly(vinyl alcohol) (PVA)/borax-based gel at various ratios. A systematic evaluation revealed that, despite their pseudo-thixotropic behavior and soft gel nature, the resulting hydrogels maintained the chemically reversible electrochemical characteristics of Fc. Moreover, the hydrogels demonstrated electrochemical reversibility in a hydrogel medium, particularly under slow potential sweep conditions. The synthesized hydrogel was used as an electroactive matrix to fabricate a single-layer gel-based electrochromic device containing methyl viologen dibromide as the electrochromic molecule. The device displayed distinct color changes upon voltage application, thereby confirming its electrochromic functionality. These findings demonstrate that the developed redox hydrogel functions effectively as a redox-active matrix for electrochemical devices, with significant potential for advanced applications in electrochemical and wearable technologies.
1. Introduction
Poly(vinyl alcohol) (PVA) ranks among the most widely produced water-soluble polymers, finding extensive use in both industrial applications and everyday products.1,2 Recently, PVA-based hydrogels have garnered considerable attention for their potential applications in biomaterials and novel hybrid materials.3–6 Hydrogels derived from PVA/DMSO7 and PVA/glycerine8 systems exhibit robust network structures where PVA crystallites function as pseudo-crosslinking points, enhancing mechanical durability. This durability, combined with their versatility, positions PVA hydrogels as promising candidates for diverse functional applications.The incorporation of appropriate electrolyte solutions into PVA hydrogels imparts ionic conductivity, rendering them valuable as electrolyte gels for rechargeable batteries and electrochemical capacitors.9 Ionically crosslinked hydrogels formed through PVA–borax interactions—commonly known as “slime” or borax gels—were initially regarded merely as toys.10 However, recent research has expanded their applications to advanced technologies, including bioelectronics that leverage Na+ ion conductivity from borax11 and ion-conductive matrices for electrochromic materials.12 Consequently, PVA hydrogels have garnered significant interest in electrochemical applications and materials science beyond their basic electrical properties.
The development of hydrogel-based materials for electronic applications represents an active research area.13–17 Their inherent softness, tuneable electronic and ionic conductivity, and biocompatibility render these materials promising candidates for next-generation gel-based electrodes. Electrochemically active hydrogels are particularly noteworthy for their potential in sensing, energy storage and bioelectronics.16,17 Such hydrogels can be engineered via the incorporation of salts or ionizable functional groups for ionic conductivity, or through the introduction of electroactive species to enable redox activity.18–23 These innovations facilitate the development of novel redox-active hydrogels with significant promise for bioelectronic applications.
In recent years, the application of hydrogels to electrochemical devices has gained considerable attention, particularly in the development of electrochromic devices. Electrochromism refers to the reversible change in optical absorption properties of a material through electrochemical redox reactions.24 This property has been extensively utilized in applications such as smart windows and antiglare mirrors for automobiles.24,25 Researchers are currently exploring various electrochemically active materials as novel electrochromic compounds.26,27 Among these efforts, redox-active hydrogels have emerged as promising candidates for electrochromic device materials due to their unique combination of redox activity and soft matter properties.28–30 In particular, their inherent softness and high ionic diffusivity—comparable to those in liquid electrolytes—allow for efficient electrochemical processes within the gel matrix. While electrolyte gels are increasingly being employed as substitutes for conventional liquid electrolytes,31 there is growing interest in utilizing redox-active hydrogels for colour-tunable wearable devices and wearable displays, where both flexibility and electrochemical functionality are required.
In this study, we developed and evaluated an electrochemically active hydrogel, specifically a redox hydrogel, and explored its application as an electrochemically active matrix. We incorporated a ferrocene (Fc)32,33/hydroxypropyl-β-cyclodextrin (CD) inclusion complex (Fc/CD) into a poly(vinyl alcohol) (PVA)/borax-based gel matrix (Fig. 1). Fc, a well-established redox-active hydrophobic molecule capable of single-electron oxidation, becomes water-soluble through inclusion within CD.34 We investigated both the viscoelastic properties and electrochemical anodic redox behaviour of the resulting hydrogel, further exploring its utility as a redox matrix for gel-based electrochromic devices.
Previous research has demonstrated that Fc/CD complexes maintain the electrochemical characteristics of Fc in aqueous electrolytes without dissociation or aggregation, despite Fc's inherent hydrophobicity.34 Herein, we integrated the Fc/CD complex into a PVA/borax hydrogel, which forms through hydrogen bonding between the hydroxyl groups of PVA and borax. We hypothesized that additional covalent, ionic, and hydrogen bonding interactions with the hydroxyl groups of CDs owing to the introduction of the Fc/CD complex into this hydrogel system would facilitate the incorporation of the complex into the gel network. Thus, this study focuses on the fabrication and characterization of this redox hydrogel and evaluates its potential as an advanced electrochemically active material.
2. Experimental section
Ferrocene (Fc, 98%), hydroxypropyl-β-cyclodextrin (CD, 98%), poly(vinyl alcohol) (PVA, degree of polymerisation 1500–1800, degree of saponification 78–82%) and sodium chloride (ethyl viologen dibromide) (EB, 98%) were purchased from Tokyo Chemical Industry Co., Ltd. and used as received. Water was purified with an Elix UV 3 Milli-Q integral water purification system (Nihon Millipore K.K., Tokyo, Japan).First, the synthesis of the Fc/CD complex was performed with reference to a previously reported procedure.34 An aqueous Fc/CD solution containing 30 mM Fc and 90 mM CD was prepared by making a mixture of a predetermined amount of Fc, CD, and water, which was mashed with a pestle and mortar. In addition, an 8 wt% PVA solution was obtained by heating and stirring the mixture of PVA and water at 90 °C for 24 h. PVA/borax gel (PVA-B) containing Fc/CD was (PVA/Fc-B) prepared in a vial at a weight ratio of 1/2 of Fc/CD solution and 8 wt% PVA solution, while PVA/Fc-B was prepared by adding 3 wt% aqueous borax solution while stirring with a vortex mixer (the stirring and mixing time was 1 minute), changing the weight ratio of borax solution to PVA solution to 1/4, 1/6, 1/8, and 1/16. As an abbreviation for these gels, the ratio of PVA/Fc-B to borax aqueous solution shall be written in the form, e.g., PVA/Fc-B 1/4. PVA/Fc-B containing NaCl as a supporting electrolyte was also prepared for electrochemical property evaluation. To prepare samples for electrochemical measurements, PVA/Fc-B gels were synthesized using three different aqueous base solutions, each containing 1 M NaCl: an Fc/CD solution, an 8 wt% PVA solution, and a borax solution. These NaCl-containing gel samples are abbreviated as PVA/Fc-B-N. These mixtures were non-uniform immediately after mixing but became uniform after standing at room temperature for 3 days. To determine whether the PVA/Fc-B sample in the sample tube was a gel or not, the sample tube was turned over and if it did not sag down, it was judged to be a gel, and if it sagged down, it was judged to be a sol or viscous wilt liquid (determination of gelation by the inversion method).
The infrared absorption spectra of the samples were recorded by attenuated total reflectance Fourier-transform infrared spectroscopy (ATR-FTIR) using a single-reflection diamond ATR system (FT/IR-6600 Type A; JASCO Corporation, Tokyo, Japan). The measurements were performed using a TGS detector with 16 scans at a resolution of 4 cm−1.
The surface microstructures and elemental compositions of the platinum-sputter-coated xerogel samples obtained by freeze-drying the hydrogels were investigated using field emission scanning electron microscopy (FE-SEM, JSM-6700FN; JEOL Ltd., Tokyo, Japan) and energy dispersive X-ray spectroscopy (EDS, SU-8020 TYPE II EDS; Hitachi High-Technologies Corp., Tokyo, Japan). FE-SEM images were acquired at an accelerating voltage of 1 kV, and EDS mappings were obtained at 10 kV.
Rheological measurements were performed using an MCR-301 rheometer (Anton Paar Japan K.K., Tokyo, Japan) with a parallel plate (8 mm diameter) at a gap of 0.50 mm at 25 °C. The strain sweeps were carried out at a constant angular frequency of 1 rad s−1. The repeated step-shear measurements were carried out with the application of a normal strain with an amplitude of 0.01% and a frequency of 1 Hz and a large strain with a shear rate of 3000 s−1 for 0.1 s.
Electrochemical characterization of the gels was performed using an electrochemical analyzer (model: 660E, B.A.S. Corporation). The electrochemical properties of the Fc/CD complexes in 1 M NaCl solution were evaluated in a three-electrode one-chamber cell using a platinum electrode (1.6 mm diameter) as the working electrode, a platinum wire (0.5 mm diameter) as the counter electrode and an Ag/AgCl electrode (3 M NaCl) as the reference electrode. Electrochemical characterization of the gel was performed by placing the gel at the bottom of a 5 ml mini cup (polypropylene, Maruemu Corporation) and bringing the working electrode, counter electrode, and reference electrode—identical to those used in the solution-phase measurements—into direct contact with the gel. The reference electrode was connected via a salt bridge consisting of an agar hydrogel containing 3 M NaCl, enclosed within a silicone tube with an inner diameter of 1 mm.
Electrochromic gel electrochemical properties were evaluated by kneading a mixture of PVA/Fc-B 1/4 15 mg/3 M NaCl solution 5 μl/1 M EB solution 5 μl, placing Kapton tape (150 μm) as a spacer between the ITO electrodes (10 mm × 10 mm, 10 Ω sq−1), and applying voltage using an electrochemical analyzer. Electrochromism was evaluated in a two-electrode system by applying voltage using an electrochemical analyzer (model: 660E, B.A.S. Corporation). Measurements of the absorbance of the electrochromic cells were performed using a measurement system consisting of an HR4000 spectrometer (Ocean Optics, Inc., Tokyo, Japan), a DH-200-BAL UV-VIS-NIR light source (Mikropack GmbH), and an FVA-UV variable attenuator (Ocean Optics, Inc., Tokyo, Japan) controlled using PC software OPwave (Ocean Photonics, Tokyo, Japan). The measurement system was constructed by Ocean Photonics.
3. Results and discussion
The PVA/borax hydrogel containing the Fc/CD complex (PVA/Fc-B) exhibited yellow coloration, reflecting the inherent colour of Fc (Fig. 2a). The material exhibited gel-like properties with notable viscosity. This enabled its lifting, stretching, and easy deformation into various shapes (Fig. 2b). Both PVA/Fc-B-N 1/4 and PVA-B 1/4 formed soft, adhesive gels. However, incorporating 1 M NaCl into the PVA/Fc-B hydrogel for electrochemical characterization significantly altered its mechanical properties. Compared to the NaCl-free PVA/Fc-B, the NaCl-containing hydrogel demonstrated increased softness. Moreover, with an increase in the NaCl concentration, the material progressively transitioned from a gel state to a viscous liquid, as observed in PVA/Fc-B-N 1/8 and PVA/Fc-B-N 1/16. This transition is quantified in subsequent rheological measurements. The phenomenon is attributable to NaCl disrupting the ionic crosslinking within the slime-type PVA/borax hydrogel. In the absence of NaCl, the hydrogel maintains its structure through electrostatic interactions that contribute to crosslinking. However, NaCl screens these electrostatic interactions. Consequently, the crosslinking network is weakened, and the number of effective crosslinking points is reduced, ultimately causing the transition from a gel to a viscous liquid. | ||
| Fig. 2 Photographs of the appearance of PVA/Fc-Bs: (a) PVA/Fc-Bs and PVA-B in the vial-inversion test and (b) PVA/Fc-B 1/4 in various shapes. | ||
To evaluate the presence of intermolecular interactions within the PVA/Fc-B hydrogel, ATR-FTIR measurements were performed. However, no significant spectral shifts or characteristic features indicative of strong intermolecular interactions were observed, and thus ATR-FTIR was not effective for characterizing the hydrogel structure in this case (Fig. S1, ESI†). Consequently, further surface analysis was carried out using FE-SEM and EDS techniques.
Surface morphology observation of the PVA/Fc-B xerogel using SEM revealed micron-scale wrinkles that were not present in the PVA-B sample, which lacks Fc/CD-based crosslinking units (Fig. 3a and b). This suggests that the PVA/Fc-B xerogel was derived from a hydrogel with a more finely structured network. Such structural refinement is likely attributable to an increased number of crosslinking points introduced by the Fc/CD interactions. Subsequent elemental analysis of the xerogel surface by EDS confirmed the presence of iron (Fe) in the PVA/Fc-B sample, whereas no Fe signal was detected in the PVA-B sample (Fig. 3c–f). Moreover, the detected Fe atoms were found to be uniformly distributed throughout the PVA/Fc-B sample. This suggests that ferrocene moieties in Fc/CD are uniformly incorporated within the hydrogel network and act as an integral component of the gel structure.
Dynamic viscoelastic measurements using a rheometer were conducted to evaluate the mechanical properties of the PVA/Fc-B-N hydrogels. Strain dispersion results revealed that PVA/Fc-B-N 1/4 and PVA/Fc-B-N 1/6, containing Fc/CD and NaCl within the PVA-B 1/4 hydrogel matrix, maintained their gel state (Fig. 4). These findings indicate that the selected PVA/borax ratio facilitates gel formation even in the presence of NaCl. At low strain, these samples exhibited storage moduli (G′) greater than their loss moduli (G′′), thereby confirming their gel-like behaviour.35 However, as the strain increased, a transition occurred where G′ became smaller than G′′. This implied a gel-to-sol transition and confirmed the original samples as being in a gel state. In contrast, PVA/Fc-B-N 1/8 and PVA/Fc-B-N 1/16 displayed G′′ greater than G′, signifying that these samples were not gels. Specifically, PVA/Fc-B-N 1/8 behaved as a highly viscous liquid, whereas PVA/Fc-B-N 1/16 exhibited low viscosity. These observations further supported the hypothesis that NaCl-induced disruption of crosslinking facilitated the breakdown of the gel structure.
Fig. 4 illustrates that incorporating Fc/CD into PVA-B 1/4 decreased the elastic modulus, resulting in a softer gel. This phenomenon is attributable to the hydroxyl groups of CDs interacting with borax, thereby reducing the number of effective crosslinking sites necessary for PVA–borax gel formation. We also assessed thixotropy36,37—a key parameter related to gel spreadability—of PVA/Fc-B-N 1/4, the most stable hydrogel among the tested samples. Despite its thixotropic behaviour being inferior to that of PVA-B, PVA/Fc-B-N 1/4 exhibited a reversible gel-to-sol transition (G′ > G′′ → G′ < G′′) under high shear stress, followed by recovery to a gel state (G′ > G′′) within seconds (Fig. 5). However, because G′ and G′′ remained nearly equivalent, the observed behaviour was classified as pseudo-thixotropy rather than true thixotropy.36 Similar but weaker pseudo-thixotropic behaviour was also observed in PVA/Fc-B-N 1/6. Thus, these findings suggest that introducing Fc/CD enables partial retention of thixotropic properties in the PVA/borax hydrogel system.
Next, we performed electrochemical characterization on the resulting gels and highly viscous liquids. CV measurements of the Fc/CD complex exhibited anodic oxidation and reduction waves involving a single electron from Fc. We observed increases in Epa (peak anodic oxidation potential of Fc) and Epc (peak anodic reduction potential of Fc) with increasing sweep rate, along with an increase in the peak-to-peak potential difference Epa − Epc (90 mV at 0.01 V s−1 to 175 mV at 0.5 V s−1) (Fig. 6a, Table 1 and Fig. S2, Table S1, ESI†). The increasing trend in Epa − Epc indicates that low sweep rates produce conditions closer to the ideal electron transfer process of Fc, although the complex formation deviates from Fc's ideal electron transfer process.32,33 Furthermore, Ipc/Ipa, a measure of electrochemical reversibility (where Ipc/Ipa = 1 indicates a fully reversible process38), approached 1 at low sweep rates. This confirmed greater electrochemical reversibility under these conditions.37 This finding aligns with previous studies of this complex33 and corroborates the peak-to-peak potential difference results. The CV curves demonstrated chemical reversibility, as evidenced by consistent traces after repeated sweeps. The trends observed in the Fc/CD complex CV measurements were also observed in the PVA/Fc-B-N 1/4, PVA/Fc-B-N 1/6, and PVA/Fc-B-N 1/8 samples containing NaCl (Fig. 6b–d, Table 1 and Fig. S2, Tables S2–S6, ESI†). These samples exhibited increases in Epa and Epc with increasing sweep rates and corresponding increases in Epa − Epc. Furthermore, the Ipc/Ipa values approached unity at lower sweep rates, indicating enhanced electrochemical reversibility, while identical CV curves across multiple sweeps confirmed chemical reversibility.38 Thus, the electrochemical properties of the Fc/CD complex, even when incorporated within the PVA/borax system, exhibited nearly identical trends to those observed in aqueous Fc/CD complex solutions. However, the Epa − Epc values were smaller in the PVA/borax system, possibly because Fc remains fixed near the electrode in the PVA/borax system, thereby reducing the shifts in Epa and Epc owing to mass diffusion effects.
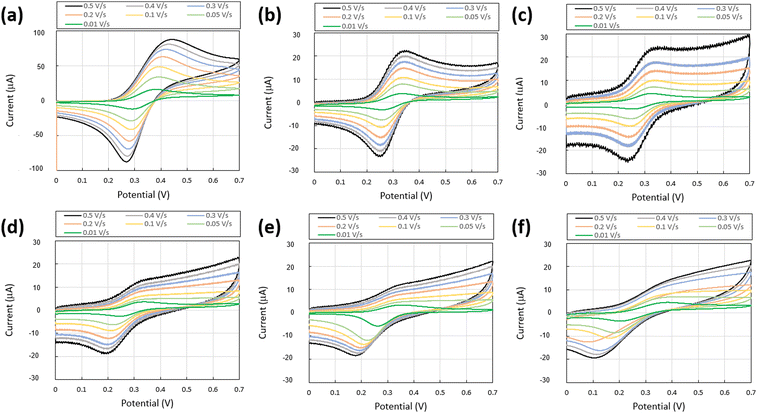 | ||
| Fig. 6 Cyclic voltammograms of PVA/Fc-B-N samples: (a) Fc/CD aqueous solution, (b) PVA/Fc-B-N 1/4, (c) PVA/Fc-B-N 1/6, (d) PVA/Fc-B-N 1/8, (e) PVA/Fc-B-N 1/16, and (f) PVA/Fc-B 1/4. | ||
| Sample | Anodic process | Cathodic process | Epa − Epc (mV) | Anodic process | Cathodic process | Isp0 (μA) | Ipc/Ipa |
|---|---|---|---|---|---|---|---|
| Epa (V) | Epc (V) | Ipa (μA) | Ipc (μA) | ||||
| Fc/CD aq. | 0.382 | 0.292 | 90 | 16.1 | 12.1 | 7.97 | 1.08 |
| PVA/Fc-B-N 1/4 | 0.335 | 0.26 | 75 | 3.78 | 3.15 | 1.46 | 1.11 |
| PVA/Fc-B-N 1/6 | 0.327 | 0.256 | 71 | 4.34 | 2.29 | 2.76 | 0.92 |
| PVA/Fc-B-N 1/8 | 0.335 | 0.25 | 85 | 3.60 | 2.61 | 1.46 | 1.01 |
| PVA/Fc-B-N 1/16 | 0.355 | 0.272 | 83 | 3.28 | 5.60 | 4.42 | 1.86 |
| PVA/Fc-B 1/4 | 0.383 | 0.214 | 169 | 4.57 | 3.45 | 2.46 | 1.10 |
For PVA/Fc-B-N 1/16 (Fig. 6e), interpretable data could only be obtained at a sweep rate of 0.01 V s−1. While the Epa − Epc trend was similar to that of other slime systems, at this low sweep rate it demonstrated characteristics closer to the ideal electron transfer than the other samples. However, with Ipc/Ipa exceeding 1, the system cannot be classified as electrochemically reversible. Nevertheless, the consistent CV curves across repeated sweeps indicate the chemical reversibility of the electrochemical process.
When measuring PVA/Fc-B 1/4 (the sample without NaCl), interpretable data could only be obtained at a sweep rate of 0.01 V s−1, similar to PVA/Fc-B 1/16. The Epa − Epc value exceeded that of the NaCl-containing PVA/borax systems, resembling values observed in aqueous solutions (Fig. 6f). This may result from insufficient electrical conductivity—while Na+ ions are present in the PVA/borax system, their concentration is inadequate for electrochemical measurements under the present conditions, rendering the application of effective voltage for electron transfer challenging. However, with Ipc/Ipa approaching 1, the process exhibits electrochemical reversibility.29 Overall, our electrochemical evaluation confirms that both PVA/Fc-B-N and PVA/Fc-B systems function as electrochemically active redox hydrogels at specific component ratios.
As demonstrated above, the initial CV cycles exhibited favourable redox behaviour. However, the cycling stability of the Fc/CD-based hydrogel system (PVA/Fc-B-N 1/4) was relatively low under the present conditions. Even after approximately 10 CV cycles, a noticeable attenuation of the redox current peaks was observed, and after 100 cycles, the decrease became pronounced (Fig. S3, ESI†). This degradation in electrochemical performance is presumed to result from the progressive dissociation of the Fc/CD inclusion complexes upon repeated potential sweeps. Improving the electrochemical cycling stability of this Fc/CD-based hydrogel remains a critical challenge for its future practical applications. Nevertheless, it is anticipated that optimizing the external solution environment to suppress dissociation of the Fc/CD complexes could lead to enhanced stability during repeated electrochemical operation.
As noted in the Introduction section, one of the promising applications of redox-active hydrogels is their use in electrochromic devices, which has attracted increasing attention in recent years. If the PVA/Fc-B hydrogel system—which was confirmed through our electrochemical evaluation to be redox-active—can function as a redox hydrogel matrix, it should enable the fabrication of a single-layer, two-electrode gel-based electrochromic device through a simple process. This process leverages the thixotropic nature of the redox hydrogel, facilitating the incorporation of electrochromic molecules via simple mixing.
We fabricated a single-layer, two-electrode gel-based electrochromic device using PVA/Fc-B 1/4 as the redox hydrogel matrix. Ethyl viologen dibromide (EB), a well-known electrochromic molecule that exhibits blue coloration upon electrochemical reduction in aqueous electrolyte solutions,24 served as the electrochromic species.39,40 The device was fabricated as a two-electrode system by sandwiching a gel composite—prepared by incorporating the electrochromic molecule EB and the supporting electrolyte NaCl into the redox hydrogel matrix PVA/Fc-B 1/4—between two transparent ITO electrodes. A square-wave voltage alternating between −1.9 V and 0.0 V, which was sufficient to induce coloration and bleaching, was applied to the device. When the voltage was applied at 60-second intervals, the absorbance change reached a steady state, indicating that this duration was sufficient for complete redox switching of the electrochromic response (Fig. S4a, ESI†). To evaluate the device's performance under faster switching conditions, subsequent experiments were conducted using 10-second intervals for voltage application. Upon voltage application, the system exhibited reversible colour changes. This confirmed the reversible electrochromic behaviour of the PVA/Fc-B/NaCl/EB system (Fig. 7a). Moreover, the UV-Vis absorption spectra revealed that these colour changes resulted from shifts in the absorption band within the visible region owing to the generation of the viologen radical cation by electrochemical reduction, consistent with previous studies on EB electrochromism (Fig. 7b).30,34 However, the presence of a shoulder around 600 nm indicates that the spectral profile differs from that of a typical viologen radical cation alone. This deviation suggests the possible formation of an additional absorption band arising from charge-transfer interactions between ferrocene and viologen moieties.41 Furthermore, we observed electrochemical current responses corresponding to the applied voltage (Fig. 7c). Unfortunately, although the electrochromic performance remained within an acceptable range over the first few cycles, a gradual decrease in absorbance was observed after approximately 30 cycles of voltage application (Fig. S4b, ESI†). As previously discussed, this degradation is likely due to the dissociation of the Fc/CD inclusion complexes under repeated electrochemical stimulation, resulting in the loss of function of the redox hydrogel matrix. Therefore, to improve the electrochromic durability of this device, it will be essential to develop an internal gel environment that suppresses the decomplexation of Fc/CD.
To further investigate the role of the PVA/Fc-B hydrogel in the device, we conducted a control experiment using a hydrogel without Fc, consisting solely of PVA-B/NaCl/EB. In this case, no EB coloration occurred upon voltage application. This result suggests that Fc's electrochemical oxidation at the anode is essential for facilitating EB's electrochemical reduction at the cathode, thereby maintaining charge balance and enabling smooth progression of the overall electrochemical reaction. These findings demonstrate that the PVA/Fc-B hydrogel functions effectively as a redox hydrogel matrix, facilitating electrochemical reactions within the device.
4. Conclusions
This study developed a redox hydrogel that served as a matrix for aqueous electrochemical reactions. We successfully synthesized a PVA/borax hydrogel containing a water-soluble ferrocene complex. The resulting ferrocene-incorporated PVA/borax hydrogel exhibited viscoelastic properties, thereby allowing it to be lifted, stretched, and moulded into various shapes, demonstrating its versatility as a functional material.Electrochemical characterization using cyclic voltammetry confirmed that the ferrocene-incorporated PVA/borax hydrogel exhibited electrochemical responses derived from ferrocene. This validated its function as a redox hydrogel, as initially intended. As a demonstration of potential applications, we successfully incorporated the material into an electrochromic device and demonstrated its capacity to serve as a matrix for electrochemical reactions.
These results indicate that the developed hydrogel holds promise for diverse electrochemical applications, including advanced electrochromic devices, electrochemical luminescent systems, and other electrochemical technologies. The combination of mechanical versatility and electrochemical functionality in this redox hydrogel offers significant potential for future innovations in flexible electronics and wearable electrochemical devices.
Author contributions
Conceptualization, Y. O.; investigation, R. E. and Y. O.; writing – original draft preparation, R. E. and Y. O.; writing – review and editing, R. E. and Y. O.; supervision, Y. O. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The authors confirm that the data supporting the findings of this study are available within the article.References
- D. Feldman, J. Compos. Sci., 2020, 4, 175 CrossRef CAS
.
- N. Ben Halima, RSC Adv., 2016, 6, 39823–39832 RSC
.
- M. Aslam, M. A. Kalyar and Z. A. Raza, Polym. Eng. Sci., 2018, 58, 2119–2132 CrossRef CAS
.
- C. Xu, Y. Chen, S. Zhao, D. Li, X. Tang, H. Zhang, J. Huang, Z. Guo and W. Liu, Chem. Rev., 2024, 124, 10435–10508 CrossRef CAS PubMed
.
- P. Bertsch, M. Diba, D. J. Mooney and S. C. G. Leeuwenburgh, Chem. Rev., 2023, 123, 834–873 CrossRef CAS PubMed
.
- Y. Ohsedo and W. Ueno, Gels, 2023, 9, 679 CrossRef CAS
.
- S.-H. Hyon, W.-I. Cha and Y. Ikada, Polym. Bull., 1989, 22, 119–122 CrossRef CAS
.
- S. Shi, X. Peng, T. Liu, Y.-N. Chen, C. He and H. Wang, Polymer, 2017, 111, 168–176 CrossRef CAS
.
- S. Alipoori, S. Mazinani, S. H. Aboutalebi and F. Sharif, J. Energy Storage, 2020, 27, 101072 CrossRef
.
- E. Z. Casassa, A. M. Sarquis and C. H. Van Dyke, J. Chem. Educ., 1986, 63, 57 CrossRef CAS
.
- K. Parida, V. Kumar, W. Jiangxin, V. Bhavanasi, R. Bendi and P. S. Lee, Adv. Mat., 2017, 29, 1702181 CrossRef PubMed
.
- Y. Alesanco, J. Palenzuela, A. Viñuales, G. Cabañero, H. J. Grande and I. Odriozola, ChemElectroChem, 2015, 2, 218–223 CrossRef CAS
.
- H. Yuk, B. Lu and X. Zhao, Chem. Soc. Rev., 2019, 48, 1642–1667 RSC
.
- S. Cheng, R. Zhu and X. Xu, Commun. Mater., 2024, 5, 1–9 CrossRef
.
- S. Lee, X. Liang, J. S. Kim, T. Yokota, K. Fukuda and T. Someya, Chem. Rev., 2024, 124, 6543–6591 CrossRef CAS PubMed
.
- Y. Zhang, Y. Tan, J. Lao, H. Gao and J. Yu, ACS Nano, 2023, 17, 9681–9693 CrossRef CAS
.
- R. Singh, R. Gupta, D. Bansal, R. Bhateria and M. Sharma, ACS Omega, 2024, 9, 7336–7356 CAS
.
- Dhanjai, A. Sinha, P. K. Kalambate, S. M. Mugo, P. Kamau, J. Chen and R. Jain, Trends Anal. Chem., 2019, 118, 488–501 CrossRef CAS
.
- S. Bonardd, M. Nandi, J. I. Hernández García, B. Maiti, A. Abramov and D. Díaz Díaz, Chem. Rev., 2023, 123, 736–810 CrossRef CAS PubMed
.
- L. Li, Y. Zhang, H. Lu, Y. Wang, J. Xu, J. Zhu, C. Zhang and T. Liu, Nat. Commun., 2020, 11, 62 CrossRef CAS PubMed
.
- Y. Ohsedo and M. Sasaki, Gels, 2022, 8, 469 CrossRef CAS PubMed
.
- Y. Ohsedo and M. Sasaki, New J. Chem., 2023, 47, 17817–17823 RSC
.
- C. Nitta and Y. Ohsedo, Chem. – Eur. J., 2024, 30, e202401469 CrossRef CAS PubMed
.
- P. M. S. Monk, R. J. Mortimer and D. R. Rosseinsky, Electrochromism Electrochromic Devices, Cambridge University Press, Cambridge, UK, 2007 Search PubMed
.
- P. M. Beaujuge and J. R. Reynolds, Chem. Rev., 2010, 110, 268–320 CrossRef CAS PubMed
.
- G.-J. Yang, Y.-M. Zhang, Y.-R. Cai, B.-G. Yang, C. Gu and S. X.-A. Zhang, Chem. Soc. Rev., 2020, 49, 8687–8720 RSC
.
- C. Gu, A.-B. Jia, Y.-M. Zhang and S. X.-A. Zhang, Chem. Rev., 2022, 122, 14679–14721 CrossRef CAS PubMed
.
- H. Fang, P. Zheng, R. Ma, C. Xu, G. Yang, Q. Wang and H. Wang, Mater. Horiz., 2018, 5, 1000–1007 RSC
.
- S. Halder, S. Roy, M. Dixit and C. Chakraborty, Chem. Commun., 2022, 58, 8368–8371 RSC
.
- T. J. Adams, A. R. Brotherton, J. A. Molai, N. Parmar, J. R. Palmer, K. A. Sandor and M. G. Walter, Adv. Funct. Mater., 2021, 31, 2103408 CrossRef CAS
.
- F. Zhao, B. Wang, J. Chen, S. Cao, Y. Yang, W. W. Yu and H. Li, Phys. Status Solidi RRL, 2024, 18, 2300345 CrossRef CAS
.
- R. R. Gagne, C. A. Koval and G. C. Lisensky, Inorg. Chem., 1980, 19, 2854–2855 CrossRef CAS
.
- R. Kaur, A. Ghoshal, P. Galav and P. C. Mondal, Chem. – Asian J., 2024, 19, e202400744 CrossRef CAS PubMed
.
- Y. Li, Z. Xu, Y. Liu, S. Jin, E. M. Fell, B. Wang, R. G. Gordon, M. J. Aziz, Z. Yang and T. Xu, ChemSusChem, 2021, 14, 745–752 CrossRef CAS PubMed
.
- G. M. Kavanagh and S. B. Ross-Murphy, Prog. Polym. Sci., 1998, 23, 533–562 CrossRef CAS
.
- J. Goodwin and R. Hughes, Rheology for Chemists: An Introduction, Royal Society of Chemistry, Cambridge, 2nd edn, 2008 Search PubMed
.
- R. G. Larson and Y. Wei, J. Rheol., 2019, 63, 477–501 CrossRef CAS
.
- A. J. Bard, L. R. Faulkner and H. S. White, Electrochemical Methods: Fundamentals and Applications, Wiley, 3rd edn, 2022, ch. 7 Search PubMed
.
- N. Ahlawat, L. Bansal, B. Sahu, D. K. Rath, S. Tiwari, A. Chaudhary, S. Kaladi Chondath and R. Kumar, ACS Appl. Electron. Mater., 2025, 7, 5712–5720 CrossRef CAS
.
- L. Bansal, F. Lübkemann-Warwas, B. Sahu, T. Ghosh, S. Kandpal, C. Rani, D. K. Rath, L. Prawitt, C. Wesemann, N. C. Bigall and R. Kumar, ACS Appl. Mater. Interfaces, 2025, 17, 31201–31211 CrossRef CAS PubMed
.
- Y. Nishikitani, S. Uchida, T. Asano, M. Minami, S. Oshima, K. Ikai and T. Kubo, J. Phys. Chem. C, 2008, 112, 4372–4377 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5tc02004b |
| This journal is © The Royal Society of Chemistry 2025 |





